The Impact of Small Incision Lenticule Extraction on the Biomechanical Properties of the Cornea: A Review
Abstract
1. Introduction
2. Methods
2.1. Review Type and Rationale
2.2. Literature Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Synthesis
3. Results
3.1. Overview of Corneal Biomechanics
Basic Concepts of Corneal Biomechanics
3.2. Measurements of Corneal Biomechanics
3.3. Effects of Keratorefractive Surgery on Corneal Biomechanics
3.4. SMILE and Corneal Biomechanics
The Effect of SMILE on Corneal Biomechanics
3.5. Comparison of Corneal Biomechanical Effects of Other Keratorefractive Surgery Versus SMILE
3.5.1. PRK/LASEK vs. SMILE
3.5.2. FS-LASIK or FLEx Versus SMILE
3.6. Exploration of Mechanisms Affecting Corneal Biomechanical Changes After SMILE
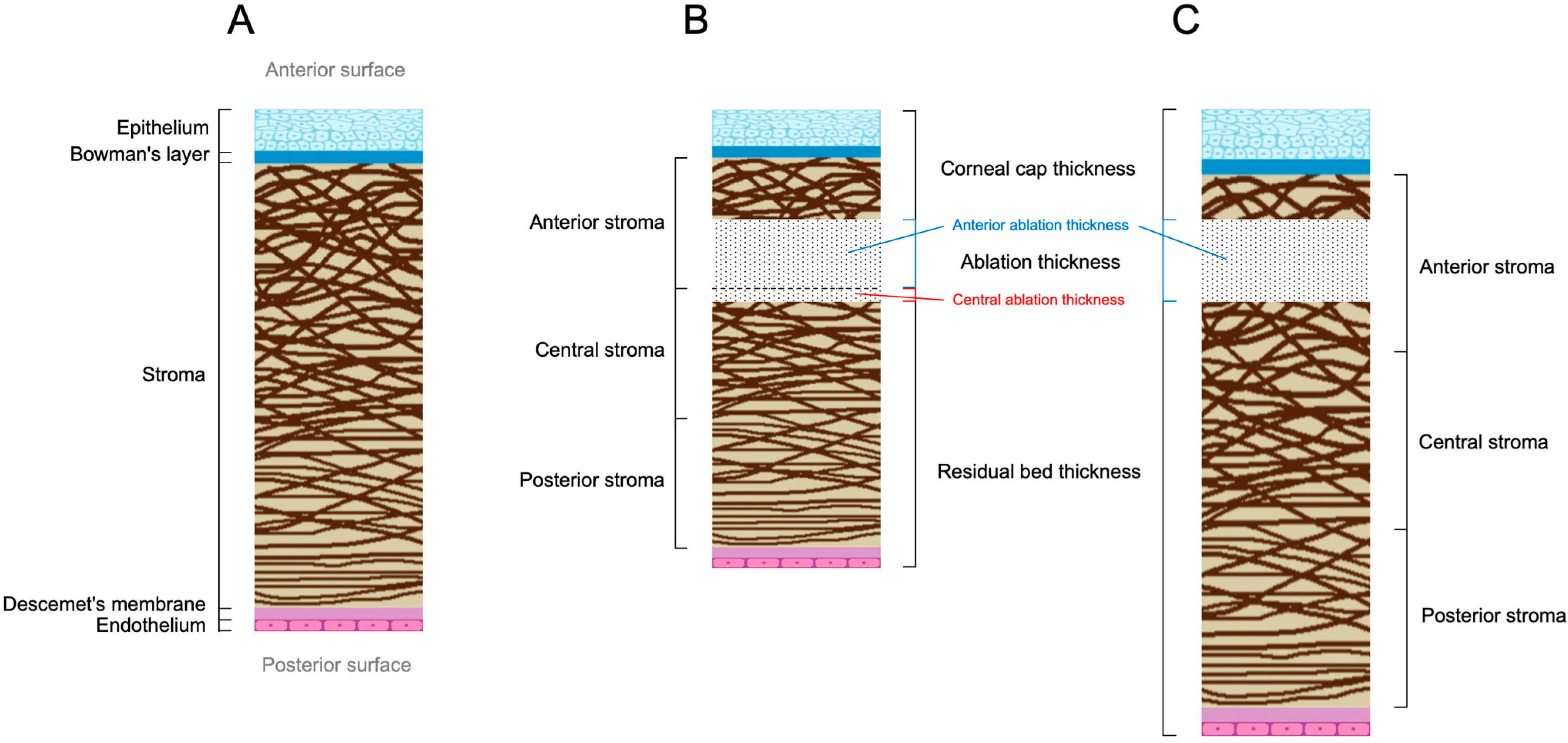
Changes in Corneal Thickness
3.7. Other Factors
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Melki, S. Small Incision Lenticule Extraction (SMILE): Myths and Realities. Semin. Ophthalmol. 2021, 36, 140–148. [Google Scholar] [CrossRef]
- Blum, M.; Kunert, K.S.; Sekundo, W. Historical overview of the clinical development of the small incision lenticule extraction surgery (SMILE). Klin. Monbl. Augenheilkd. 2017, 234, 117–122. [Google Scholar] [PubMed]
- Krueger, R.R.; Meister, C.S. A review of small incision lenticule extraction complications. Curr. Opin. Ophthalmol. 2018, 29, 292–298. [Google Scholar] [CrossRef]
- Santhiago, M.R.; Giacomin, N.T.; Smadja, D.; Bechara, S.J. Ectasia risk factors in refractive surgery. Clin. Ophthalmol. 2016, 10, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wolle, M.A.; Randleman, J.B.; Woodward, M.A. Complications of Refractive Surgery: Ectasia After Refractive Surgery. Int. Ophthalmol. Clin. 2016, 56, 127–139. [Google Scholar] [CrossRef]
- Sutton, G.; Lawless, M.; Hodge, C. Laser in situ keratomileusis in 2012: A review. Clin. Exp. Optom. 2014, 97, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Geraghty, B.; Wang, Q.; Elsheikh, A. Consideration of corneal biomechanics in the diagnosis and management of keratoconus: Is it important? Eye Vis. 2016, 3, 18. [Google Scholar] [CrossRef]
- Bao, F.; Lopes, B.T.; Zheng, X.; Zheng, X.; Ji, Y.; Wang, J.; Elsheikh, A. Corneal Biomechanics Losses Caused by Refractive Surgery. Curr. Eye Res. 2023, 48, 137–143. [Google Scholar] [CrossRef]
- Chong, J.; Dupps, W.J., Jr. Corneal biomechanics: Measurement and structural correlations. Exp. Eye Res. 2021, 205, 108508. [Google Scholar] [CrossRef]
- Wilson, A.; Marshall, J. A review of corneal biomechanics: Mechanisms for measurement and the implications for refractive surgery. Indian J. Ophthalmol. 2020, 68, 2679–2690. [Google Scholar] [CrossRef]
- Kling, S.; Hafezi, F. Corneal biomechanics—A review. Ophthalmic. Physiol. Opt. 2017, 37, 240–252. [Google Scholar] [CrossRef]
- Winkler, M.; Shoa, G.; Xie, Y.; Petsche, S.J.; Pinsky, P.M.; Juhasz, T.; Brown, D.J.; Jester, J.V. Three-dimensional distribution of transverse collagen fibers in the anterior human corneal stroma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7293–7301. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: Implications and association with corneal disorders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, G.; Yan, Y.; Wang, Z.; Liu, X.; Shi, H. Biomechanical analysis of ocular diseases and its in vitro study methods. Biomed. Eng. Online 2022, 21, 49. [Google Scholar] [CrossRef]
- Li, F.; Wang, K.; Liu, Z. In Vivo Biomechanical Measurements of the Cornea. Bioengineering 2023, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Yu, M.; Zhang, H.; Chen, X.; Li, L. The Mechanical Interpretation of Ocular Response Analyzer Parameters. Biomed. Res. Int. 2019, 2019, 5701236. [Google Scholar] [CrossRef]
- Miao, Y.Y.; Ma, X.M.; Qu, Z.X.; Eliasy, A.; Wu, B.W.; Xu, H.; Wang, P.; Zheng, X.B.; Wang, J.J.; Ye, Y.F.; et al. Performance of Corvis ST Parameters Including Updated Stress-Strain Index in Differentiating Between Normal, Forme-Fruste, Subclinical, and Clinical Keratoconic Eyes. Am. J. Ophthalmol. 2024, 258, 196–207. [Google Scholar] [CrossRef]
- Prevedel, R.; Diz-Muñoz, A.; Ruocco, G.; Antonacci, G. Brillouin microscopy: An emerging tool for mechanobiology. Nat. Methods 2019, 16, 969–977. [Google Scholar] [CrossRef]
- Lan, G.; Twa, M.D.; Song, C.; Feng, J.; Huang, Y.; Xu, J.; Qin, J.; An, L.; Wei, X. In vivo corneal elastography: A topical review of challenges and opportunities. Comput. Struct. Biotechnol. J. 2023, 21, 2664–2687. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Touboul, D.; Gennisson, J.L.; Bercoff, J.; Fink, M. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans. Med. Imaging 2009, 28, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, C.; Li, B.; Wang, D.; Fang, X. Influence of Cap Thickness on Corneal Curvature and Corneal Biomechanics After SMILE: A Prospective, Contralateral Eye Study. J. Refract. Surg. 2020, 36, 82–88. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Zhang, J.; Chan, T.C.Y.; Ng, A.L.K.; Cheng, G.P.M.; Jhanji, V. Comparison of corneal biomechanics after microincision lenticule extraction and small incision lenticule extraction. Br. J. Ophthalmol. 2017, 101, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, A.H.; Grauslund, J.; Ivarsen, A.R.; Hjortdal, J.Ø. Central corneal sublayer pachymetry and biomechanical properties after refractive femtosecond lenticule extraction. J. Refract. Surg. 2014, 30, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Kang, D.S.Y.; Roberts, C.J.; Lee, H.; Jean, S.K.; Kim, E.K.; Seo, K.Y.; Kim, T.I. Comparison of Clinical and Biomechanical Outcomes of Small Incision Lenticule Extraction With 120- and 140-µm Cap Thickness. Transl. Vis. Sci. Technol. 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Pniakowska, Z.; Jurowski, P.; Wierzbowska, J. Clinical Evaluation of Corneal Biomechanics following Laser Refractive Surgery in Myopic Eyes: A Review of the Literature. J. Clin. Med. 2022, 12, 243. [Google Scholar] [CrossRef]
- Fu, D.; Li, M.; Knorz, M.C.; Wei, S.; Shang, J.; Zhou, X. Intraocular pressure changes and corneal biomechanics after hyperopic small-incision lenticule extraction. BMC Ophthalmol. 2020, 20, 129. [Google Scholar] [CrossRef]
- Cao, K.; Liu, L.; Yu, T.; Chen, F.; Bai, J.; Liu, T. Changes in corneal biomechanics during small-incision lenticule extraction (SMILE) and femtosecond-assisted laser in situ keratomileusis (FS-LASIK). Lasers Med. Sci. 2020, 35, 599–609. [Google Scholar] [CrossRef]
- Qu, Z.; Li, X.; Yuan, Y.; Wang, P.; Li, Y.; Lin, S.; Lian, H.; Chen, S.; Ye, Y.; Wang, J.; et al. In Vivo Corneal Biomechanical Response to Three Different Laser Corneal Refractive Surgeries. J. Refract. Surg. 2024, 40, e344–e352. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.A.; El Dorghamy, A.A.; Ghoneim, A.M.; Saad, H.A. Comparison of corneal biomechanical changes after LASIK and F-SMILE with CorVis ST. Eur. J. Ophthalmol. 2021, 31, 1762–1770. [Google Scholar] [CrossRef]
- Yu, M.; Chen, M.; Dai, J. Comparison of the posterior corneal elevation and biomechanics after SMILE and LASEK for myopia: A short- and long-term observation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 601–606. [Google Scholar] [CrossRef]
- He, S.; Luo, Y.; Ye, Y.; Chen, P.; Liu, C.; Lei, L.; Zhuang, J.; Yu, K. A comparative and prospective study of corneal biomechanics after SMILE and FS-LASIK performed on the contralateral eyes of high myopia patients. Ann. Transl. Med. 2022, 10, 730. [Google Scholar] [CrossRef]
- Xin, Y.; Lopes, B.T.; Wang, J.; Wu, J.; Zhu, M.; Jiang, M.; Miao, Y.; Lin, H.; Cao, S.; Zheng, X.; et al. Biomechanical Effects of tPRK, FS-LASIK, and SMILE on the Cornea. Front. Bioeng. Biotechnol. 2022, 10, 834270. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Jafarpour, S.; Hassanzadeh, S.; Bakhtiari, E.; Daraee, G.; Monadi, S.D.; Ziaei, M. Changes in Corneal Biomechanical Properties After Small-Incision Lenticule Extraction and Photorefractive Keratectomy, Using a Noncontact Tonometer. Cornea 2022, 41, 886–893. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, F.; Song, Y.; Zhai, C.; Guo, N.; Lai, L.; Xu, Y. Corneal biomechanical characteristics following small incision lenticule extraction for myopia and astigmatism with 3 different cap thicknesses. BMC Ophthalmol. 2023, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, N.; Chen, T.; Tian, G.; Lin, Y.; Gao, H.; Shi, W. Comparison of Corneal Biomechanics Treated with Femtosecond Laser-Assisted in Situ Keratomileusis and Small-Incision Lenticule Extraction by New Corneal Biomechanical Parameters of Corvis ST II. Cornea 2023, 42, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Roberts, C.J.; Elsheikh, A.; Mehravaran, S.; Panahi, P.; Asgari, S. Corneal Biomechanics After SMILE, Femtosecond-Assisted LASIK, and Photorefractive Keratectomy: A Matched Comparison Study. Transl. Vis. Sci. Technol. 2023, 12, 12. [Google Scholar] [CrossRef]
- Sedaghat, M.R.; Momeni-Moghaddam, H.; Yekta, A.A.; Maddah, N.; Roberts, C.J.; Savardashtaki, M. Early elastic and viscoelastic corneal biomechanical changes after photorefractive keratectomy and small incision lenticule extraction. Int. Ophthalmol. 2024, 44, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, W.; Liu, X.; Li, N.; Chen, T.; Gao, H. Postoperative corneal biomechanics and influencing factors during femtosecond-assisted laser in situ keratomileusis (FS-LASIK) and laser-assisted subepithelial keratomileusis (LASEK) for high myopia. Lasers Med. Sci. 2021, 36, 1709–1717. [Google Scholar] [CrossRef]
- Guo, H.; Hosseini-Moghaddam, S.M.; Hodge, W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: A systematic review and meta-analysis. BMC Ophthalmol. 2019, 19, 167. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Z.; Knorz, M.C.; Li, M.; Zhao, J.; Zhou, X. Comparison of corneal deformation parameters after SMILE, LASEK, and femtosecond laser-assisted LASIK. J. Refract. Surg. 2014, 30, 310–318. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Zhang, L.; Wei, S.; Tang, X. Corneal biomechanical effects: Small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J. Cataract. Refract. Surg. 2014, 40, 954–962. [Google Scholar] [CrossRef]
- Elmohamady, M.N.; Abdelghaffar, W.; Daifalla, A.; Salem, T. Evaluation of femtosecond laser in flap and cap creation in corneal refractive surgery for myopia: A 3-year follow-up. Clin. Ophthalmol. 2018, 12, 935–942. [Google Scholar] [CrossRef]
- Yan, H.; Gong, L.Y.; Huang, W.; Peng, Y.L. Clinical outcomes of small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: A Meta-analysis. Int. J. Ophthalmol. 2017, 10, 1436–1445. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Naidu, R.K.; Chu, R.; Dai, J.; Qu, X.; Yu, Z.; Zhou, H. Comparison of the change in posterior corneal elevation and corneal biomechanical parameters after small incision lenticule extraction and femtosecond laser-assisted LASIK for high myopia correction. Cont. Lens Anterior Eye 2016, 39, 191–196. [Google Scholar] [CrossRef]
- Raevdal, P.; Grauslund, J.; Vestergaard, A.H. Comparison of corneal biomechanical changes after refractive surgery by noncontact tonometry: Small-incision lenticule extraction versus flap-based refractive surgery—A systematic review. Acta Ophthalmol. 2019, 97, 127–136. [Google Scholar] [CrossRef]
- Petsche, S.J.; Chernyak, D.; Martiz, J.; Levenston, M.E.; Pinsky, P.M. Depth-dependent transverse shear properties of the human corneal stroma. Invest. Ophthalmol. Vis. Sci. 2012, 53, 873–880. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Igarashi, A.; Kobashi, H.; Sato, N.; Ishii, R. Intraindividual comparison of changes in corneal biomechanical parameters after femtosecond lenticule extraction and small-incision lenticule extraction. J. Cataract Refract. Surg. 2014, 40, 963–970. [Google Scholar] [CrossRef]
- Agca, A.; Ozgurhan, E.B.; Demirok, A.; Bozkurt, E.; Celik, U.; Ozkaya, A.; Cankaya, I.; Yilmaz, O.F. Comparison of corneal hysteresis and corneal resistance factor after small incision lenticule extraction and femtosecond laser-assisted LASIK: A prospective fellow eye study. Cont. Lens Anterior Eye 2014, 37, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, I.B.; Reffat, M.; Hjortdal, J. Review of Corneal Biomechanical Properties Following LASIK and SMILE for Myopia and Myopic Astigmatism. Open Ophthalmol. J. 2018, 12, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J. Comparison of corneal biomechanics after myopic small-incision lenticule extraction compared to LASIK: An ex vivo study. Clin. Ophthalmol. 2018, 12, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Cheng, G.P.; Zhang, J.; Ng, A.L.; Chan, T.C.; Jhanji, V.; Wang, Y. Changes in Corneal Volume at Different Areas and Its Correlation with Corneal Biomechanics after SMILE and FS-LASIK Surgery. J. Ophthalmol. 2020, 2020, 1713979. [Google Scholar] [CrossRef]
- Jia, Y.; He, R.; Li, X.; Song, Y.; Wei, J.; Qin, H.; Yang, X.; Chen, W. Effects of SMILE with different residual stromal thicknesses on corneal biomechanical properties of rabbits in vivo. J. Biomedical. Eng. 2022, 39, 679–684. [Google Scholar]
- Liu, J.; Wang, Y.; Zou, H.H.; Li, M.D. Relation between corneal biomechanical alteration after small incision lenticule extraction and intraoperative cutting thickness. Chin. J. Ophthalmol. 2021, 57, 104–112. [Google Scholar]
- Damgaard, I.B.; Ivarsen, A.; Hjortdal, J. Refractive Correction and Biomechanical Strength Following SMILE With a 110- or 160-μm Cap Thickness, Evaluated Ex Vivo by Inflation Test. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1836–1843. [Google Scholar] [CrossRef]
- Liu, T.; Yu, T.; Liu, L.; Chen, K.; Bai, J. Corneal Cap Thickness and Its Effect on Visual Acuity and Corneal Biomechanics in Eyes Undergoing Small Incision Lenticule Extraction. J. Ophthalmol. 2018, 2018, 6040873. [Google Scholar] [CrossRef] [PubMed]
- El-Massry, A.A.; Goweida, M.B.; Shama, A.E.S.; Elkhawaga, M.H.; Abdalla, M.F. Contralateral Eye Comparison Between Femtosecond Small Incision Intrastromal Lenticule Extraction at Depths of 100 and 160 μm. Cornea 2015, 34, 1272–1275. [Google Scholar] [CrossRef]
- Hosny, M.; Aboalazayem, F.; El Shiwy, H.; Salem, M. Comparison of different intraocular pressure measurement techniques in normal eyes and post small incision lenticule extraction. Clin. Ophthalmol. 2017, 11, 1309–1314. [Google Scholar] [CrossRef]
- Viswanathan, D.; Kumar, N.L.; Males, J.J.; Graham, S.L. Relationship of Structural Characteristics to Biomechanical Profile in Normal, Keratoconic, and Crosslinked Eyes. Cornea 2015, 34, 791–796. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, X.; Zheng, Z.Y.; Lu, Q. Correlation of the changes in corneal volume with the corneal biomechanical parameters after small incision lenticule extraction. Eye Sci. 2022, 37, 609–619. [Google Scholar]
- Ma, J.N.; Wang, Y.; Song, Y.; Shao, T.; Cai, Y. The effect of corneal biomechanical properties on opaque bubble layer in small incision lenticule extraction (SMILE). Chin. J. Ophthalmol. 2019, 55, 115–121. [Google Scholar]

| Measurement Principles | Measurement Process | Main Observed Indicators | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| ORA | The bi-directional movement of the cornea as well as the deformation of the cornea is monitored by the reflection of the infrared beam | A rapid pulse of air is applied to a 3–6 mm area in the centre of the cornea to measure the process of inward depression of the cornea due to the pulse of air, flattening of the cornea to form a slight depression, and rebound of the cornea back to its normal state | CH, CRF, and 37 new parameters describing ORA curve waveforms | Non-contact, rapid and more accurate detection of corneal biomechanics | Does not directly provide standardised biomechanical indications of the cornea, does not reflect the specific location and severity of the cornea, and may not reflect the biomechanical tensile strength of the cornea in its physiological state when measured by indentation |
| Corvis ST | Corneal deformation was monitored using an ultra-high speed Scheimpflug camera (4300 frames per second) | Similarly to ORA, a pulse of air with a maximum pressure of 25 kPa was applied, and the process of corneal inward depression due to the action of the pulsed air flow, flattening and then forming a slight depression, and corneal rebound back to its normal state was measured | DA, HCPD, SP-A1, IR, DAR, CBI, and other parameters | Non-contact, rapid and more accurate detection of corneal biomechanics, higher visualisation compared to ORA | Same as ORA |
| OCE | An elastography method developed based on optical coherence tomography | (1) inducing soft tissue deformation or vibration; (2) detecting the propagation of deformation, displacement, vibration, or oscillation; (3) assessing soft tissue elasticity | Modulus of elasticity, which means that the measured data on the elastic response of the tissue is mathematically modelled and finally presented in the form of Young’s modulus | Non-invasive imaging, real-time image processing performance and high resolution | Lack of harmonised clinical standards and non-existence of commercially available devices |
| BOM | Based on the Brillouin scattering principle | A low-power focused laser beam and a high-resolution confocal spectrometer were used to measure the Brillouin frequency at the focal point | Differences are shown by comparing the Brillouin frequency shift of the cornea, but there is no uniformity in the current range of reference values | Good spatial resolution, no contact during imaging, no external loads required | Long collection time, highly influenced by the degree of corneal hydration, and the lack of commercially available equipment |
| SSWI | Elastography based on ultrasound imaging | Quantification of transverse wave velocities generated by focused ultrasound in tissues using high frame rate (up to 20,000 frames/s) ultrasound imaging and linking them to elastic moduli through mathematical modelling | Modulus of Elasticity. Similar to OCE, through mathematical modelling, and ultimately in the form of Young’s modulus | Real-time mapping of tissue elasticity and good spatial resolution | Requires liquid coupling medium to contact the cornea, long image acquisition times, and complex data processing |
| PhD-OCT | Corneal biomechanics measurements based on dynamic light scattering theory | Image acquisition is achieved using two OCT devices with different centre wavelengths, and post-acquisition processing is performed using the Fourier Transform | The parameter Γ. For the cornea, the parameter Γ is inversely proportional to the degree of collagen restriction. That is, the higher the parameter Γ, the lower the biomechanics | Good soft-tissue spatial resolution without any perturbation of the cornea, relatively simple measurements, less affected by IOP, short acquisition time, can be achieved using existing clinical OCT systems | High signal-to-noise ratio, susceptible to corneal hydration and eye movement artefacts |
| Study | Eyes (n) | Country | Age (y) | Sphere (D) | Cylinder (D) | CCT (μm) | Follow-Up | Instruments | Assessment Parameters |
|---|---|---|---|---|---|---|---|---|---|
| Fu et al. [26] | 13 | China | 32.8 ± 9.0 | 4.17 ± 1.55 | −0.90 ± 0.75 | 546.7 ± 25.3 | 1 w, 1 m, 3 m | ORA, Corvis ST | bIOP, CH, CRF, A1T, DA, SP-A1 |
| Cao et al. [27] | 80 | China | 26.9 ± 5.6 | −4.67 ± 1.31 * | 539.8 ± 27.0 | 3 m | Corvis ST | bIOP, IR, HCR, DAR1, DAR2 | |
| Qu et al. [28] | 69 | China | 27.3 ± 5.8 | −5.17 ± 1.80 | −0.74 ± 0.57 | 552.5 ± 24.2 | 3 m, 6 m | Corvis ST | DAR2, IR, SP-A1, HCT, SSIv2 |
| Abd et al. [29] | 30 | Egypt | 26.4 ± 5.4 | −4.95 ± 1.32 * | 556.8 ± 28.4 | 3 m | Corvis ST | bIOP, A1V, A2V, DAR, IR, ARTH, SP-A1, CBI | |
| Yu et al. [30] | 32 | China | 23.4 ± 4.6 | −4.1 ± 0.8 * | 551.1 ± 23.1 | 3 m, 3 y | ORA | IOPcc, CH, CRF | |
| He et al. [31] | 50 | China | 25.9 ± 4.9 | −7.96 ± 1.11 | −1.03 ± 0.69 | 552.8 ± 38.8 | 1 m, 3 m, 6 m, 15 m | Corvis ST | A1T, A1L, A1V, A2T, A2L, A2V, HCT, HCR, HCPD, DAR2, IR, ARTH, SP-A1, CBI, SSI |
| Xin et al. [32] (SMILE) | 72 * | China | 26.9 ± 5.7 | −5.93 ± 1.05 | −0.84 ± 0.49 | 547.0 ± 22.7 | 1 m, 3 m, 6 m | Corvis ST | SP-A1, IR, DA, DAR2 |
| Xin et al. [32] (FS-LASIK) | 72 * | China | 25.7 ± 6.3 | −3.38 ± 0.72 | −0.81 ± 0.77 | 546.0 ± 20.3 | 1 m, 3 m, 6 m | Corvis ST | SP-A1, IR, DA, DAR2 |
| Jun et al. (120 μm corneal cap) [24] | 91 | Korea | 27.8 ± 6.0 | −3.18 ± 1.28 | −0.93 ± 0.69 | 565.2 ± 24.4 | 6 m | Corvis ST | DAR, SP-A1, IR, ARTH, SSI, bIOP |
| Jun et al. (140 μm corneal cap) [24] | 59 | Korea | 27.3 ± 7.0 | −3.26 ± 1.49 | −0.97 ± 0.94 | 563.9 ± 22.0 | 6 m | Corvis ST | DAR, SP-A1, IR, ARTH, SSI, bIOP |
| Ghanavati et al. [33] | 37 | Iran | 32.3 ± 6.7 | −3.70 ± 1.92 | −0.93 ± 0.93 | 523.8 ± 37.8 | 3 m | Corvis ST | bIOP, A1T, A1L, A1V, A2T, A2L, A2V, SP-A1, SP-HC, DA, DAmax, DAR2, HCT, HCPD, HCR, IRmax, ARTH, SSI, WEMmax |
| Lv et al. (110 μm corneal cap) [34] | 48 | China | 24.0 ± 4.4 | −5.02 ± 0.99 | −0.55 ± 0.51 | 545.3 ± 15.0 | 1 w, 1 m, 3 m, 6 m | Corvis ST | IR, DAR1, DAR2, ARTH, SP-A1, SSI, bIOP |
| Lv et al. (120 μm corneal cap) [34] | 49 | China | 25.5 ± 4.4 | −4.87 ± 1.01 | −0.79 ± 0.69 | 546.6 ± 15.0 | 1 w, 1 m, 3 m, 6 m | Corvis ST | IR, DAR1, DAR2, ARTH, SP-A1, SSI, bIOP |
| Lv et al. (130 μm corneal cap) [34] | 49 | China | 24.0 ± 4.1 | −4.60 ± 0.82 | −0.75 ± 0.81 | 551.3 ± 9.9 | 1 w, 1 m, 3 m, 6 m | Corvis ST | IR, DAR1, DAR2, ARTH, SP-A1, SSI, bIOP |
| Liu et al. [35] | 45 | China | 25.2 ± 6.5 | −4.99 ± 1.06 | 544.7 ± 20.8 | 1 m, 6 m | Corvis ST | IR, DAR2, ARTH, SP-A1 | |
| Wu et al. (110 μm corneal cap) [21] | 50 | China | 25.0 ± 5.1 * | −4.54 ± 0.95 | 551.2 ± 26.0 | 6 m | Corvis ST | bIOP, A1T, A1L, A1V, A2T, A2L, A2V, SP-A1, HCPD, HCR, DA, IR, DAR | |
| Wu et al. (140 μm corneal cap) [21] | 50 | China | 25.0 ± 5.1 * | −4.50 ± 1.03 | 550.9 ± 25.1 | 6 m | Corvis ST | bIOP, A1T, A1L, A1V, A2T, A2L, A2V, SP-A1, HCPD, HCR, DA, IR, DAR | |
| Hashemi et al. [36] | 120 | Iran | 28.0 ± 5.3 | −4.66 ± 0.85 * | 567.0 ± 25.3 | 3 m, 1 y | Corvis ST | SSI, SP-A1, IR, DAR1, DAR2 | |
| Sedaghat et al. [37] | 62 | Iran | 26.4 ± 5.2 | −3.73 ± 1.18 * | 552.1 ± 25.0 | ORA, Corvis ST | A1L, A1V, A2L, A2V, HCPD, HCR, DA, SP-A1, ARTH, IR, DAR, CH, CRF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Jiang, H.; Mo, F.; Jiang, Y. The Impact of Small Incision Lenticule Extraction on the Biomechanical Properties of the Cornea: A Review. Bioengineering 2025, 12, 1199. https://doi.org/10.3390/bioengineering12111199
Du Y, Jiang H, Mo F, Jiang Y. The Impact of Small Incision Lenticule Extraction on the Biomechanical Properties of the Cornea: A Review. Bioengineering. 2025; 12(11):1199. https://doi.org/10.3390/bioengineering12111199
Chicago/Turabian StyleDu, Yifan, Hanyu Jiang, Fei Mo, and Yang Jiang. 2025. "The Impact of Small Incision Lenticule Extraction on the Biomechanical Properties of the Cornea: A Review" Bioengineering 12, no. 11: 1199. https://doi.org/10.3390/bioengineering12111199
APA StyleDu, Y., Jiang, H., Mo, F., & Jiang, Y. (2025). The Impact of Small Incision Lenticule Extraction on the Biomechanical Properties of the Cornea: A Review. Bioengineering, 12(11), 1199. https://doi.org/10.3390/bioengineering12111199






