MRI-Based Machine Learning and Radiomics Methods for Assessing Spinal Cord Function in Patients with Mild Cervical Spondylotic Myelopathy
Abstract
1. Introduction
- We prospectively collected a cohort of mild CSM patients and elucidated the relationship between decreased SUVmax and prognosis.
- We constructed a model based on radiomics capable of identifying compressed cervical segments.
- We developed a model based on radiomics capable of identifying segments with decreased SUVmax.
- We conducted feature analysis on the models, yielding radiomic indicators with clinical relevance to guide clinical practice.
2. Methods
2.1. Study Population
2.2. Image Scanning Process and Analysis
2.3. Radiomic Feature Extraction
2.4. Clinical Task Setting
2.5. Construction of the Machine Learning Model
2.6. Model Interpretation
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics
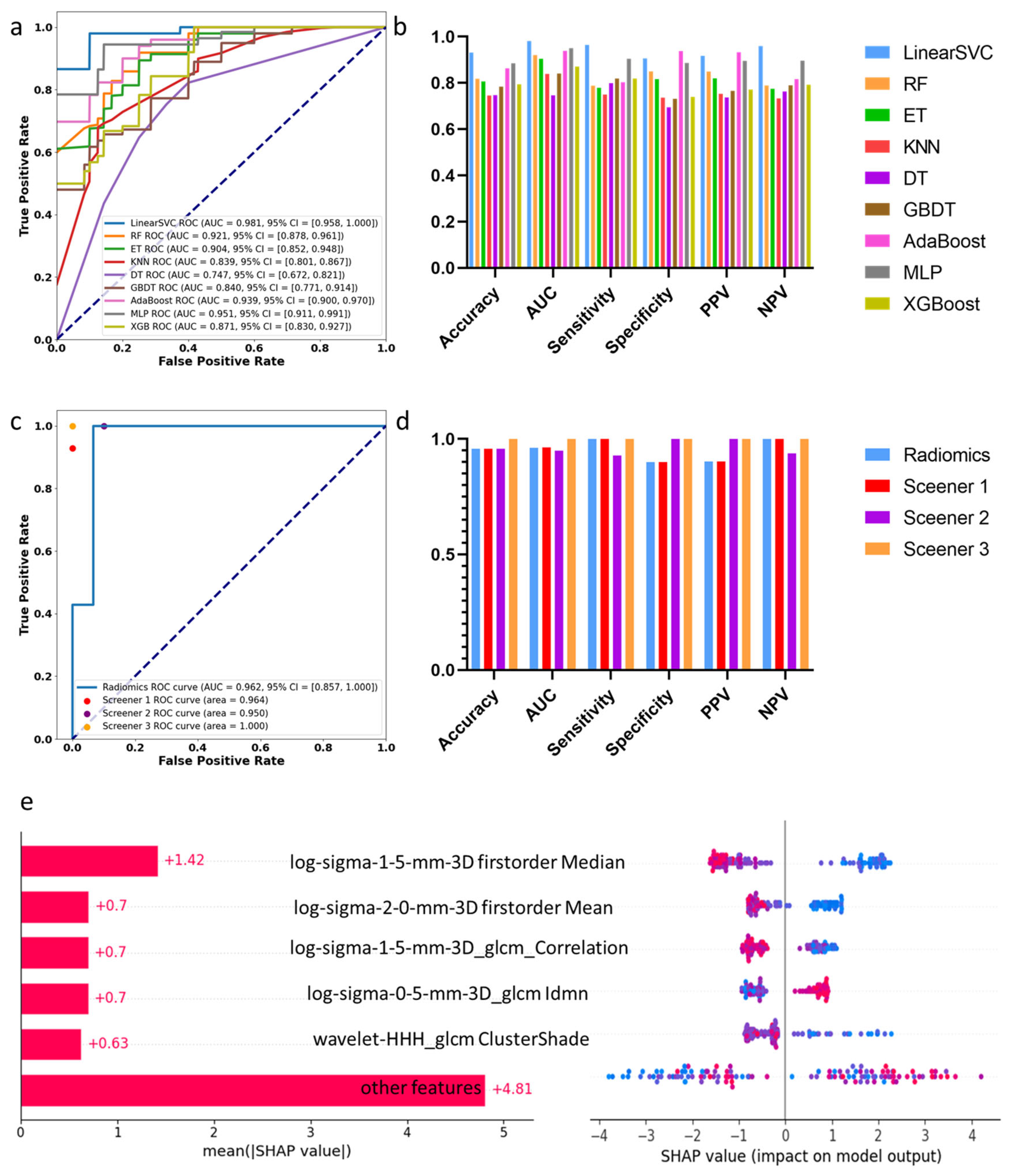
3.2. Machine Learning Assessment of Spinal Cord Compression
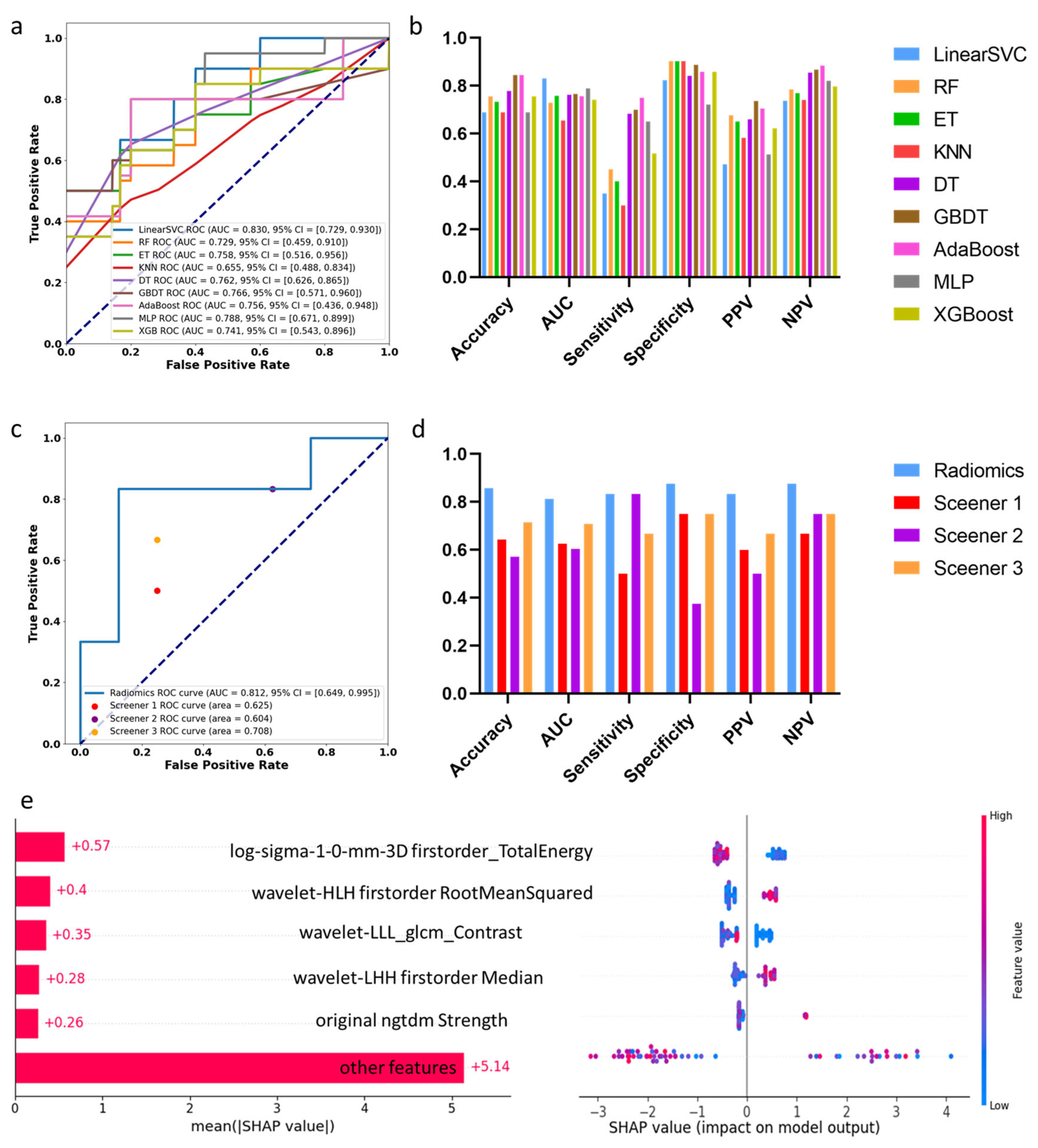
3.3. Machine Learning Assessment of Segments with Decreased SUVmax
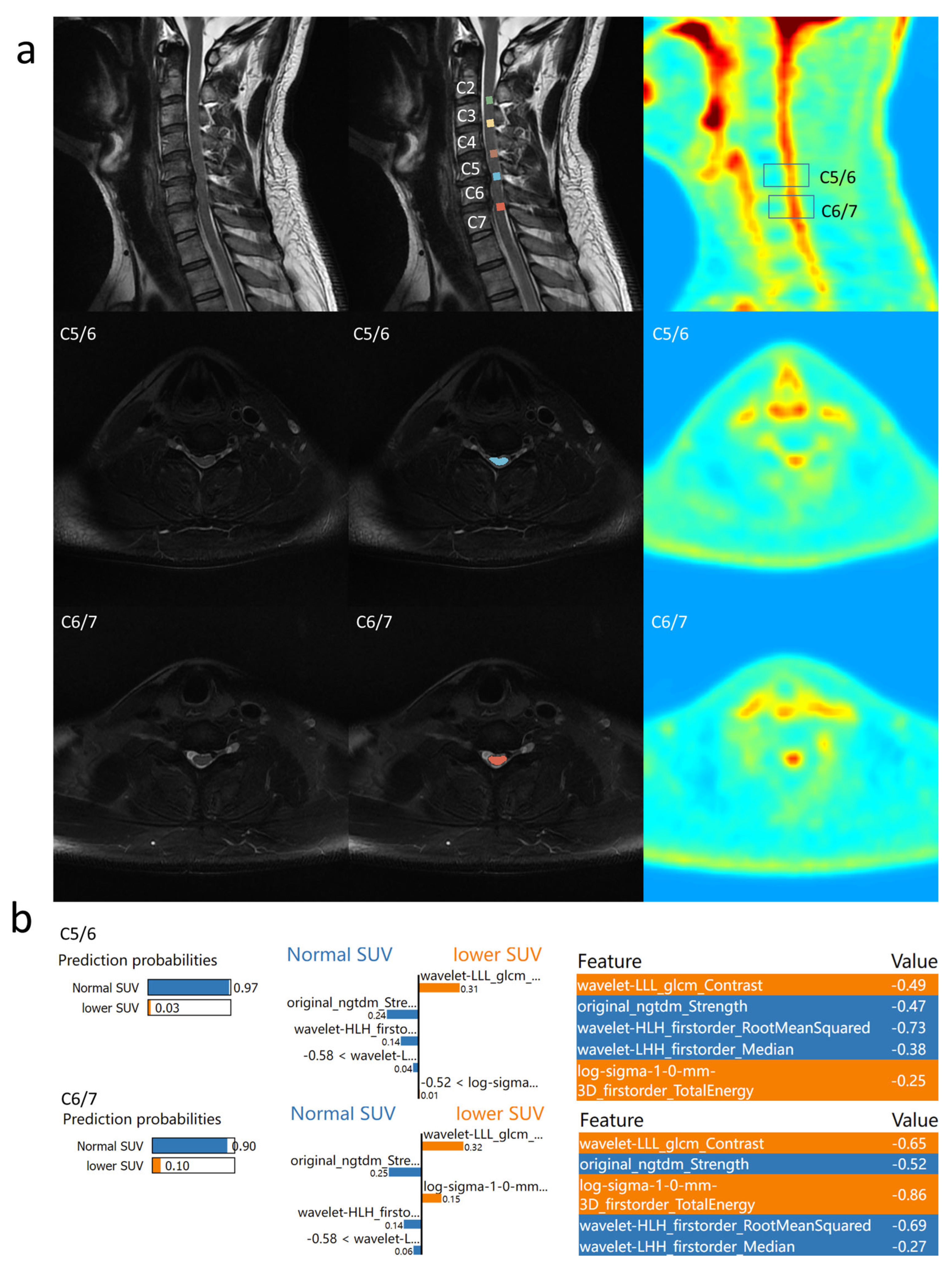
3.4. Individual Feature Interpretation Based on LIME
4. Discussion
4.1. Deep Learning and Machine Learning Applications for CSM Patients
4.2. Association Between PET/MRI and Prognosis
4.3. Treatment Options for Patients with Cervical Spondylotic Myelopathy
4.4. Other Methods for the Assessment of Cervical Spondylotic Myelopathy
4.5. Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSM | Cervical spondylotic myelopathy |
| mJOA | Modified Japanese Orthopaedic Association scale |
| SHAPLEY | Shapley Additive exPlanations |
| LIME | Local Interpretable Model-Agnostic Explanations |
| SUVmax | The maximum standardized uptake value |
References
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative cervical myelopathy—Update and future directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Kwon, B.K.; Tetreault, L.A. Guidelines for the management of degenerative cervical myelopathy and spinal cord injury: An introduction to a focus issue. Glob. Spine J. 2017, 7, 6S–7S. [Google Scholar] [CrossRef] [PubMed]
- Karadimas, S.K.; Erwin, W.M.; Ely, C.G.; Dettori, J.R.; Fehlings, M.G. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine 2013, 38, S21–S36. [Google Scholar] [CrossRef]
- Rhee, J.; Tetreault, L.A.; Chapman, J.R.; Wilson, J.R.; Smith, J.S.; Martin, A.R.; Dettori, J.R.; Fehlings, M.G. Nonoperative versus operative management for the treatment degenerative cervical myelopathy: An updated systematic review. Glob. Spine J. 2017, 7 (Suppl. 3), 35S–41S. [Google Scholar] [CrossRef]
- Aiello, M.; Alfano, V.; Salvatore, E.; Cavaliere, C.; Picardi, M.; Della Pepa, R.; Nicolai, E.; Soricelli, A.; Vella, A.; Salvatore, M.; et al. [18F] FDG uptake of the normal spinal cord in PET/MR imaging: Comparison with PET/CT imaging. EJNMMI Res. 2020, 10, 91. [Google Scholar] [CrossRef]
- Lam, M.; Burke, C.J.; Walter, W.R.J.D.; Radiology, I. Correlation of 18F-FDG PET/CT uptake with severity of MRI findings and epidural steroid injection sites in patients with symptomatic degenerative disease of the lumbar spine: A retrospective study. Diagn. Interv. Radiol. 2021, 27, 580–586. [Google Scholar] [CrossRef]
- Tan, X.; Li, D.; Wu, X.; Yang, Y.; Hou, Q.; He, L.; Jiang, L. Physiologically intense FDG uptake of distal spinal cord on total-body PET/CT. Ann. Nucl. Med. 2022, 36, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Borrelli, P.; Alfano, V.; Picardi, M.; Mascalchi, M.; Nicolai, E.; Salvatore, M.; Aiello, M. The impact of MR-based attenuation correction in spinal cord FDG-PET/MR imaging for neurological studies. Med Phys. 2021, 48, 5924–5934. [Google Scholar] [CrossRef]
- Floeth, F.W.; Stoffels, G.; Herdmann, J.; Jansen, P.; Meyer, W.; Steiger, H.-J.; Langen, K.-J. Regional impairment of 18 F-FDG uptake in the cervical spinal cord in patients with monosegmental chronic cervical myelopathy. Eur. Radiol. 2010, 20, 2925–2932. [Google Scholar] [CrossRef]
- Floeth, F.W.; Stoffels, G.; Herdmann, J.; Eicker, S.; Galldiks, N.; Steiger, H.-J.; Langen, K.-J. Prognostic value of 18F-FDG PET in monosegmental stenosis and myelopathy of the cervical spinal cord. J. Nucl. Med. 2011, 52, 1385–1391. [Google Scholar] [CrossRef]
- Uchida, K.; Nakajima, H.; Yayama, T.; Kobayashi, S.; Shimada, S.R.; Tsuchida, T.; Okazawa, H.; Mwaka, E.M.; Baba, H. High-resolution magnetic resonance imaging and 18FDG-PET findings of the cervical spinal cord before and after decompressive surgery in patients with compressive myelopathy. Spine 2009, 34, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Nakajima, H.; Okazawa, H.; Kimura, H.; Kudo, T.; Watanabe, S.; Yoshida, A.; Baba, H. Clinical significance of MRI/18 F-FDG PET fusion imaging of the spinal cord in patients with cervical compressive myelopathy. Eur. J. Nucl. Med. 2012, 39, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H.J.R. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Y.; Liang, C.; Zhao, Y.; Lv, X.; Zou, Y.; Yan, K.; Zheng, H.; Liang, D.; Li, Z.-C. Biologic pathways underlying prognostic radiomics phenotypes from paired MRI and RNA sequencing in glioblastoma. Radiology 2021, 301, 654–663. [Google Scholar] [CrossRef]
- Ligero, M.; Garcia-Ruiz, A.; Viaplana, C.; Villacampa, G.; Raciti, M.V.; Landa, J.; Matos, I.; Martin-Liberal, J.; Ochoa-De-Olza, M.; Hierro, C.; et al. A CT-based radiomics signature is associated with response to immune checkpoint inhibitors in advanced solid tumors. Radiology 2021, 299, 109–119. [Google Scholar] [CrossRef]
- Benzel, E.C.; Lancon, J.; Kesterson, L.; Hadden, T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J. Spinal Disord. 1991, 4, 286–295. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef]
- Quinlan, J.R. Induction of Decision Trees. Mach. Learn. 1986, 1, 81–106. [Google Scholar] [CrossRef]
- Friedman, J.H.; Analysis, D. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Schapire, R.E.; Singer, Y. BoosTexter: A Boosting-based System for Text Categorization. Mach. Learn. 2000, 39, 135–168. [Google Scholar] [CrossRef]
- Gardner, M.W.; Dorling, S.R. Artificial neural networks (the multilayer perceptron)—A review of applications in the atmospheric sciences. Atmos. Environ. 1998, 32, 2627–2636. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of machine learning models using shapley values: Application to compound potency and multi-target activity predictions. J. Comput. Aided Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- de Sousa, I.P.; Maria Bernardes Rebuzzi Vellasco, M.; da Silva, E.C. Local interpretable model-agnostic explanations for classification of lymph node metastases. Sensors 2019, 19, 2969. [Google Scholar] [CrossRef]
- Qu, B.; Cao, J.; Qian, C.; Wu, J.; Lin, J.; Wang, L.; Ou-Yang, L.; Chen, Y.; Yan, L.; Hong, Q.; et al. Current development and prospects of deep learning in spine image analysis: A literature review. Quant. Imaging Med. Surg. 2022, 12, 3454–3479. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Wu, J.; Liu, C.; Makmur, A.; Ting, Y.H.; Nor, F.E.M.; Tan, L.Y.; Ong, W.; Tan, W.C.; Lee, Y.J.; et al. Deep learning model for automated diagnosis of degenerative cervical spondylosis and altered spinal cord signal on MRI. Spine J. 2025, 25, 255–264. [Google Scholar] [CrossRef]
- Xie, Y.; Nie, Y.; Lundgren, J.; Yang, M.; Zhang, Y.; Chen, Z. Cervical spondylosis diagnosis based on convolutional neural network with x-ray images. Sensors 2024, 24, 3428. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Zhou, C.; Zhu, J.; Chen, T.; Feng, S.; Huang, C.; Wang, Z.; Wu, S.; Liu, C.; et al. Prediction of additional hospital days in patients undergoing cervical spine surgery with machine learning methods. Comput. Assist. Surg. 2024, 29, 2345066. [Google Scholar] [CrossRef]
- Karabacak, M.; Bhimani, A.D.; Schupper, A.J.; Carr, M.T.; Steinberger, J.; Margetis, K. Machine learning models on a web application to predict short-term postoperative outcomes following anterior cervical discectomy and fusion. BMC Musculoskelet. Disord. 2024, 25, 401. [Google Scholar] [CrossRef]
- Niemeyer, F.; Galbusera, F.; Tao, Y.; Kienle, A.; Beer, M.; Wilke, H. A deep learning model for the accurate and reliable classification of disc degeneration based on MRI data. Investig. Radiol. 2020, 56, 78–85. [Google Scholar] [CrossRef]
- Floeth, F.W.; Galldiks, N.; Eicker, S.; Stoffels, G.; Herdmann, J.; Steiger, H.-J.; Antoch, G.; Rhee, S.; Langen, K.-J. Hypermetabolism in 18F-FDG PET predicts favorable outcome following decompressive surgery in patients with degenerative cervical myelopathy. J. Nucl. Med. 2013, 54, 1577–1583. [Google Scholar] [CrossRef]
- Cho, N.; Al-Shawwa, A.; Jacobs, W.B.; Evaniew, N.; Bouchard, J.; Casha, S.; Duplessis, S.; Lewkonia, P.; Nicholls, F.; Soroceanu, A.; et al. Spinal Cord Tract Integrity in Degenerative Cervical Myelopathy. Neurosurgery 2022, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, S.; Wang, J.; Zhang, Z.; Jiang, W.; Wang, C.; Jiang, Y.; Guo, H.; Han, X.; Tian, W. Diagnostic efficacy of tract-specific diffusion tensor imaging in cervical spondylotic myelopathy with electrophysiological examination validation. Eur. Spine J. 2024, 33, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, X.; Zhang, Y.; Zhao, B.; Wang, N.; Gao, T.; Zhang, L. Predictive value of dynamic diffusion tensor imaging for surgical outcomes in patients with cervical spondylotic myelopathy. BMC Med. Imaging 2024, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, B.; Dou, Y.; Wang, Y.; Ma, J.; Huang, C.; Zhang, Y.; Cao, P. Aided diagnosis of cervical spondylotic myelopathy using deep learning methods based on electroencephalography. Med Eng. Phys. 2023, 121, 104069. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Shu, T.; Ji, Q.; Wang, X.; Li, R.; Sui, Y.; He, D.; Xu, Z. Preliminarily exploring the intraoperative ultrasonography characteristics of patients with degenerative cervical myelopathy. BMC Musculoskelet. Disord. 2024, 25, 538. [Google Scholar] [CrossRef]


| Decreased SUVmax | Normal SUVmax | p-Value | |
|---|---|---|---|
| Num | 10 | 14 | |
| Age (years) | 56.9 ± 11.4 | 57.9 ± 8.3 | 0.801 |
| Gender (M, %) | 7 (70%) | 7 (50%) | 0.349 |
| Course (months) | 42 (6–114) | 12 (5.5–66) | 0.465 |
| BMI | 26.2 ± 1.5 | 25.1 ± 3.0 | 0.284 |
| Pre-op mJOA | 15 (14–16) | 15.5 (14–16) | 0.732 |
| Post-op mJOA | 16 (15.75–17) | 17 (16–17) | 0.24 |
| mJOA improvement | 1 (1–1.25) | 2 (1–2) | 0.043 |
| Task 1 | Training Dataset | Test Dataset | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Level | Normal | Percent | Compressed | Percent | Normal | Percent | Compressed | Percent | |
| Total | 45 | 51.72% | 42 | 48.28% | 15 | 51.72% | 14 | 48.28% | |
| C2/3 | 16 | 100.00% | 0 | 0.00% | 5 | 100.00% | 0 | 0.00% | |
| C3/4 | 12 | 66.67% | 6 | 33.33% | 5 | 83.33% | 1 | 16.67% | |
| C4/5 | 4 | 22.22% | 14 | 77.78% | 2 | 33.33% | 4 | 66.67% | |
| C5/6 | 2 | 11.11% | 16 | 88.89% | 1 | 16.67% | 5 | 83.33% | |
| C6/7 | 11 | 64.71% | 6 | 35.29% | 2 | 33.33% | 4 | 66.67% | |
| Task 2 | Training Dataset | Test Dataset | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Level | Normal | Percent | Decreased | Percent | Normal | Percent | Decreased | Percent | |
| Total | 32 | 76.19% | 10 | 23.81% | 8 | 57.14% | 6 | 42.86% | |
| C2/3 | 0 | / | 0 | / | 0 | / | 0 | / | |
| C3/4 | 6 | 100.00% | 0 | 0.00% | 1 | 100.00% | 0 | 0.00% | |
| C4/5 | 12 | 85.71% | 2 | 14.29% | 3 | 75.00% | 1 | 25.00% | |
| C5/6 | 10 | 62.50% | 6 | 37.50% | 2 | 40.00% | 3 | 60.00% | |
| C6/7 | 4 | 66.67% | 2 | 33.33% | 2 | 50.00% | 2 | 50.00% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, K.; Wang, Y.; Liu, Z.; Zhang, L.; Jia, S.; He, K.; Zhang, X.; Wu, H. MRI-Based Machine Learning and Radiomics Methods for Assessing Spinal Cord Function in Patients with Mild Cervical Spondylotic Myelopathy. Bioengineering 2025, 12, 666. https://doi.org/10.3390/bioengineering12060666
Wang H, Wang K, Wang Y, Liu Z, Zhang L, Jia S, He K, Zhang X, Wu H. MRI-Based Machine Learning and Radiomics Methods for Assessing Spinal Cord Function in Patients with Mild Cervical Spondylotic Myelopathy. Bioengineering. 2025; 12(6):666. https://doi.org/10.3390/bioengineering12060666
Chicago/Turabian StyleWang, He, Kai Wang, Yutian Wang, Zhenlei Liu, Lei Zhang, Shanhang Jia, Kun He, Xiangyu Zhang, and Hao Wu. 2025. "MRI-Based Machine Learning and Radiomics Methods for Assessing Spinal Cord Function in Patients with Mild Cervical Spondylotic Myelopathy" Bioengineering 12, no. 6: 666. https://doi.org/10.3390/bioengineering12060666
APA StyleWang, H., Wang, K., Wang, Y., Liu, Z., Zhang, L., Jia, S., He, K., Zhang, X., & Wu, H. (2025). MRI-Based Machine Learning and Radiomics Methods for Assessing Spinal Cord Function in Patients with Mild Cervical Spondylotic Myelopathy. Bioengineering, 12(6), 666. https://doi.org/10.3390/bioengineering12060666






