Biomechanical Analysis and Clinical Study of Augmented Versus Conventional Endoscopic Orbital Decompression for Dysthyroid Optic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
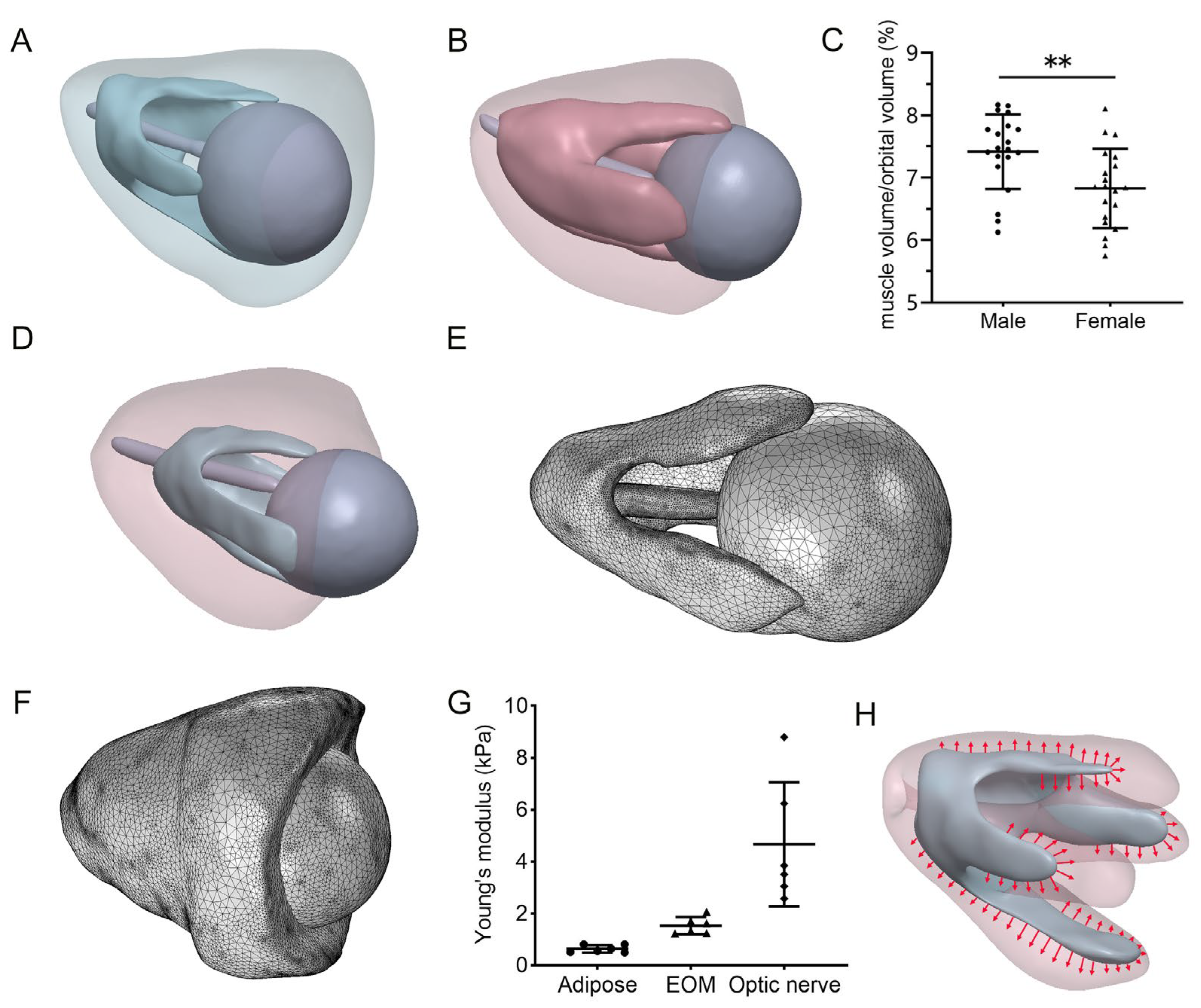
2.1. MRI Data Collection
2.2. Geometry and Finite Element Model
2.3. Material Properties
2.4. Finite Element Model Simulation
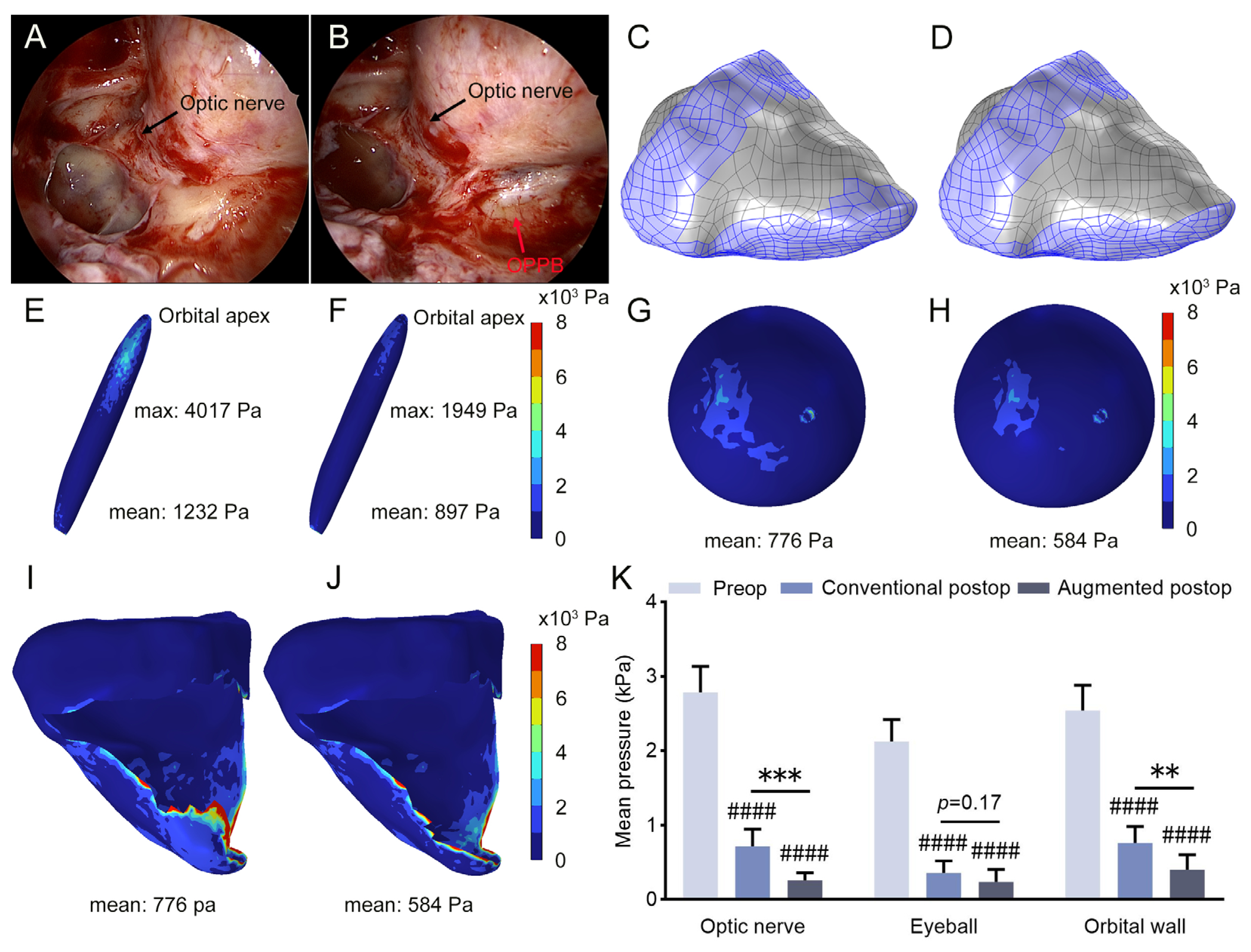
2.5. Orbital Decompression Surgery and Clinical Data Collection
2.6. Statistical Analysis
3. Results
3.1. Increased Intraorbital Pressure in DON Patients
3.2. Pronounced Pressure Reduction Following Augmented Orbital Decompression Surgery
3.3. Prediction of Postoperative Soft Tissue Displacement
3.4. More Effective Visual Function Improvement Following Augmented Orbital Decompression Surgery
4. Discussion
4.1. Prediction of Mechanical Stress Distribution Within the Orbit for DON
4.2. Prediction of Postoperative Stress Distribution Within the Orbit
4.3. Prediction of Postoperative Complications and Exophthalmos Reduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Conventional Group | Augmented Group | p-Value | |
|---|---|---|---|
| No. of participants | 11 | 11 | |
| No. of eyes | 18 | 16 | |
| Sex, male/female | 6/5 | 3/8 | 0.39 a |
| Age, years | 52.36 ± 7.87 (39–64) | 56.36 ± 10.5 (38–70) | 0.32 b |
| CAS | 3.9 ± 1.22 (3–6) | 4.9 ± 1.14 (3–6) | 0.09 c |
| Duration of thyroid disease, months | 18 ± 11.36 (6–39) | 16.09 ± 12.49 (5–45) | 0.66 c |
| Duration of TED, months | 13.45 ± 11.64 (3–39) | 11 ± 8.28 (4–33) | 0.88 c |
| Duration of VA impairment, months | 5 ± 2.9 (1–10) | 6.23 ± 4.7 (0.5–17) | 0.47 b |
| Hyperthyroid | 2 (18.19%) | 1 (9.09%) | >0.99 a |
| Hypothyroid | 1 (9.09%) | 1 (9.09%) | >0.99 a |
| Euthyroid | 8 (72.73%) | 9 (81.82%) | >0.99 a |
| History of I131 treatment | 2 (18.19%) | 1 (9.09%) | >0.99 a |
| History of radiotherapy | 1 (9.09%) | 3 (27.27%) | 0.59 a |
| History of glucocorticoid pulse therapy | 5 (45.45%) | 10 (90.9%) | 0.06 a |
| Male | Female | p-Value | |
|---|---|---|---|
| No. of participants | 20 | 20 | |
| Age, years | 45.1 ± 12.75 (18–63) | 46.35 ± 10.45 (25–71) | 0.74 a |
| Unilateral orbital tumor | 16 (80%) | 16 (80%) | >0.99 b |
| Unilateral ocular tumor | 3 (15%) | 2 (10%) | >0.99 b |
| Unilateral orbital trauma | 1 (5%) | 2 (10%) | >0.99 b |
| Patient ID | Age (Years) | Gender | OD/OS | Diagnosis |
|---|---|---|---|---|
| Patient 1 | 19 | Male | OS | Phthisis bulbi |
| Patient 2 | 49 | Female | OS | Corneal ulcer |
| Patient 3 | 29 | Male | OD | Corneal staphyloma |
| Patient 4 | 51 | Male | OD | Bullous keratopathy |
| Patient 5 | 25 | Female | OS | Corneal staphyloma |
| Patient 6 | 68 | Female | OD | Corneal ulcer |
References
- Potvin, A.; Pakdel, F.; Saeed, P. Dysthyroid Optic Neuropathy. Ophthalmic Plast. Reconstr. Surg. 2023, 39, S65–S80. [Google Scholar] [CrossRef] [PubMed]
- Blandford, A.D.; Zhang, D.; Chundury, R.V.; Perry, J.D. Dysthyroid optic neuropathy: Update on pathogenesis, diagnosis, and management. Expert Rev. Ophthalmol. 2017, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Weis, E.; Heran, M.K.; Jhamb, A.; Chan, A.K.; Chiu, J.P.; Hurley, M.C.; Rootman, J. Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: A volumetric analysis. Ophthalmology 2012, 119, 2174–2178. [Google Scholar] [CrossRef] [PubMed]
- Soni, C.R.; Johnson, L.N. Visual neuropraxia and progressive vision loss from thyroid-associated stretch optic neuropathy. Eur. J. Ophthalmol. 2010, 20, 429–436. [Google Scholar] [CrossRef]
- Anderson, R.L.; Tweeten, J.P.; Patrinely, J.R.; Garland, P.E. Thiese SM: Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg. 1989, 20, 568–574. [Google Scholar]
- Dosso, A.; Safran, A.B.; Sunaric, G.; Burger, A. Anterior ischemic optic neuropathy in Graves’ disease. J. Neuroophthalmol. 1994, 14, 170–174. [Google Scholar] [CrossRef]
- Yanik, B.; Conkbayir, I.; Acaroglu, G.; Hekimoglu, B. Graves’ ophthalmopathy: Comparison of the Doppler sonography parameters with the clinical activity score. J. Clin. Ultrasound 2005, 33, 375–380. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Dolman, P.J. Dysthyroid optic neuropathy: Evaluation and management. J. Endocrinol. Investig. 2021, 44, 421–429. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Zhang, Z.; Lv, X.; Bao, Y.; Ma, Y.; Chen, R.; Cheng, C.; Li, J.; Liu, Y.; et al. Surgical Outcomes of Standardized Endoscopical Deep Medial Orbital Decompression in Dysthyroid Optic Neuropathy. Ophthalmic Res. 2024, 67, 39–50. [Google Scholar] [CrossRef]
- Singh, S.; Curragh, D.S.; Selva, D. Augmented endoscopic orbital apex decompression in dysthyroid optic neuropathy. Eye 2019, 33, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Yinghong, Z.; Jichao, Z.; Zhidi, Z.; Chiyu, X.; Haipeng, Z.; Yanrong, R.; Li, Z.; Yi, W. Combined endonasal and orbital approach for annulus of Zinn area decompression in dysthyroid optic neuropathy. Am. J. Otolaryngol. 2023, 44, 103692. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wu, S.; Pan, Z.; Hu, X.; Zhou, G.; Shi, J.; Xu, M.; Liu, W.; Wu, W. Endoscopic Transconjunctival Deep Lateral Wall Decompression for Thyroid-associated Orbitopathy: A Minimally Invasive Alternative: Transconjunctival Endoscopic with Wall Decompression for TAO. Am. J. Ophthalmol. 2022, 235, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.A.; Gout, T. Orbital Decompression: Conceptual Approach for Orbital Volume Expansion. Ophthalmic Plast. Reconstr. Surg. 2023, 39, S105–S111. [Google Scholar] [CrossRef]
- Gupta, A.; Garcia, M.D.; North, V.; Kazim, M.; Rose, G.E. Residual Apical Lamina Papyracea as a Cause for Persistent Dysthyroid Optic Neuropathy After Orbital Decompression. Ophthalmic Plast. Reconstr. Surg. 2023, 39, e37–e40. [Google Scholar] [CrossRef]
- Kauh, C.Y.; Gupta, S.; Douglas, R.S.; Elner, V.M.; Nelson, C.C.; Niziol, L.M.; Kahana, A. Compressive Optic Neuropathy and Repeat Orbital Decompression: A Case Series. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 385–390. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, G.; Yan, Y.; Wang, Z.; Liu, X.; Shi, H. Biomechanical analysis of ocular diseases and its in vitro study methods. Biomed. Eng. Online 2022, 21, 49. [Google Scholar] [CrossRef]
- Jafari, S.; Park, J.; Lu, Y.; Demer, J.L. Finite element model of ocular adduction with unconstrained globe translation. Biomech. Model. Mechanobiol. 2024, 23, 601–614. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Y.; Sun, H.; Kikkawa, D.O.; Lu, W. Biomechanical Study on the Comparison of Synthetic Materials for Cranio-Orbital Fracture Repair. J. Craniofac. Surg. 2024, 35, 398–402. [Google Scholar] [CrossRef]
- Li, Y.; Wu, P.; Liu, S.; Tang, M.; Yu, S.; Kikkawa, D.O.; Lu, W. Finite Element Analysis of 2- and 3-Point Internal Fixation Methods for the Treatment of Zygomaticomaxillary Complex Fracture. J. Craniofac. Surg. 2020, 31, 2208–2212. [Google Scholar] [CrossRef]
- Gladilin, E.; Hierl, T.; Sterker, I.; Hümpfner-Hierl, H.; Hemprich, A.; Krause, M. Retrospective cohort study of three-wall orbital resection for treatment of endocrine orbitopathy using 3D tomographic data and biomechanical modeling. Clin. Biomech. 2020, 71, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Neuhaus, M.T.; Sterker, I.; Bartella, A.K.; Schönfeld, A.; Lethaus, B.; Zimmerer, R.; Gladilin, E. Consideration of specific key points improves outcome of decompression treatment in patients with endocrine orbitopathy: Pre-/post-OP comparison and biomechanical simulation. Eur. J. Med. Res. 2022, 27, 92. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.C.; Lee, C.; Park, J.; Ryu, D. Identification of optimal surgical plan for treatment of extraocular muscle damage in thyroid eye disease patients based on computational biomechanics. Front. Bioeng. Biotechnol. 2022, 10, 969636. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M.; Regensburg, N.I.; Mourits, M.P. Differential involvement of orbital fat and extraocular muscles in graves’ ophthalmopathy. Eur. Thyroid. J. 2013, 2, 14–21. [Google Scholar] [CrossRef]
- Schutte, S.; van den Bedem, S.P.; van Keulen, F.; van der Helm, F.C.; Simonsz, H.J. A finite-element analysis model of orbital biomechanics. Vision Res. 2006, 46, 1724–1731. [Google Scholar] [CrossRef]
- Wang, X.; Rumpel, H.; Lim, W.E.; Baskaran, M.; Perera, S.A.; Nongpiur, M.E.; Aung, T.; Milea, D.; Girard, M.J. Finite Element Analysis Predicts Large Optic Nerve Head Strains During Horizontal Eye Movements. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2452–2462. [Google Scholar] [CrossRef]
- Liu, T.; Hung, P.T.; Wang, X.; Girard, M.J.A. Effect of Eye Globe and Optic Nerve Morphologies on Gaze-Induced Optic Nerve Head Deformations. Investig. Ophthalmol. Vis. Sci. 2024, 65, 48. [Google Scholar] [CrossRef]
- Karami, A.; Eghtesad, M.; Haghpanah, S.A. Prediction of muscle activation for an eye movement with finite element modeling. Comput. Biol. Med. 2017, 89, 368–378. [Google Scholar] [CrossRef]
- Jafari, S.; Lu, Y.; Park, J.; Demer, J.L. Finite Element Model of Ocular Adduction by Active Extraocular Muscle Contraction. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1. [Google Scholar] [CrossRef]
- Dolman, P.J. Evaluating Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 229–248. [Google Scholar] [CrossRef]
- Dolman, P.J. Grading Severity and Activity in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2018, 34 (Suppl. 1), S34–S40. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.; Schittkowski, M.; Esser, J. Surgical treatment of Graves’ ophthalmopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Enz, T.J.; Tschopp, M. Assessment of Orbital Compartment Pressure: A Comprehensive Review. Diagnostics 2022, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.J.; Koornneef, L.; Mourits, M.P.; Deen-van Leeuwen, L. Retrobulbar pressures measured during surgical decompression of the orbit. Br. J. Ophthalmol. 1996, 80, 1042–1045. [Google Scholar] [CrossRef]
- Riemann, C.D.; Foster, J.A.; Kosmorsky, G.S. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology 1999, 106, 1296–1302. [Google Scholar] [CrossRef]
- Kratky, V.; Hurwitz, J.J.; Avram, D.R. Orbital compartment syndrome. Direct measurement of orbital tissue pressure: 1. Technique. Can. J. Ophthalmol. 1990, 25, 293–297. [Google Scholar]
- Nugent, R.A.; Belkin, R.I.; Neigel, J.M.; Rootman, J.; Robertson, W.D.; Spinelli, J.; Graeb, D.A. Graves orbitopathy: Correlation of CT and clinical findings. Radiology 1990, 177, 675–682. [Google Scholar] [CrossRef]
- Zah-Bi, G.; Abeillon-du Payrat, J.; Vie, A.L.; Bournaud-Salinas, C.; Jouanneau, E.; Berhouma, M. Minimal-Access Endoscopic Endonasal Management of Dysthyroid Optic Neuropathy: The Dysthone Study. Neurosurgery 2019, 85, E1059–E1067. [Google Scholar] [CrossRef]
- Neigel, J.M.; Rootman, J.; Belkin, R.I.; Nugent, R.A.; Drance, S.M.; Beattie, C.W.; Spinelli, J.A. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 1988, 95, 1515–1521. [Google Scholar] [CrossRef]
- Rana, K.; Garg, D.; Yong, L.S.S.; Macri, C.; Tong, J.Y.; Patel, S.; Slattery, J.; Chan, W.O.; Davis, G.; Selva, D. Extraocular muscle enlargement in dysthyroid optic neuropathy. Can. J. Ophthalmol. 2024, 59, e542–e546. [Google Scholar] [CrossRef]
- Oropesa, S.; Dunbar, K.E.; Godfrey, K.J.; Callahan, A.B.; Campbell, A.A.; Kazim, M. Predominant Contribution of Superior Rectus-Levator Complex Enlargement to Optic Neuropathy and Inferior Visual Field Defects in Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Dagi, L.R.; Zoumalan, C.I.; Konrad, H.; Trokel, S.L.; Kazim, M. Correlation between extraocular muscle size and motility restriction in thyroid eye disease. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Cristofani-Mencacci, L.; Fiacchini, G.; Benettini, G.; Picariello, M.; Lanzolla, G.; Lazzerini, F.; Rocchi, R.; Turri-Zanoni, M.; Menconi, F.; et al. Functional outcomes and complications in refractory dysthyroid optic neuropathy management: Experience with 3 different surgical protocols. Am. J. Otolaryngol. 2022, 43, 103451. [Google Scholar] [CrossRef] [PubMed]
- Baeg, J.; Choi, H.S.; Kim, C.; Kim, H.; Jang, S.Y. Update on the surgical management of Graves’ orbitopathy. Front. Endocrinol. 2022, 13, 1080204. [Google Scholar] [CrossRef]
- Jellema, H.M.; Althaus, M.; Merckel-Timmer, E.; Hartong, D.T.; Kloos, R.; Saeed, P. Effects of orbital decompression on duction, cyclotorsion and diplopia. Br. J. Ophthalmol. 2024, 108, 1075–1080. [Google Scholar] [CrossRef]





| Tissue | Constitutive Model | Biomechanical Properties | Source |
|---|---|---|---|
| Optic nerve | Isotropic elastic | Elastic modulus = 4663 Pa | Experimentally determined |
| Poisson’s ratio = 0.49 | Liu et al. [27] | ||
| Adipose tissue | Isotropic elastic | Elastic modulus = 636.5 Pa | Experimentally determined |
| Poisson’s ratio = 0.49 | Liu et al. [27] | ||
| Eyeball | Isotropic elastic | Elastic modulus = 500 KPa | Schutte et al. [25] |
| Poisson’s ratio = 0.4 | Schutte et al. [25] | ||
| EOMs | Isotropic elastic | Elastic modulus = 1529 Pa | Experimentally determined |
| Poisson’s ratio = 0.49 | Wang et al. [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Wu, Y.; Rao, J.; Yang, S.; Yao, H.; Liu, Q.; Wu, Y.; Mi, S.; Liu, G. Biomechanical Analysis and Clinical Study of Augmented Versus Conventional Endoscopic Orbital Decompression for Dysthyroid Optic Neuropathy. Bioengineering 2025, 12, 618. https://doi.org/10.3390/bioengineering12060618
Wu P, Wu Y, Rao J, Yang S, Yao H, Liu Q, Wu Y, Mi S, Liu G. Biomechanical Analysis and Clinical Study of Augmented Versus Conventional Endoscopic Orbital Decompression for Dysthyroid Optic Neuropathy. Bioengineering. 2025; 12(6):618. https://doi.org/10.3390/bioengineering12060618
Chicago/Turabian StyleWu, Pengsen, Yiheng Wu, Jing Rao, Shenglan Yang, Hongyi Yao, Qingjiang Liu, Yuqing Wu, Shengli Mi, and Guiqin Liu. 2025. "Biomechanical Analysis and Clinical Study of Augmented Versus Conventional Endoscopic Orbital Decompression for Dysthyroid Optic Neuropathy" Bioengineering 12, no. 6: 618. https://doi.org/10.3390/bioengineering12060618
APA StyleWu, P., Wu, Y., Rao, J., Yang, S., Yao, H., Liu, Q., Wu, Y., Mi, S., & Liu, G. (2025). Biomechanical Analysis and Clinical Study of Augmented Versus Conventional Endoscopic Orbital Decompression for Dysthyroid Optic Neuropathy. Bioengineering, 12(6), 618. https://doi.org/10.3390/bioengineering12060618







