Ex Vivo and Simulation Comparison of Leakage in End-to-End Versus End-to-Side Anastomosed Porcine Large Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
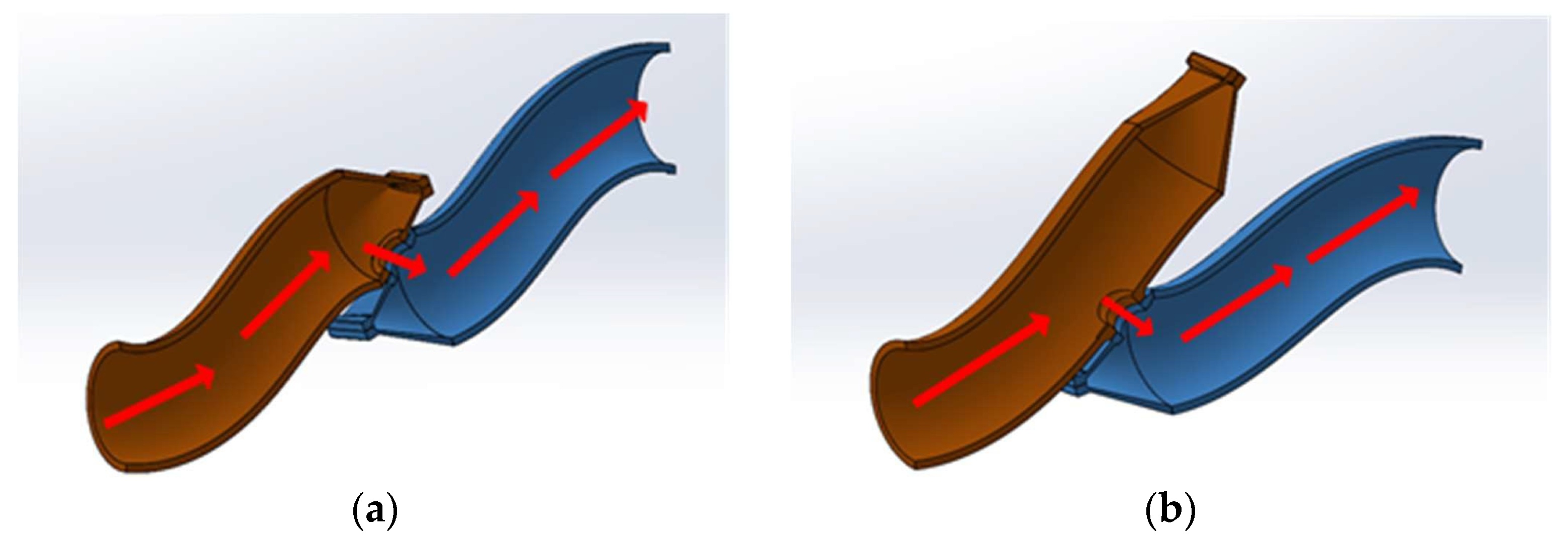
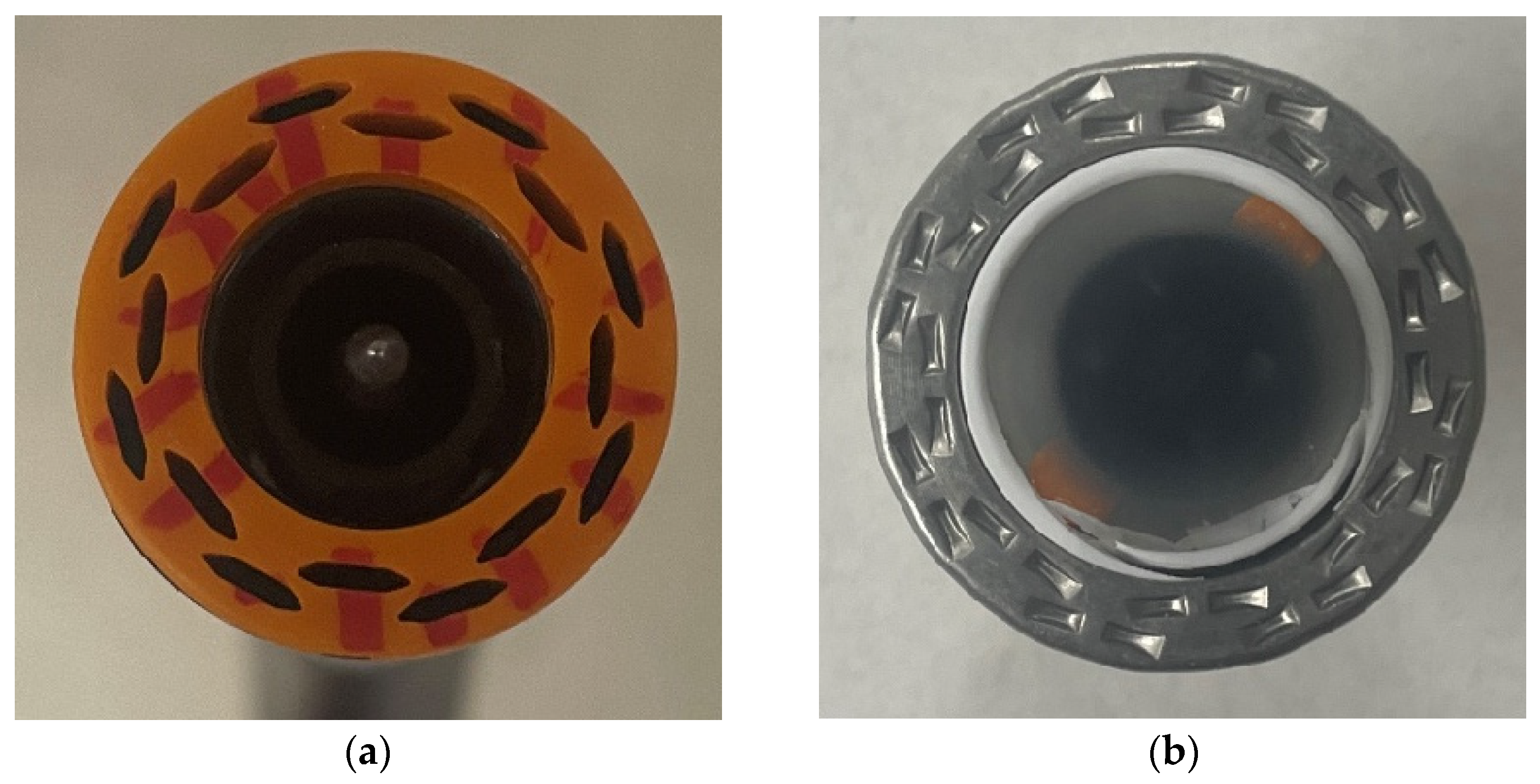
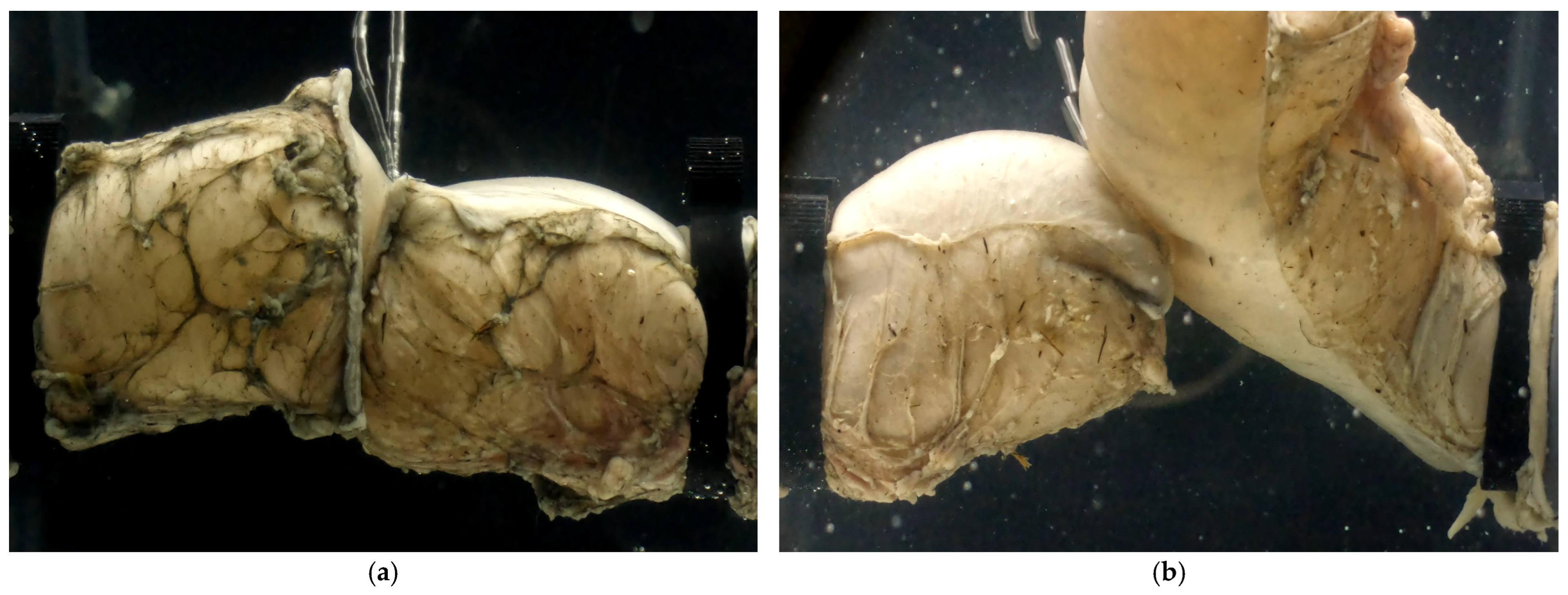
2.2. Specimen Preparation
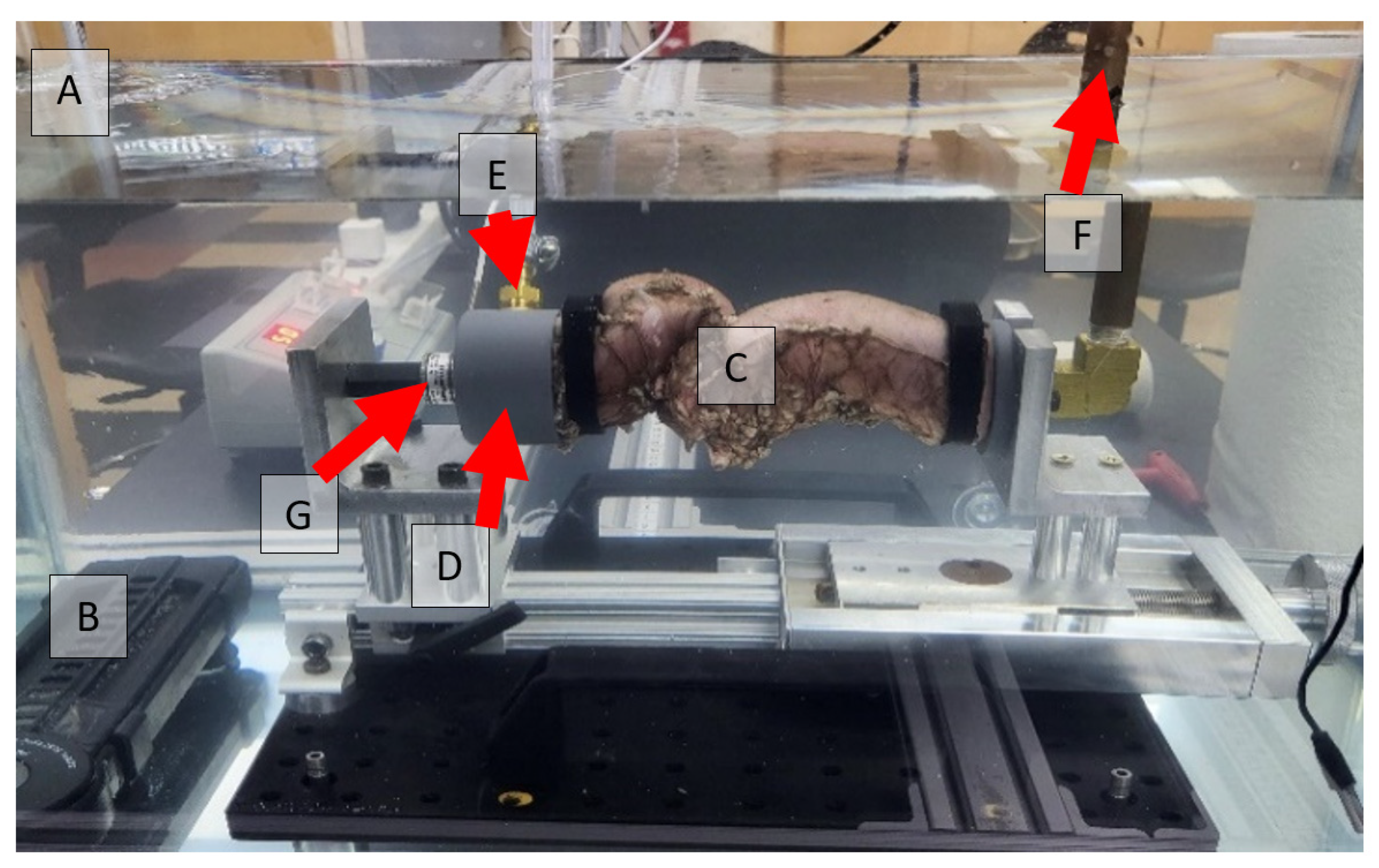
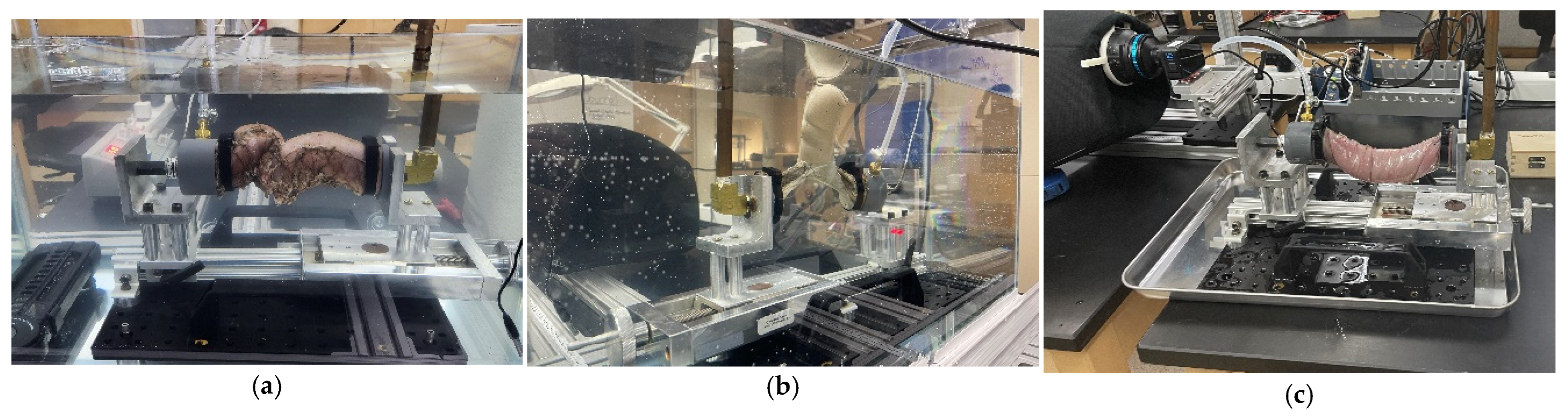
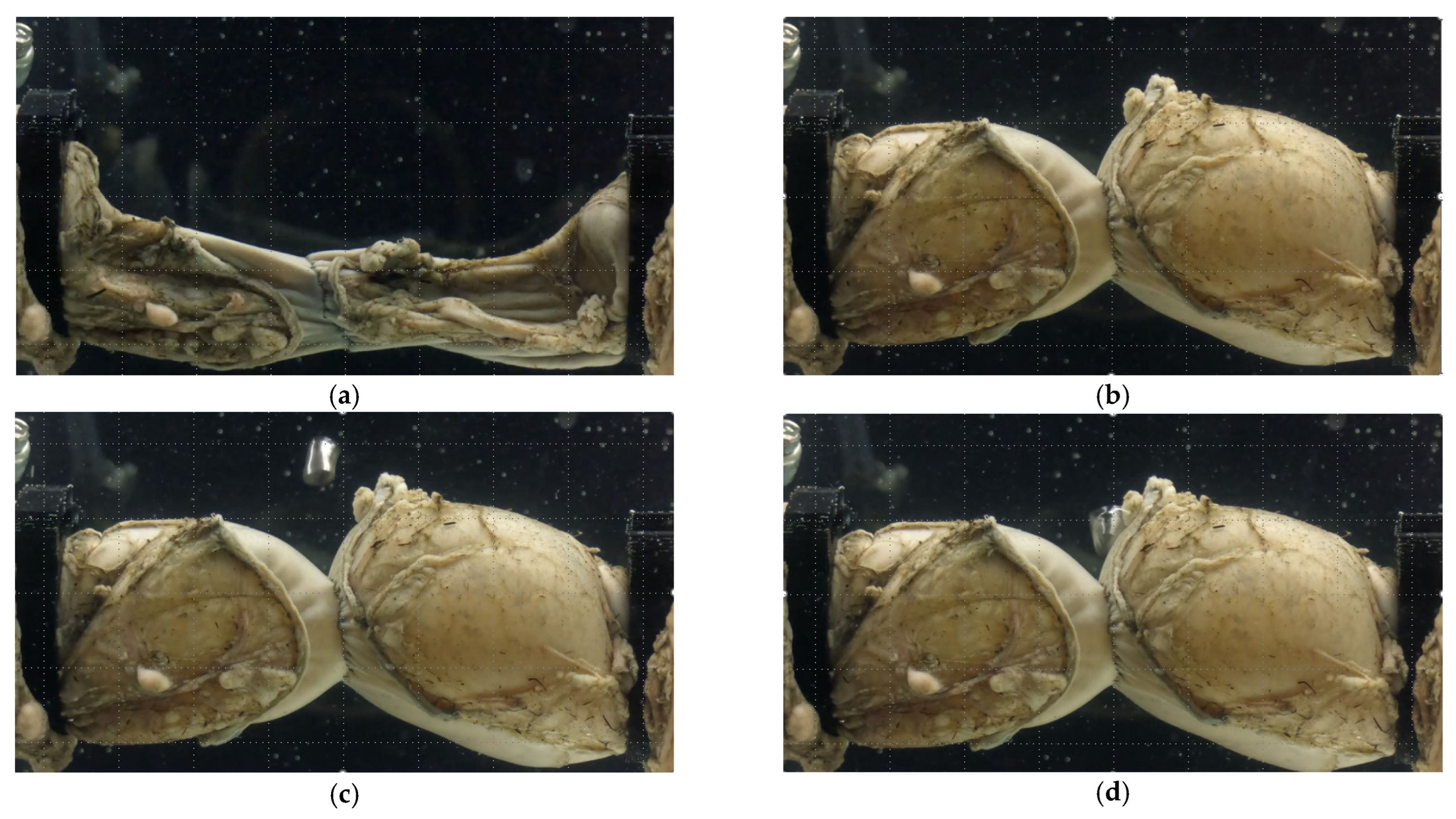
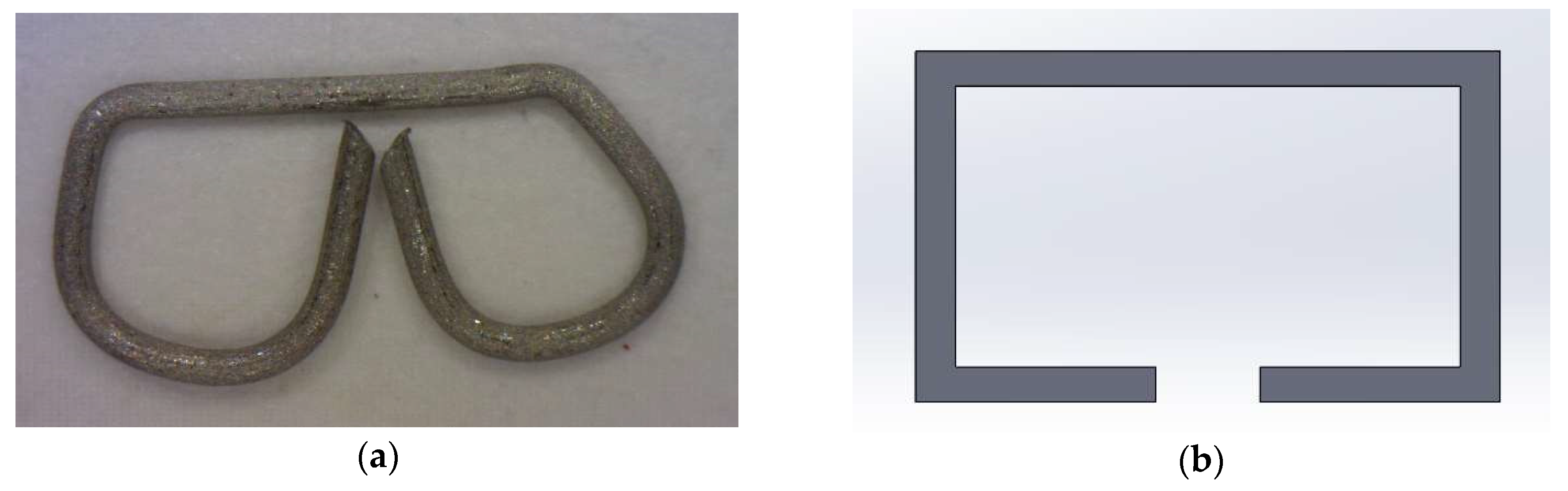
2.3. Anastomotic Leakage Experimental Setup
2.4. Data Extraction and Processing
2.5. Statistical Analysis
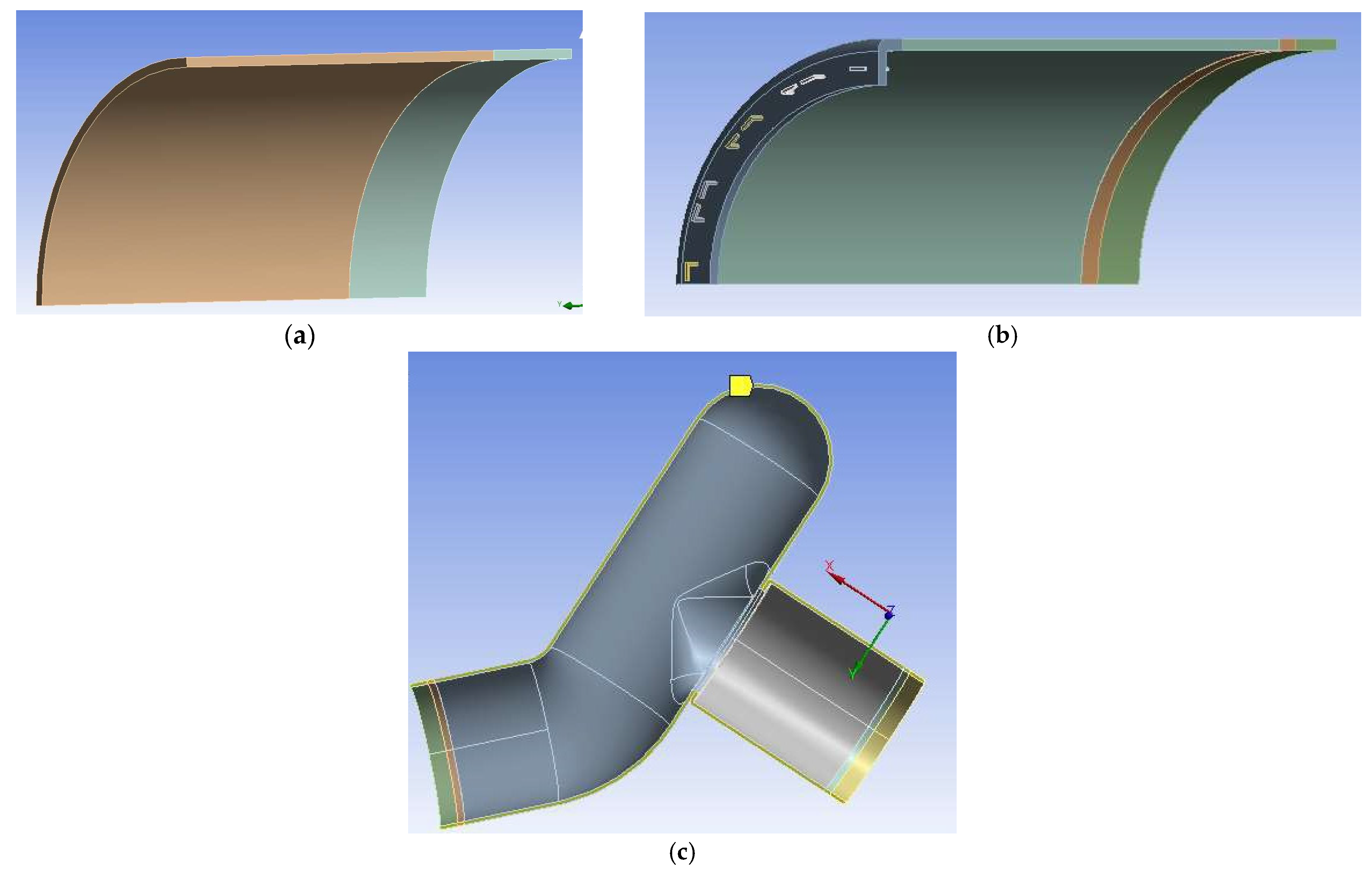
2.6. Simulation of Anastomotic Leak Testing Experiments
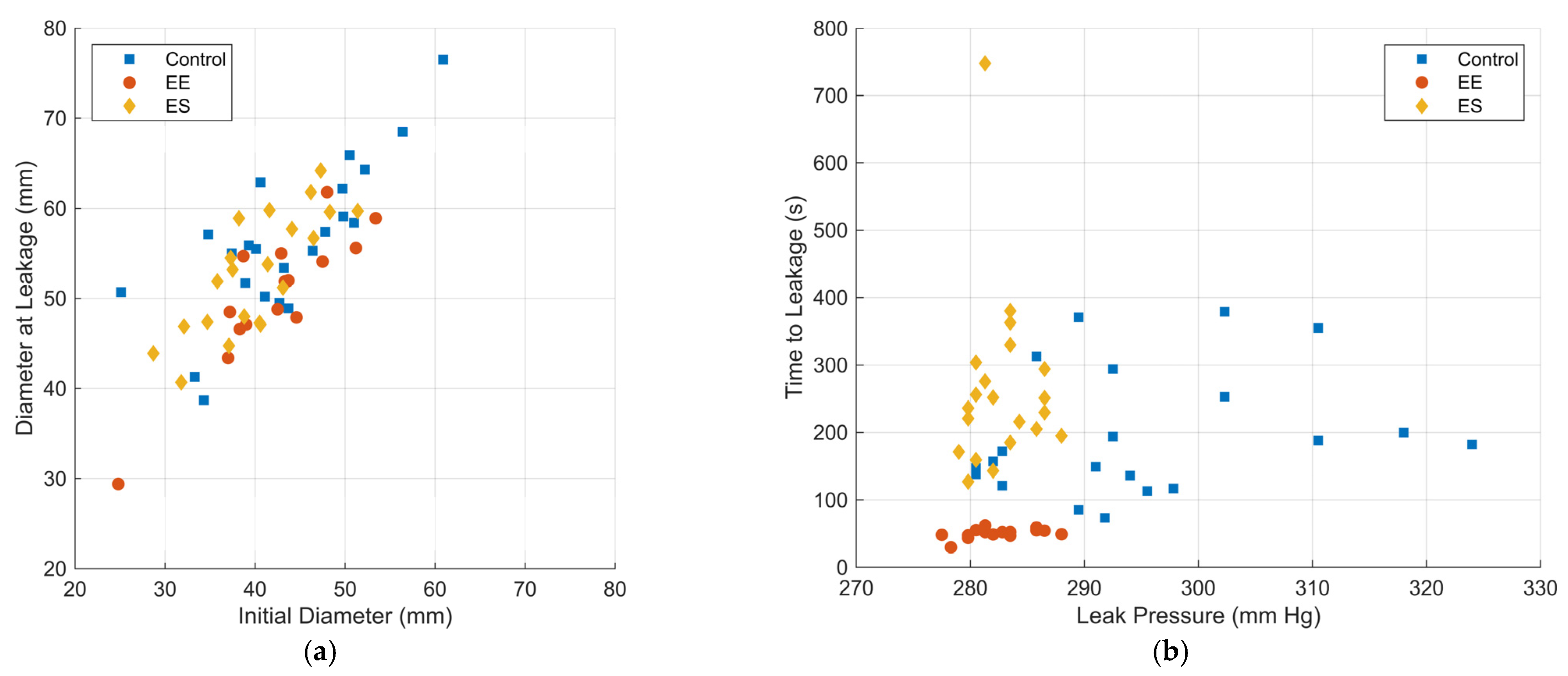
3. Results
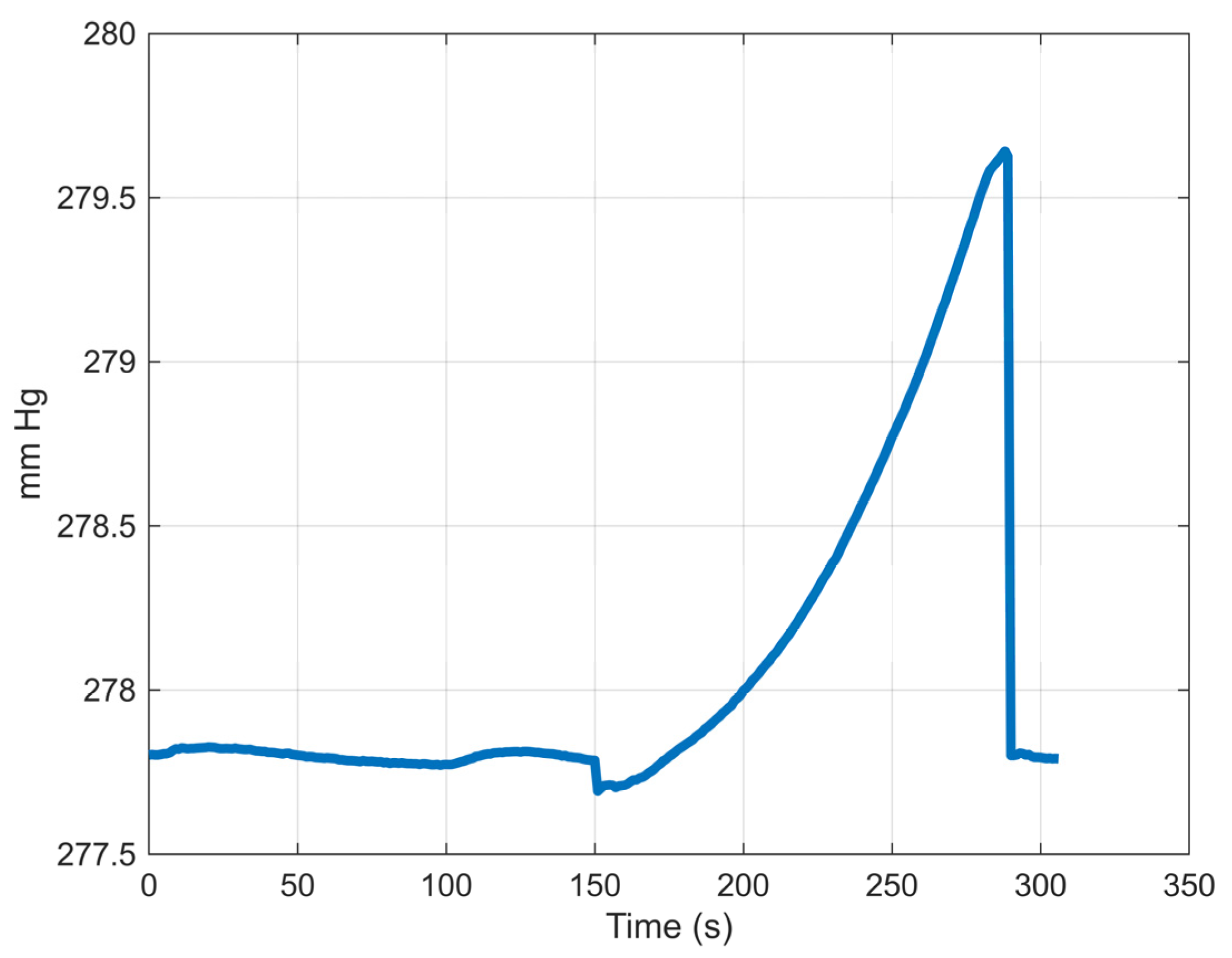
3.1. Experimental Results
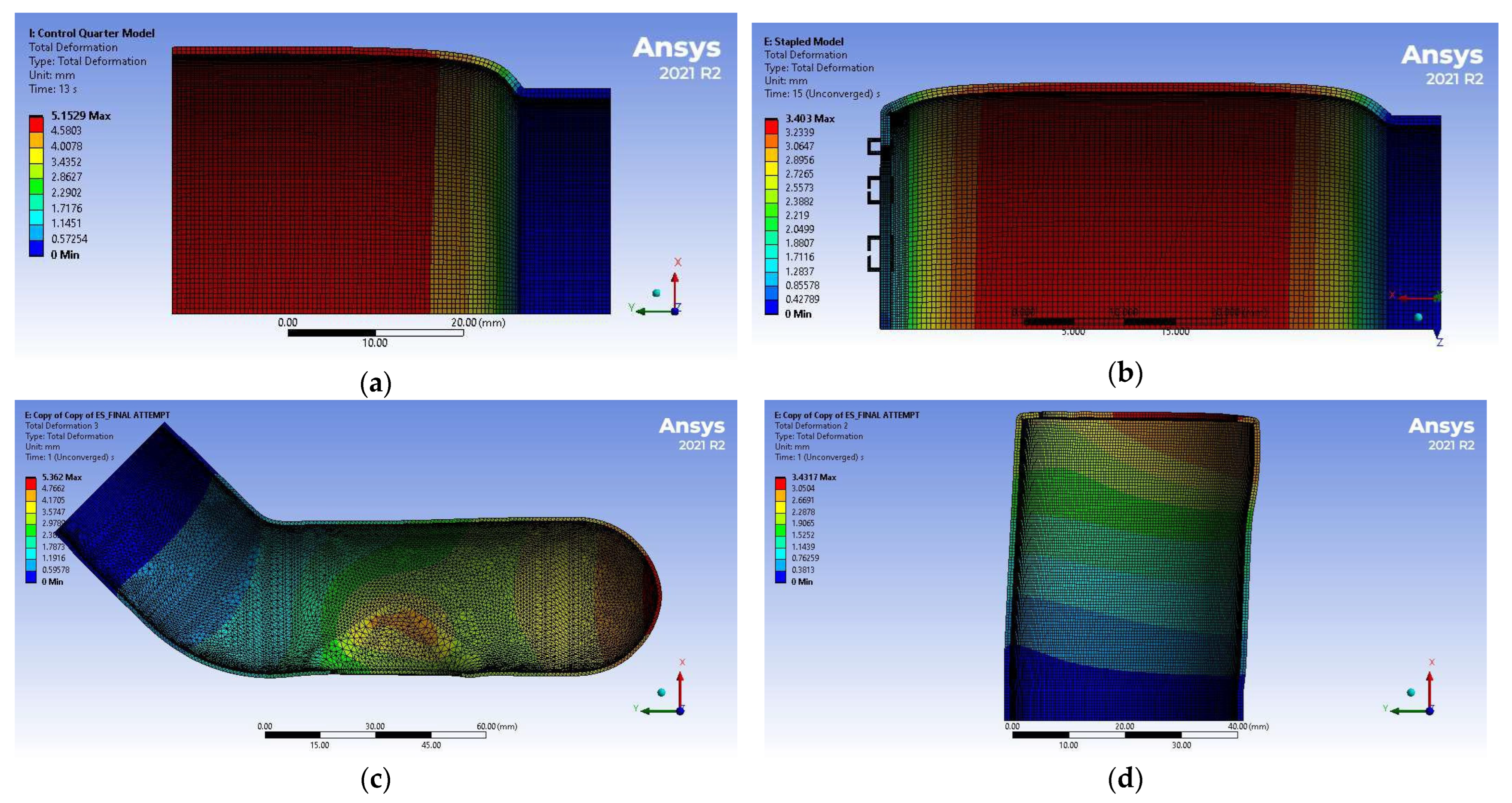
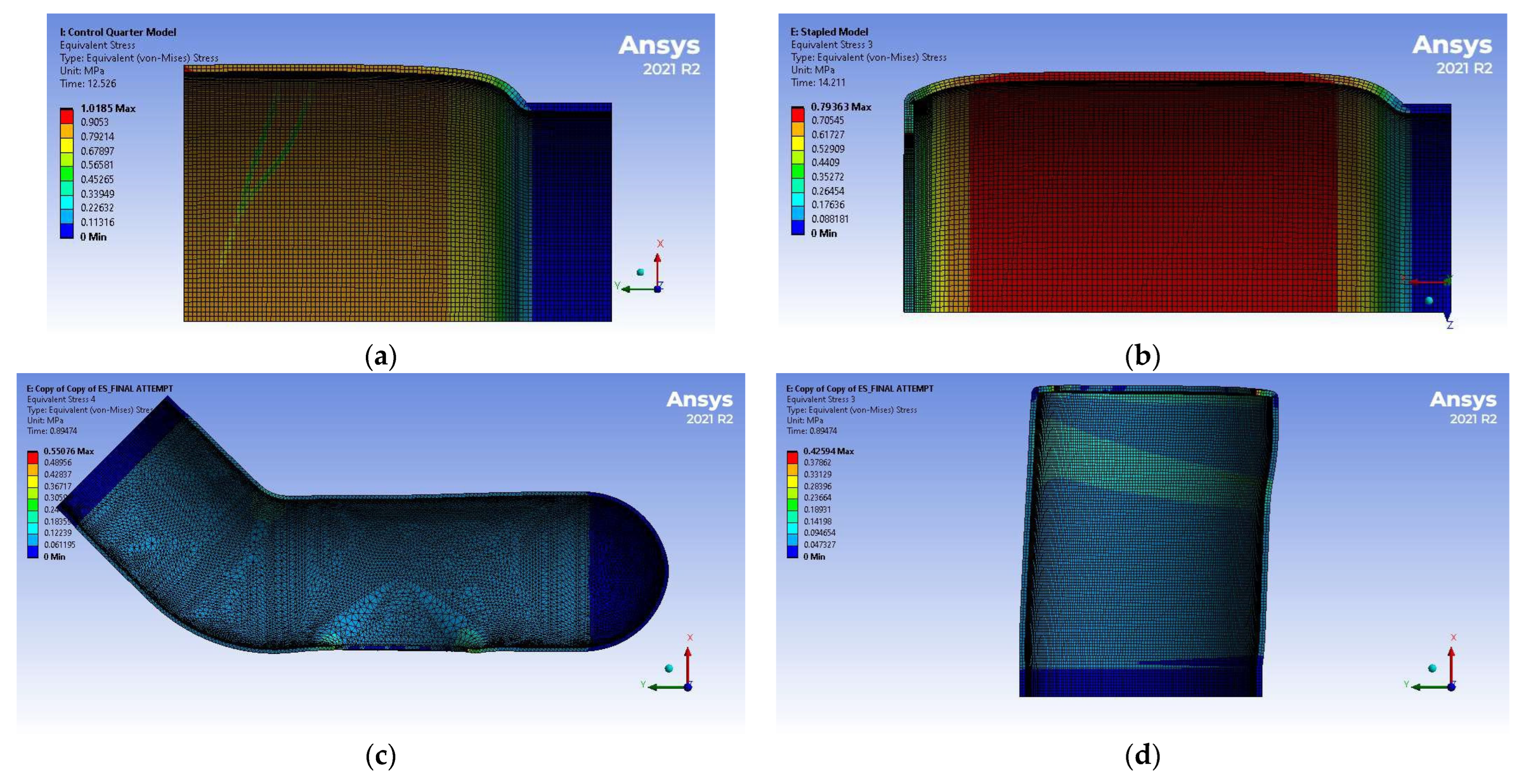
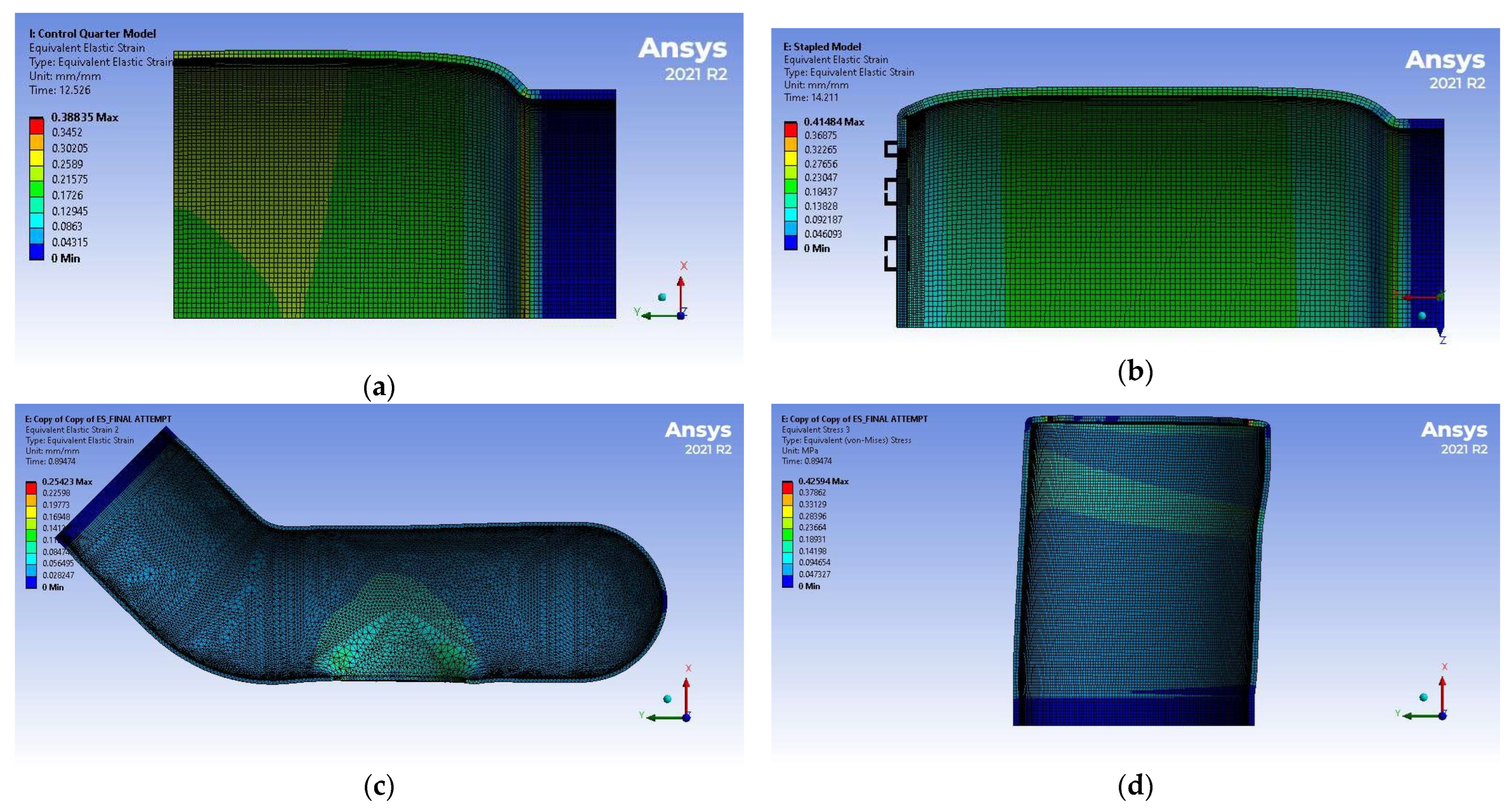
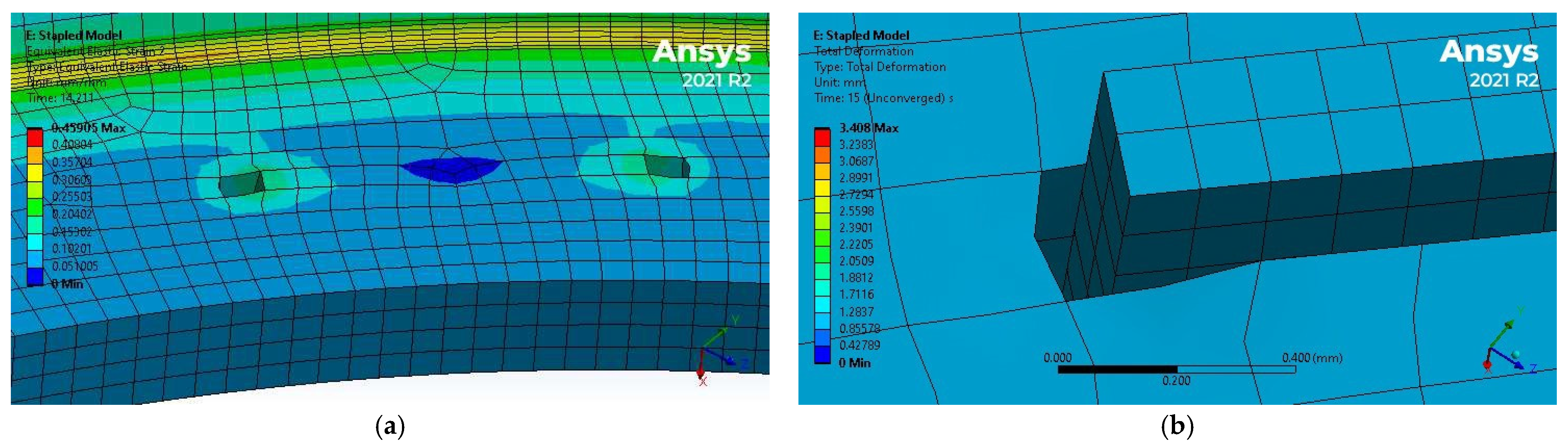
3.2. Simulation Results
4. Discussion
4.1. Experimental Results Discussion
4.2. Discussion of FEM Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Anastomotic leakage |
| EE | End-to-end colorectal anastomosis |
| ES | End-to-side colorectal anastomosis |
| FEM | Finite element method |
References
- Ho, Y.H.; Ashour, M.A.T. Techniques for colorectal anastomosis. World J. Gastroenterol. 2010, 16, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.H.; Chao, W.; Ecker, M. Review of colonic anastomotic leakage and prevention methods. J. Clin. Med. 2020, 9, 4061. [Google Scholar] [CrossRef]
- Rullier, E.; Laurent, C.; Garrelon, J.; Michel, P.; Saric, J.; Parneix, M. Risk factors for anastomotic leakage after rectal cancer surgery. Br. J. Surg. 1998, 85, 355–358. [Google Scholar] [CrossRef]
- Jestin, P.; Påhlman, L.; Gunnarsson, U. Risk factors for anastomotic leakage after rectal cancer surgery: A case-control study. Color. Dis. 2008, 10, 715–721. [Google Scholar] [CrossRef]
- Kang, C.Y.; Halabi, W.J.; Chaudhry, O.O.; Nguyen, V.; Pigazzi, A.; Carmichael, J.C.; Mills, S.; Stamos, M.J. Risk factors for anastomotic leakage after anterior resection for rectal cancer. Arch. Surg. 2013, 148, 65–71. [Google Scholar] [CrossRef]
- Hammond, J.; Lim, S.; Wan, Y.; Gao, X.; Patkar, A. The Burden of Gastrointestinal Anastomotic Leaks: An Evaluation of Clinical and Economic Outcomes. J. Gastrointest. Surg. 2014, 18, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Pollack, E.; Johnston, S.; Petraiuolo, W.J.; Roy, S.; Galvain, T. Economic analysis of leak complications in anastomoses performed with powered versus manual circular staplers in left-sided colorectal resections: A us-based cost analysis. Clin. Outcomes Res. 2021, 13, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Bundy, C.A.; Jacobs, D.M.; Zera, R.T.; Bubrick, M.P. Comparison of bursting pressure of sutured, stapled and BAR anastomoses. Int. J. Colorectal Dis. 1993, 8, 1–3. [Google Scholar] [CrossRef]
- Wu, Z.; van de Haar, R.C.J.; Sparreboom, C.L.; Boersema, G.S.A.; Li, Z.; Ji, J.; Jeekel, J.; Lange, J.F. Is the intraoperative air leak test effective in the prevention of colorectal anastomotic leakage? A systematic review and meta-analysis. Int. J. Color. Dis. 2016, 31, 1409–1417. [Google Scholar] [CrossRef]
- Vardhan, S.; Deshpande, S.G.; Singh, A.; Aravind Kumar, C.; Bisen, Y.T.; Dighe, O.R. Techniques for Diagnosing Anastomotic Leaks Intraoperatively in Colorectal Surgeries: A Review. Cureus 2023, 15, e34168. [Google Scholar] [CrossRef]
- An, V.; Chandra, R.; Lawrence, M. Anastomotic Failure in Colorectal Surgery: Where Are We at? Indian J. Surg. 2018, 80, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hansen, O.; Schwenk, W.; Hucke, H.P.; Stock, W. Colorectal stapled anastomoses: Experiences and results. Dis. Colon Rectum 1996, 39, 30–36. [Google Scholar] [CrossRef]
- Neutzling, C.B.; Lustosa, S.A.; Proenca, I.M.; da Silva, E.M.; Matos, D. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst. Rev. 2012, 2012, CD003144. [Google Scholar] [CrossRef]
- Slieker, J.C.; Daams, F.; Mulder, I.M.; Jeekel, J.; Lange, J.F. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 2013, 148, 190–201. [Google Scholar] [CrossRef]
- Kosuge, M.; Eto, K.; Hashizume, R.; Takeda, M.; Tomori, K.; Neki, K.; Mitsumori, N.; Yanaga, K. Which Is the Safer Anastomotic Method for Colon Surgery?—Ten-year results. In Vivo 2017, 31, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Luglio, G.; Corcione, F. Stapled versus handsewn methods for ileocolic anastomoses. Tech. Coloproctol. 2019, 23, 1093–1095. [Google Scholar] [CrossRef]
- Planellas, P.; Farrés, R.; Cornejo, L.; Rodríguez-Hermosa, J.I.; Pigem, A.; Timoteo, A.; Ortega, N.; Codina-Cazador, A. Randomized clinical trial comparing side to end vs. end to end techniques for colorectal anastomosis. Int. J. Surg. 2020, 83, 220–229. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Sharma, S.; Daniel, R.; Eskicioglu, C. End-to-end versus end-to-side anastomosis for low anterior resection: A systematic review and meta-analysis of randomized controlled trials. Surgery 2021, 170, 397–404. [Google Scholar] [CrossRef]
- Wang, T.; Sadowsky, M.; Blakney, R.; Coplan, P.; Petraiuolo, W.; Soberman, M.; Tomaszewski, J.; Rene, L.; Wood, J. Risk of anastomotic leakage with two-row versus three-row manual circular staplers in colorectal anastomosis: A U.S. cohort study. Int. J. Color. Dis. 2023, 38, 264. [Google Scholar] [CrossRef]
- McKechnie, T.; Shi, V.; Huang, E.; Huo, B.; Doumouras, A.; Amin, N.; Eskicioglu, C.; Hong, D. Double-row staple technology versus triple-row staple technology for colorectal surgery: A systematic review and meta-analysis. Surgery 2024, 176, 633–644. [Google Scholar] [CrossRef]
- Christensen, H.; Langfelt, S.; Laurberg, S. Bursting strength of experimental colonic anastomoses: A methodological study. Eur. Surg. Res. 1993, 25, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Trunzo, A.; Schomisch, S.; Mishra, T.; Andrews, J.; Ponsky, J.; Marks, J. The Validity of Bursting Pressure As An Objective Measure in Natural Orifice Translumenal Endoscopic Surgery (NOTES) Closure Testing. Gastrointest. Endosc. 2009, 69, 169. [Google Scholar] [CrossRef]
- Norbury, K.C.; Kilpadi, D.V.; Collins, B.A.; Cunningham, M.R. Burst strength testing of porcine intestinal anastomoses following negative pressure therapy. Surg. Innov. 2012, 19, 181–186. [Google Scholar] [CrossRef]
- Schwab, R.; Weßendorf, S.; Gutcke, A.; Becker, H. Early bursting strength of human colon anastomoses—An in vitro study comparing current anastomotic techniques. Langenbeck’s Arch. Surg. 2002, 386, 507–511. [Google Scholar] [CrossRef]
- Stewart, D.; Hunt, S.; Pierce, R.; Mao, D.; Frisella, M.; Cook, K.; Starcher, B.; Fleshman, J. Validation of the NITI endoluminal compression anastomosis ring (EndoCAR) device and comparison to the traditional circular stapled colorectal anastomosis in a porcine model. Surg. Innov. 2007, 14, 252–260. [Google Scholar] [CrossRef]
- Trần, T.; Nováček, V.; Tolba, R.; Klinge, U.; Turquier, F.; Staat, M. Experimental and Computational Approach to Study Stapled Colorectal Anastomosis. In Proceedings of the 13rd Congress of the International Society of Biomechanics, Brussels, Belgium, 3–7 July 2011. [Google Scholar]
- Ikeda, T.; Kumashiro, R.; Oki, E.; Taketani, K.; Ando, K.; Aishima, S.; Akahoshi, T.; Morita, M.; Maehara, Y. Evaluation of techniques to prevent colorectal anastomotic leakage. J. Surg. Res. 2015, 194, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.Y.; Ge, S.C.; Xu, J.J.; Li, M.Y.; Mao, L.; Song, C.L. Finite element analysis and experiment on large intestine end-to-end anastomosis. In Frontiers in Biomedical Devices, BIOMED—2018 Design of Medical Devices Conference, DMD 2018, Minneapolis, MN, USA, 9–12 April 2018; American Society of Mechanical Engineers: New York, NY, USA, 2018; Volume 3, pp. 7–9. [Google Scholar] [CrossRef]
- Sapora, J.A.; Hafez, A.; Monnet, E. Ex vivo comparison of hand-sutured versus circular stapled anastomosis in canine large intestine. Vet. Surg. 2021, 50, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.B.; Oberg, K.; Wolchok, J.C. Tensile properties of the rectal and sigmoid colon: A comparative analysis of human and porcine tissue. Springerplus 2015, 4, 142. [Google Scholar] [CrossRef]
- Ethicon PROXIMATE® Linear Cutters, TLC75. Available online: https://www.ethicon.com/na/epc/code/tlc75?lang=en-default (accessed on 15 December 2022).
- Ethicon Circular Stapler, ECS21A. Available online: https://www.ethicon.com/emea/epc/code/ecs21a?lang=en-default (accessed on 16 June 2025).
- Fahmy, Y.; Trabia, M.B.; Ward, B.; Gallup, L.; Froehlich, M. Development of an Anisotropic Hyperelastic Material Model for Porcine Colorectal Tissues. Bioengineering 2024, 11, 64. [Google Scholar] [CrossRef]
- De Keulenaer, B.L.; De Waele, J.J.; Powell, B.; Malbrain, M.L.N.G. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med. 2009, 35, 969–976. [Google Scholar] [CrossRef]
- Caulk, A.W.; Chatterjee, M.; Barr, S.J.; Contini, E.M. Mechanobiological considerations in colorectal stapling: Implications for technology development. Surg. Open Sci. 2023, 13, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, J. Simulation and Experimental Study of Staple Line Reinforcement Surgery. J. Biomed. Sci. Eng. 2024, 17, 83–95. [Google Scholar] [CrossRef]
- Kalvach, J.; Ryska, O.; Martinek, J.; Hucl, T.; Pazin, J.; Hadac, J.; Foltan, O.; Kristianova, H.; Ptacnik, J.; Juhasova, J.; et al. Randomized experimental study of two novel techniques for transanal repair of dehiscent low rectal anastomosis. Surg. Endosc. 2021, 36, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, S.; Peña, E.; Herrera, A.; Ibarz, E.; Gracia, L. A comparative study of hyperelastic constitutive models for colonic tissue fitted to multiaxial experimental testing. J. Mech. Behav. Biomed. Mater. 2020, 102, 103507. [Google Scholar] [CrossRef]
- Patel, B.; Chen, H.; Ahuja, A.; Krieger, J.F.; Noblet, J.; Chambers, S.; Kassab, G.S. Constitutive modeling of the passive inflation-extension behavior of the swine colon. J. Mech. Behav. Biomed. Mater. 2018, 77, 176–186. [Google Scholar] [CrossRef]


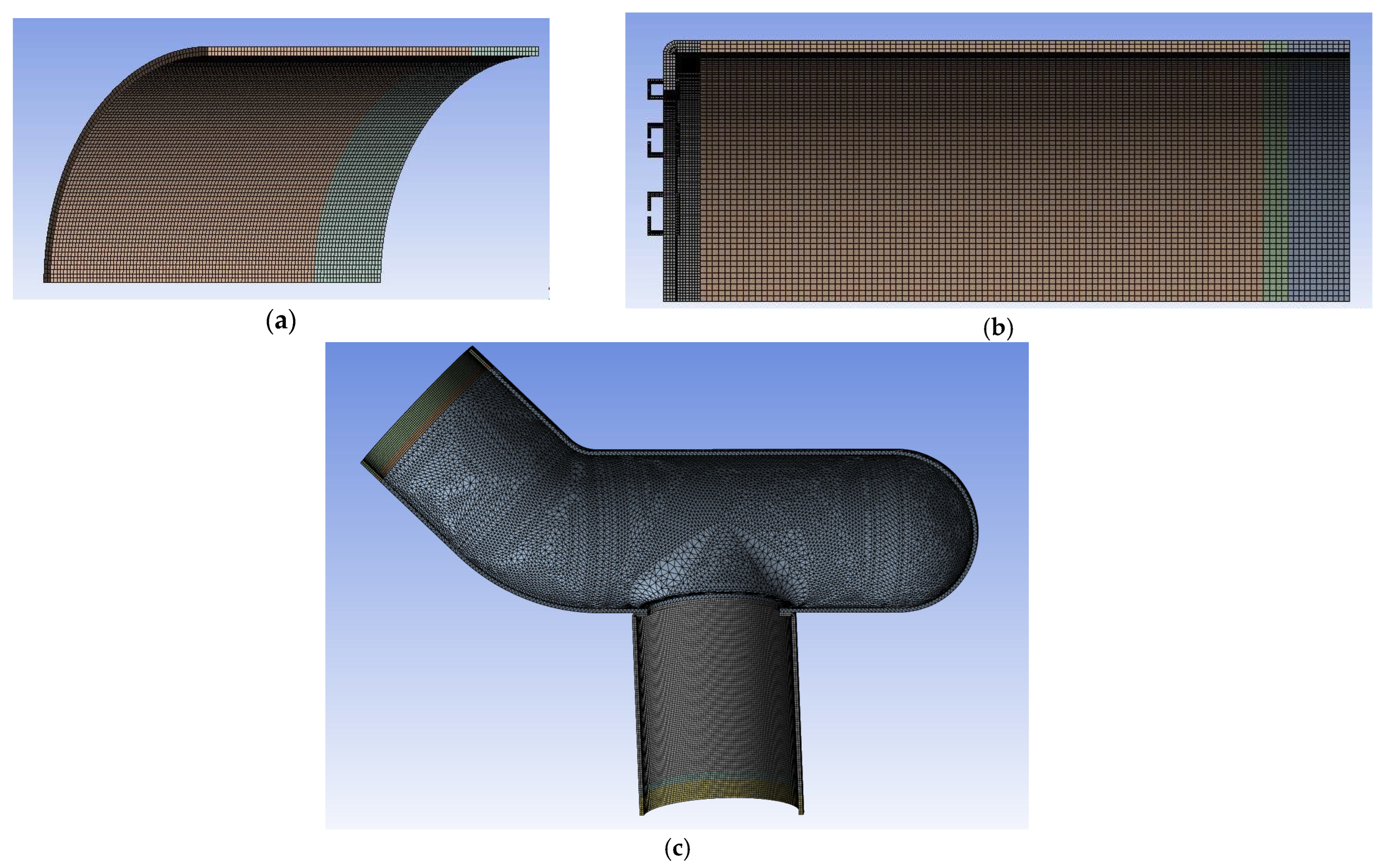
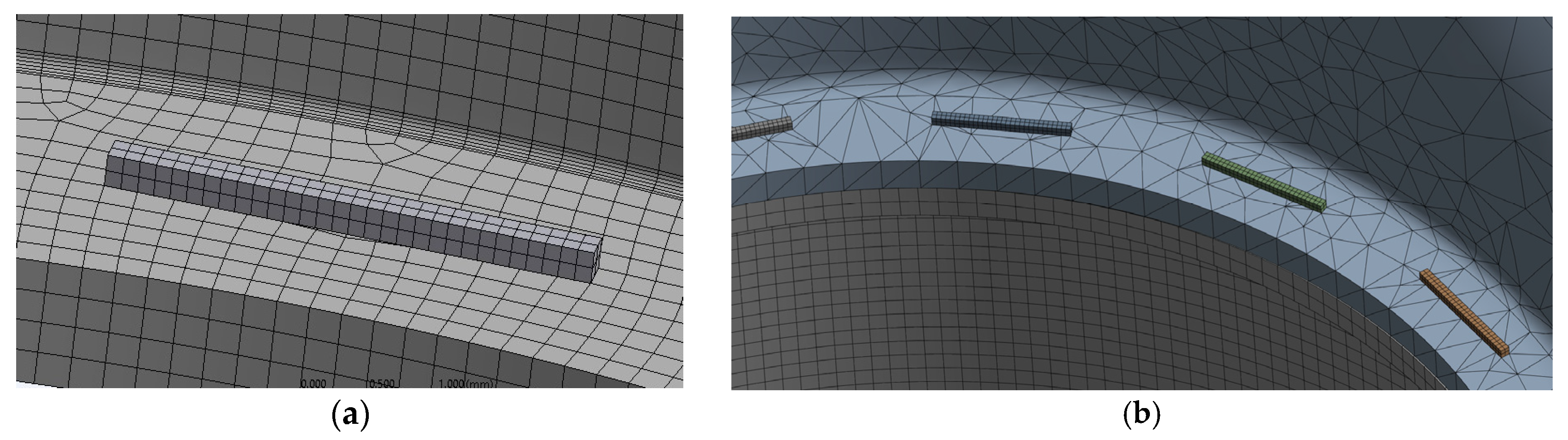
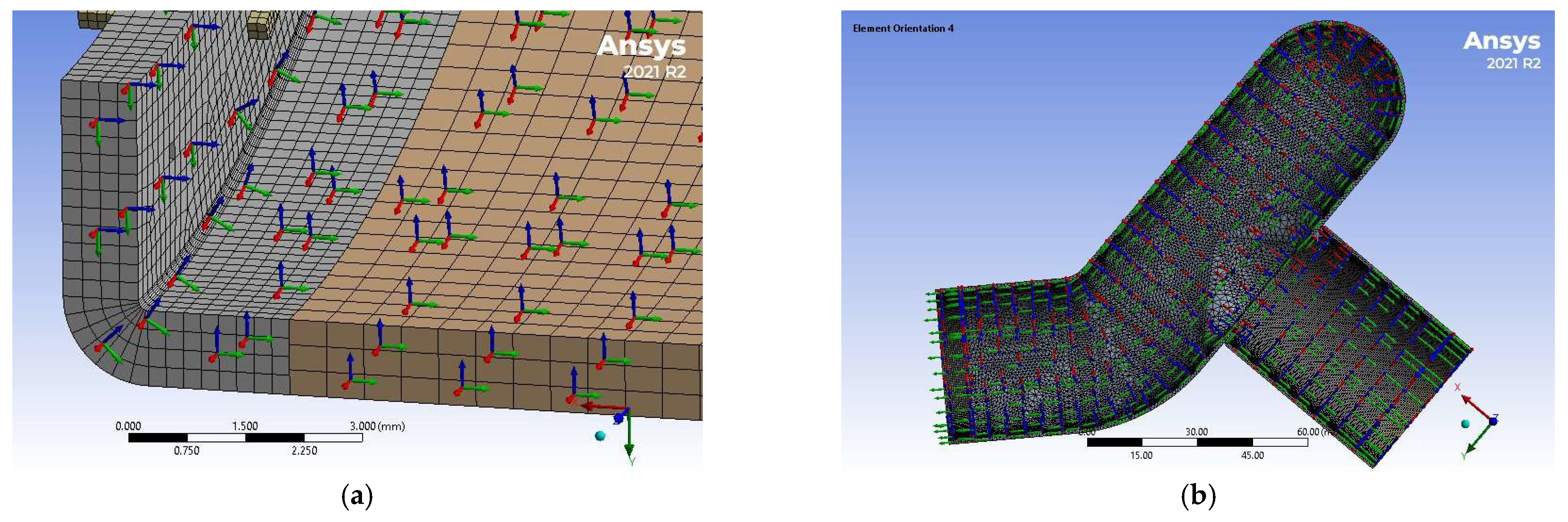
| Yield Strength (MPa) | Poisson’s Ratio | Modulus of Elasticity (GPa) | Tangent Modulus (MPa) |
|---|---|---|---|
| 930 | 0.3 | 96 | 2150 |
| Model | Meshing Technique | Element Type | Element Size (mm) | Element Count |
|---|---|---|---|---|
| Control | Multi-zone | Hexahedral | 0.5 | 16,362 |
| EE | Multi-zone | Hexahedral-Dominant Hexahedral-Dominant Hexahedral | 0.5 (Tissues, general) 0.5 (Tissues, around staples) 0.125 (Staples) | 36,035 |
| ES | Patch Conforming Method | Tetrahedral (Proximal End) Tetrahedral (Proximal End) Hexahedral | 1.0 (Proximal End) 0.5 (Distal End) 0.125 (Staples) | 98,434 |
| a1 (Pa) | a2 (Pa) | a3 (Pa) | |
|---|---|---|---|
| 1.400 × 105 | −9.585 × 103 | −1.976 × 103 | |
| c2 (Pa) | c3 (Pa) | c4 (Pa) | β1 (Degrees) |
| 4.929 × 105 | −2.981 × 105 | 6.553 × 103 | 0.790 |
| e2 (Pa) | e3 (Pa) | e4 (Pa) | β2 (Degrees) |
| 3.0146 × 104 | −2.9556 × 104 | −739.324 | 90.100 |
| Control | EE | ES | |
|---|---|---|---|
| Tissue failure | 22 | 2 | 1 |
| Slippage | 0 | 0 | 2 |
| Corrupt/low quality video | 8 | 11 | 3 |
| Linear staple leakage | N/A | 1 | 3 |
| Circular staple leakage | N/A | 16 | 21 |
| Control (n = 22) | EE (n = 16) | ES (n = 21) | |
|---|---|---|---|
| Pre-testing thickness (mm) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Leak pressure (mm Hg) | 294.4 ± 12.1 | 282.6 ± 3.0 | 282.8 ± 2.6 |
| Initial diameter (mm) | 43.6 ± 8.1 | 42.2 ± 6.5 | 40.1 ± 5.7 |
| Diameter at leakage (mm) | 56.3 ± 8.4 | 50.5 ± 7.1 | 52.8 ± 6.5 |
| Time to leakage (s) | 194.5 ± 90.2 | 106.3 ± 28.1 | 263.9 ± 127.0 |
| p -Value | Confidence Interval | |||
|---|---|---|---|---|
| Lower | Upper | |||
| Initial diameter | EE vs. Control | 0.387 | 0.566 | 3.931 |
| ES vs. Control | 0.125 | 0.819 | 4.862 | |
| Diameter at leakage | EE vs. Control | 0.560 | 0.492 | 3.416 |
| ES vs. Control | 0.255 | 0.684 | 4.058 | |
| Time to leakage (s) | EE vs. Control | 1.160 × 10−13 | 57.109 | 396.518 |
| ES vs. Control | 0.127 | 0.206 | 1.221 | |
| Group | Control Group | Lower Limit | Difference | Upper Limit | p -Value | |
|---|---|---|---|---|---|---|
| Leak pressure | EE | Control | −206.279 | −197.941 | −189.603 | 1.110 × 10−16 |
| ES | Control | −19.366 | −11.624 | −3.882 | 0.002 | |
| Initial diameter | EE | Control | −6.678 | −1.369 | 3.941 | 0.787 |
| ES | Control | −8.387 | −3.458 | 1.477 | 0.204 | |
| Diameter at leakage | EE | Control | −11.499 | −5.82 | −0.134 | 0.044 |
| ES | Control | −8.756 | −3.479 | 1.797 | 0.242 | |
| Time to leakage | EE | Control | 10.348 | 21.372 | 32.397 | 9.415 × 10−5 |
| ES | Control | 15.744 | 25.980 | 36.216 | 7.082 × 10−7 |
| Control (n = 22) | EE (n = 16) | ES (n = 21) | |
|---|---|---|---|
| (mm Hg) | 374 ± 15 | 235 ± 2 | 359 ± 3 |
| (MPa) | 0.0498 ± 0.0020 | 0.0313 ± 0.0003 | 0.0479 ± 0.0004 |
| 0.29 ± 0.04 | 0.20 ± 0.09 | 0.32 ± 0.14 |
| Control | EE | ES, Proximal End | |
|---|---|---|---|
| Initial diameter (mm) | 43.6 | 42.2 | 40.1 |
| Average maximum diameter (mm) | 53.8 | 49.0 | 48.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, Y.; Trabia, M.; Ward, B.; Gallup, L.; Elks, W. Ex Vivo and Simulation Comparison of Leakage in End-to-End Versus End-to-Side Anastomosed Porcine Large Intestine. Bioengineering 2025, 12, 676. https://doi.org/10.3390/bioengineering12070676
Fahmy Y, Trabia M, Ward B, Gallup L, Elks W. Ex Vivo and Simulation Comparison of Leakage in End-to-End Versus End-to-Side Anastomosed Porcine Large Intestine. Bioengineering. 2025; 12(7):676. https://doi.org/10.3390/bioengineering12070676
Chicago/Turabian StyleFahmy, Youssef, Mohamed Trabia, Brian Ward, Lucas Gallup, and Whitney Elks. 2025. "Ex Vivo and Simulation Comparison of Leakage in End-to-End Versus End-to-Side Anastomosed Porcine Large Intestine" Bioengineering 12, no. 7: 676. https://doi.org/10.3390/bioengineering12070676
APA StyleFahmy, Y., Trabia, M., Ward, B., Gallup, L., & Elks, W. (2025). Ex Vivo and Simulation Comparison of Leakage in End-to-End Versus End-to-Side Anastomosed Porcine Large Intestine. Bioengineering, 12(7), 676. https://doi.org/10.3390/bioengineering12070676







