Fast and Accurate Sperm Detection Algorithm for Micro-TESE in NOA Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Differential Interference Microscopy
- Objective lens: UPlanFL10x NA0.30 ∞/− (10× magnification, semi-apochromat lens);
- Eyepiece lens: WH10x/22, 2 pieces (10× magnification, field number 22, one for each eye);
- Filter: Color temperature conversion filter + ND filter + Frost filter;
- Illumination: 100 W halogen transmission lighting.
2.2. Detection Using MB-LBP
2.3. Proposed SD-CLIP Algorithm
2.3.1. Sperm Head Shape-Based Candidate Detection
2.3.2. Sperm Determination
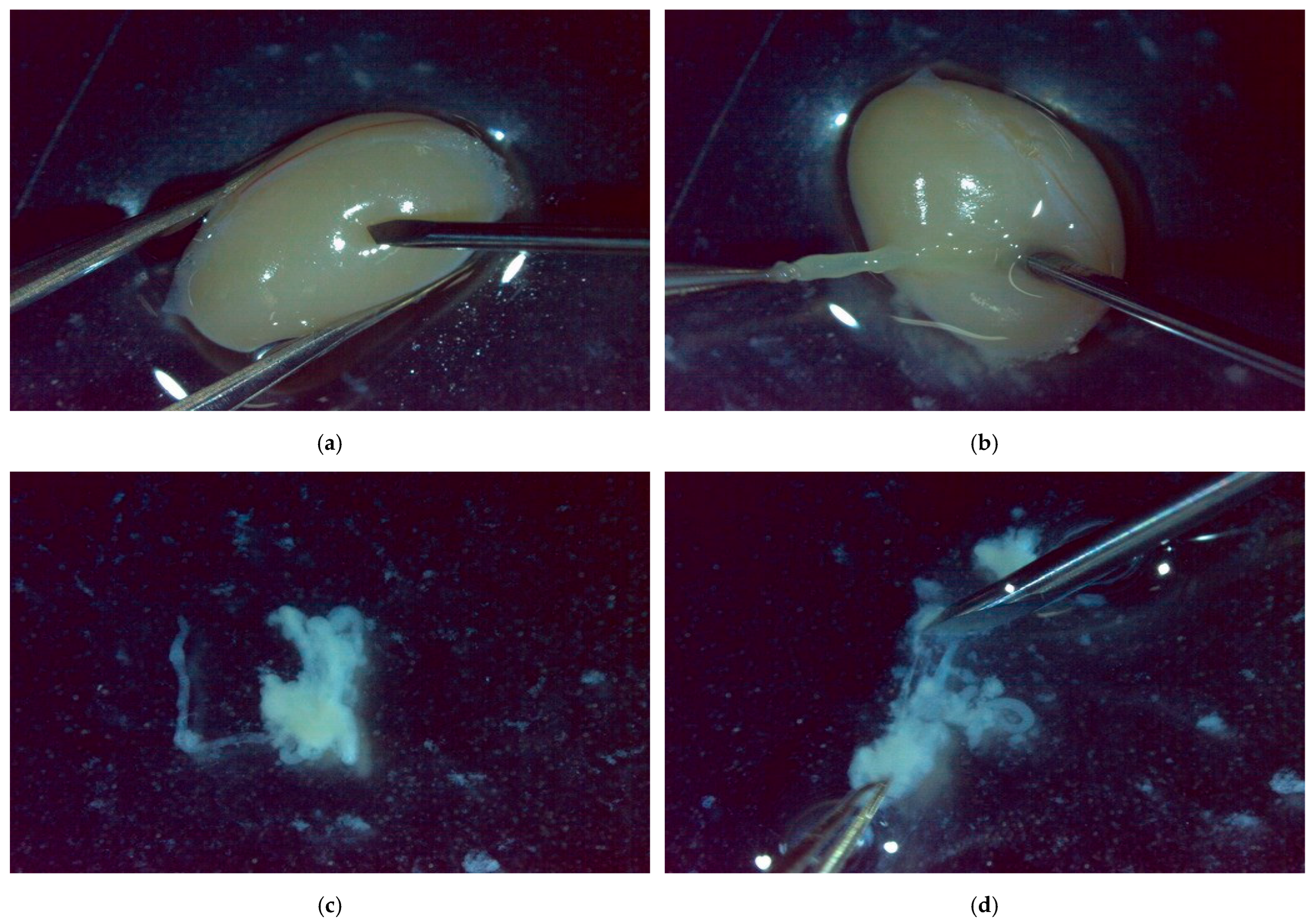
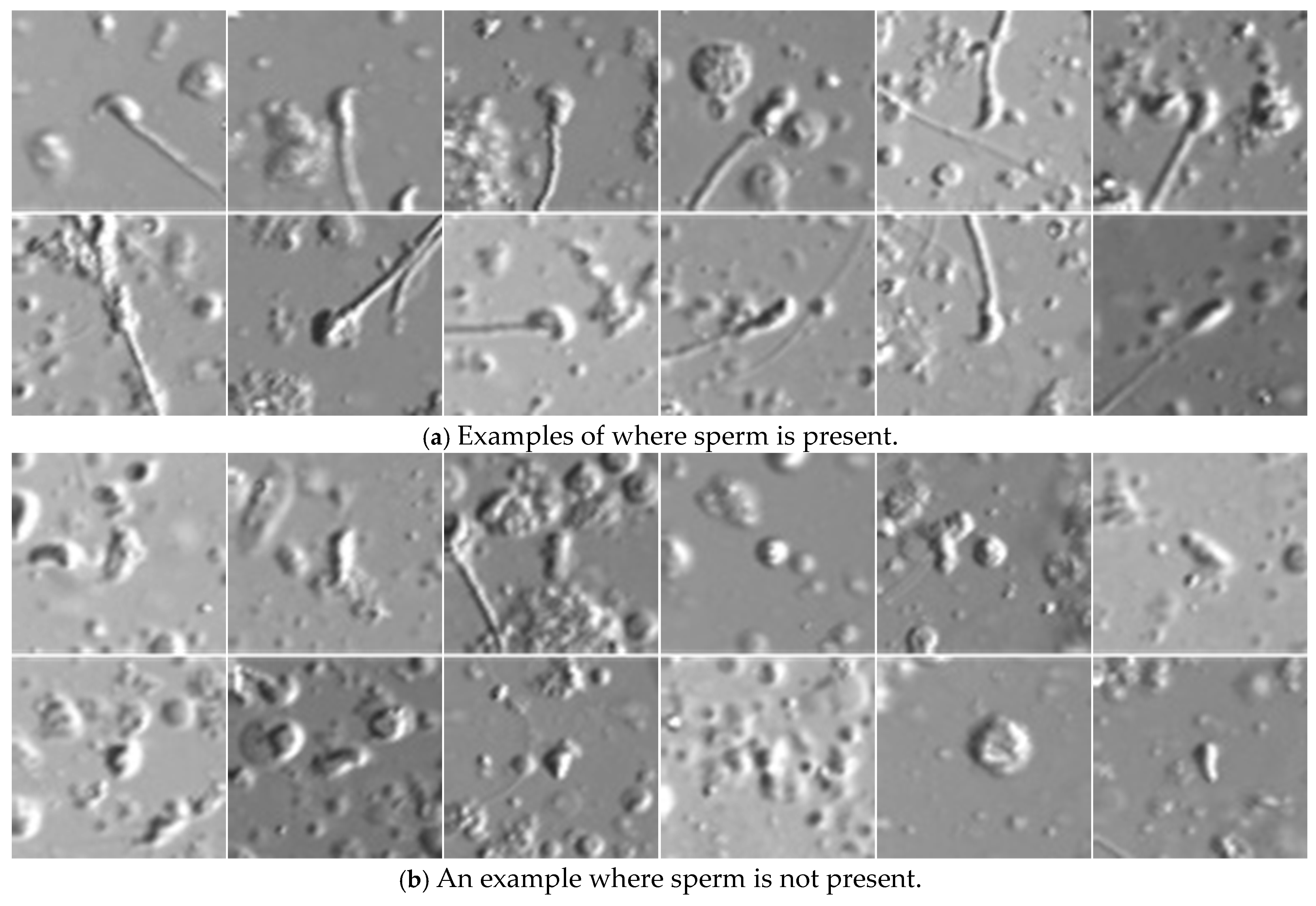
2.4. Sperm Detection Evaluation Experiment Using Simulated TESE Samples
3. Results
3.1. Results of Sperm Detection Evaluation Experiment Using Simulated TESE Samples
3.2. Performance Comparison with Existing Methods
3.2.1. Confusion Matrix and Posterior Probability Ratio (PPR)
3.2.2. Relevance to NOA: TPR–FPR Trade-Off
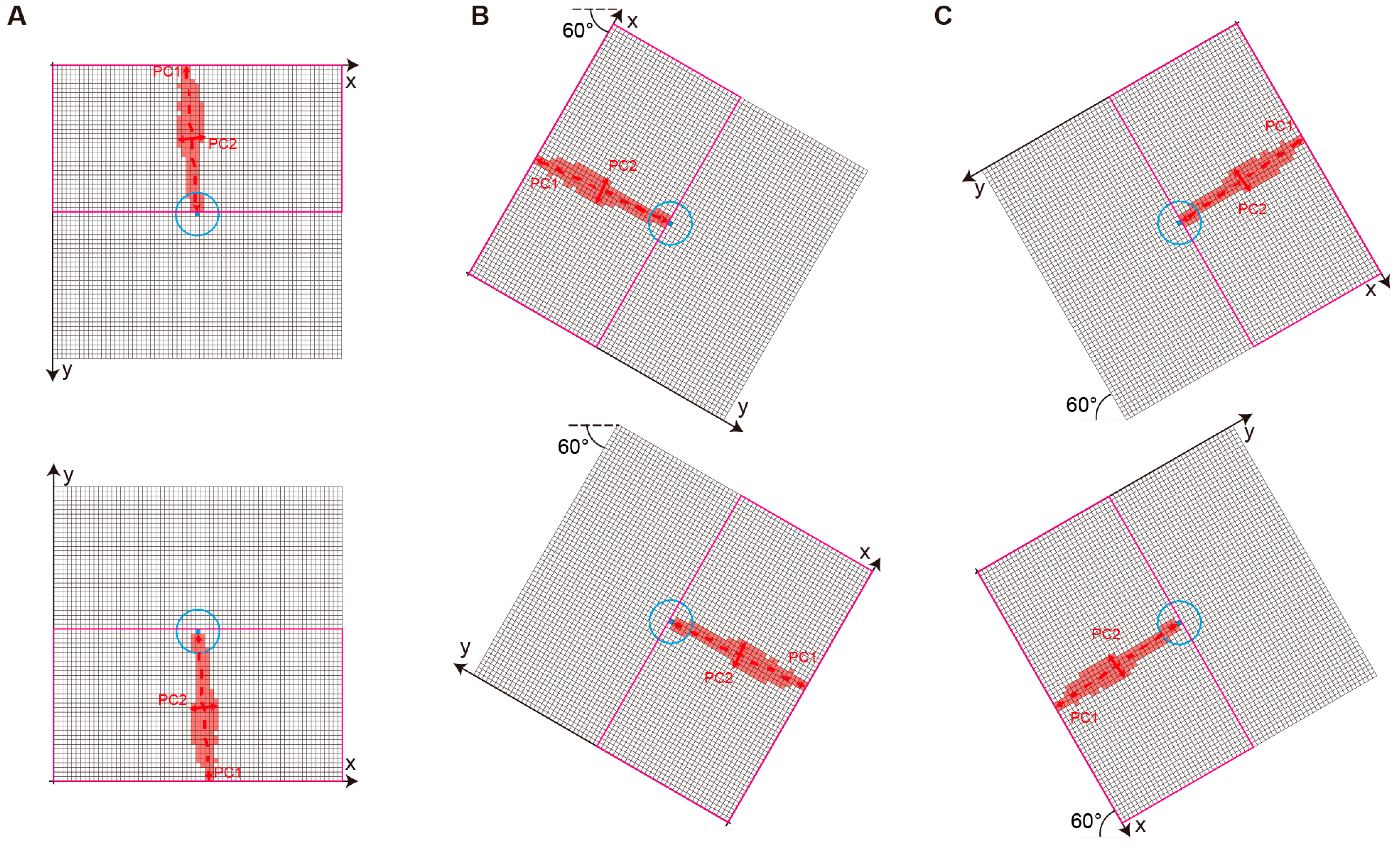
3.3. Justification of Component Contribution in SD-CLIP
- Edge-Based Candidate Detection:
- 2.
- Width-Based Alignment Filtering:
- 3.
- Area-Based Noise Removal:
- 4.
- PCA-Based Tail Verification:
4. Conclusions and Future Work
- Reduced candidate points and faster processing:By leveraging domain-specific information—namely, the approximate size and convex shape of sperm heads—our candidate detection step markedly decreased the number of false positive keypoints compared to conventional methods. Execution time for candidate detection was improved by approximately fourfold, and the algorithm remains amenable to further acceleration via parallelization or general-purpose GPU computing.
- High specificity in low-sperm environments:Although our approach has a modest TPR relative to a previously proposed method, it achieves a substantially lower FPR. Under the extremely low sperm density conditions characteristic of NOA, the resulting positive predictive value (PPV) of our method is significantly higher. Specifically, the ratio of TPR to FPR—a crucial metric in NOA contexts—showed that our method was roughly 3.8 times more effective than the conventional method.
- Applicability to Clinical Micro-TESEBy detecting sperm swiftly and minimizing the burden on embryologists, our approach has the potential to shorten operative times and reduce patient discomfort. The algorithm’s reliance on fundamental image-processing operations also facilitates integration into existing clinical workflows with minimal hardware requirements.
- Adaptive Parameter Tuning:Developing automated methods for selecting optimal thresholds in the Sobel filtering and principal component analysis (PCA) steps to further balance sensitivity and specificity.
- Future versions of SD-CLIP could benefit from using the Scharr operator, which offers improved rotational symmetry and edge accuracy compared to Sobel.
- The current comparison in Table 2 is based on fixed threshold settings optimized for specificity. A full ROC analysis was not performed due to limited positive cases and data diversity. Future work will include ROC-based evaluation to enable more rigorous comparative benchmarking.
- Edge misalignment caused by tilted imaging planes or irregular lighting can reduce the consistency of head detection. Although diagonal edge gradients are already included, future implementations could benefit from affine image registration or rotation-normalization preprocessing.
- Advanced Parallelization:Implementing real-time parallel processing pipelines on GPU or specialized hardware (e.g., FPGA) for speed-critical clinical scenarios.
- Hybrid Approaches:A potential hybrid system could integrate a lightweight neural network classifier after candidate extraction by SD-CLIP. For instance, once bounding boxes are generated from edge and shape features, a shallow CNN could be used to validate sperm presence based on learned appearance. This modular design would allow robust generalization to diverse imaging conditions while maintaining processing speed.
- Automation of Sperm Collection:Integrating our detection framework with a robotic arm and pipette system to automatically collect the detected sperm. Such an automated system would further reduce the manual workload on embryologists and could enhance the speed and precision of sperm retrieval in Micro-TESE procedures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Brugo-Olmedo, S.; Chillik, C.; Kopelman, S. Definition and causes of infertility. Reprod. Biomed. Online 2001, 2, 173–185. [Google Scholar] [CrossRef]
- Tharakan, T.; Luo, R.; Jayasena, C.N.; Minhas, S. Non-obstructive azoospermia: Current and future perspectives. Fac. Rev. 2021, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Fisher, E.S.; Ricci, J.A.; Leung, R.A.; Schlegel, P.N. Duration of microdissection testicular sperm extraction procedures: Relationship to sperm retrieval success. J. Urol. 2011, 185, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2015, 4, e979623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, R.; Xiang, S.; Liao, S.; Li, S.Z. Face detection based on multi-block LBP representation. In Advances in Biometrics, ICB 2007; Lecture Notes in Computer, Science; Lee, S.W., Li, S.Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4642, pp. 11–18. [Google Scholar] [CrossRef]
- Sasaki, H.; Nakata, M.; Yamamoto, M.; Takeshima, T.; Yumura, Y.; Hamagami, T. Investigation about control of false positive rate for automatic sperm detection in assisted reproductive technology. In Proceedings of the 2018 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; pp. 1964–1969. [Google Scholar] [CrossRef]
- Petersson, F.; Aberg, L.; Swärd-Nilsson, A.M.; Laurell, T. Free flow acoustophoresis: Microfluidic-based mode of particle and cell separation. Anal. Chem. 2007, 79, 5117–5123. [Google Scholar] [CrossRef] [PubMed]
- Valihrach, L.; Androvic, P.; Kubista, M. Platforms for single-cell collection and analysis. Int. J. Mol. Sci. 2018, 19, 807. [Google Scholar] [CrossRef] [PubMed]
- Lang, W. Differential Interference Contrast Microscopy; Reprint S-41-210-2-5-e; Carl Zeiss: Oberkochen, Germany, 1969. [Google Scholar]
- Alcantarilla, P.F.; Nuevo, J.; Bartoli, A. Fast explicit diffusion for accelerated features in nonlinear scale spaces. In Proceedings of the BMVC 2013: Proceedings of the British Machine Vision Conference, Bristol, UK, 9–13 September 2013. [Google Scholar]
- Sobel, I.; Feldman, G. A 3×3 isotropic gradient operator for image processing. A Talk at Stanford Artificial Intelligence Project (SAIL), 1968; 271–272. [Google Scholar]
- Grana, C.; Borghesani, D.; Cucchiara, R. Optimized block-based connected components labeling with decision trees. IEEE Trans. Image Process. 2010, 19, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Otoo, E.J.; Suzuki, K. Two Strategies to Speed Up Connected Component Labeling Algorithms; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2005. [Google Scholar]



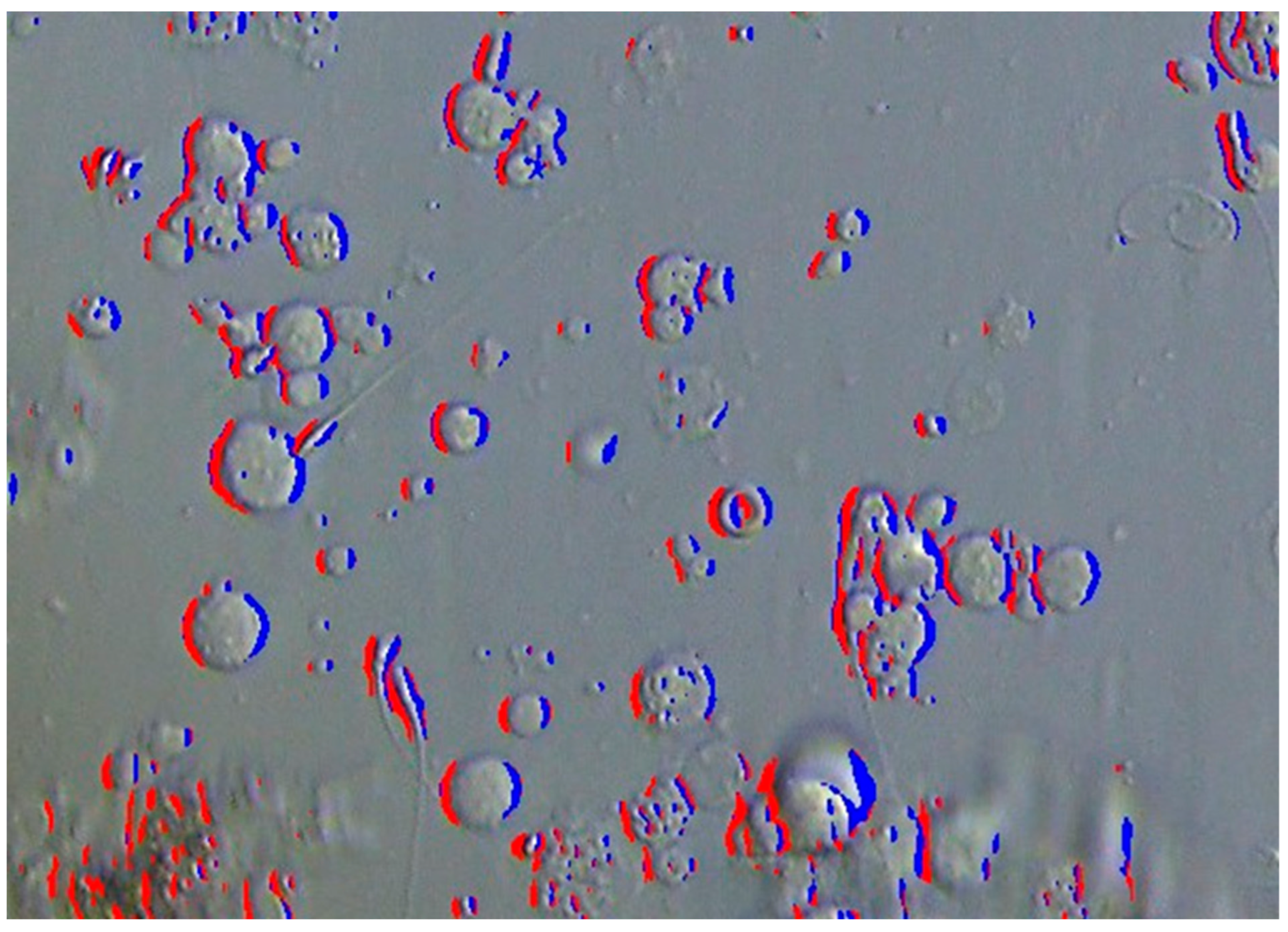
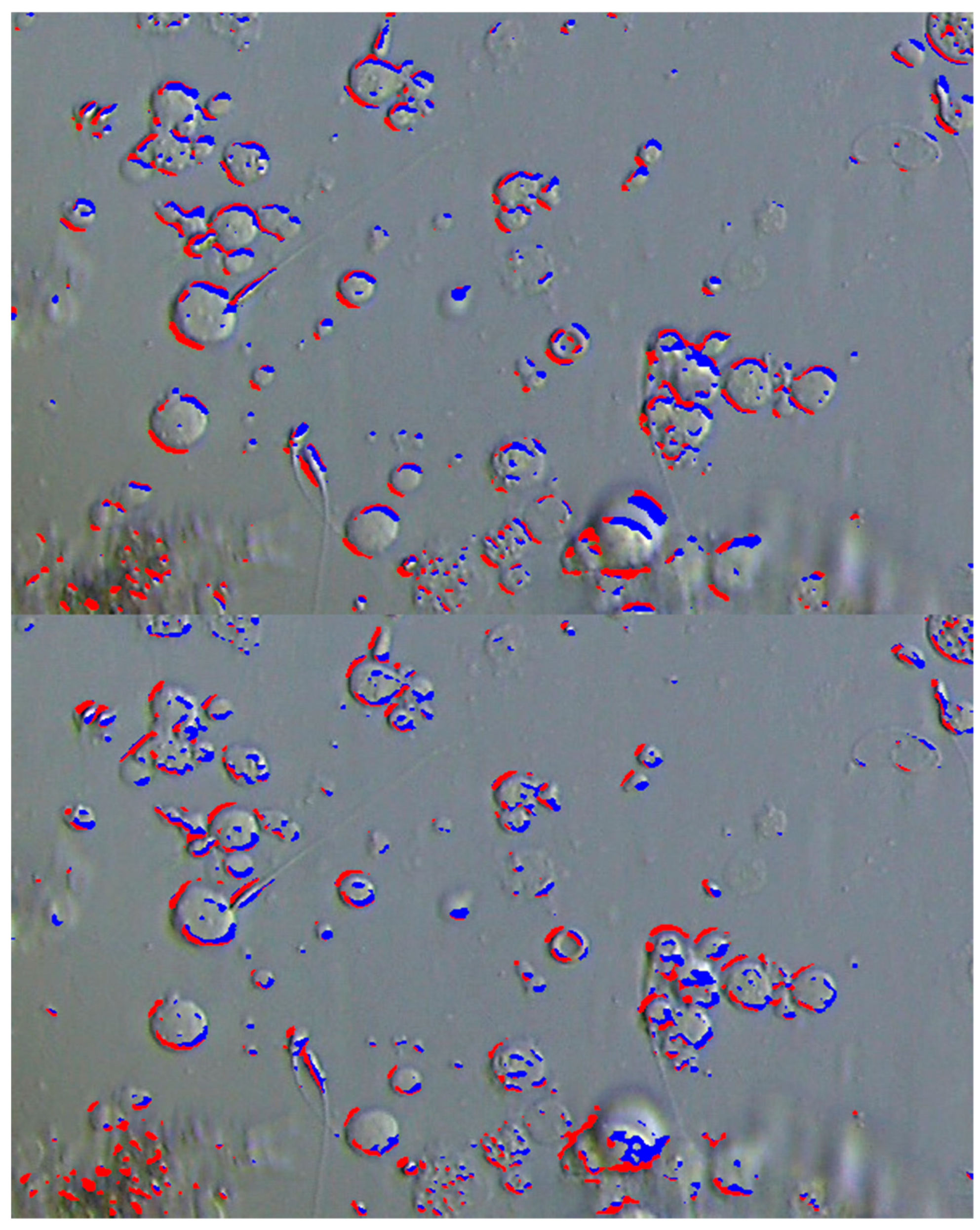

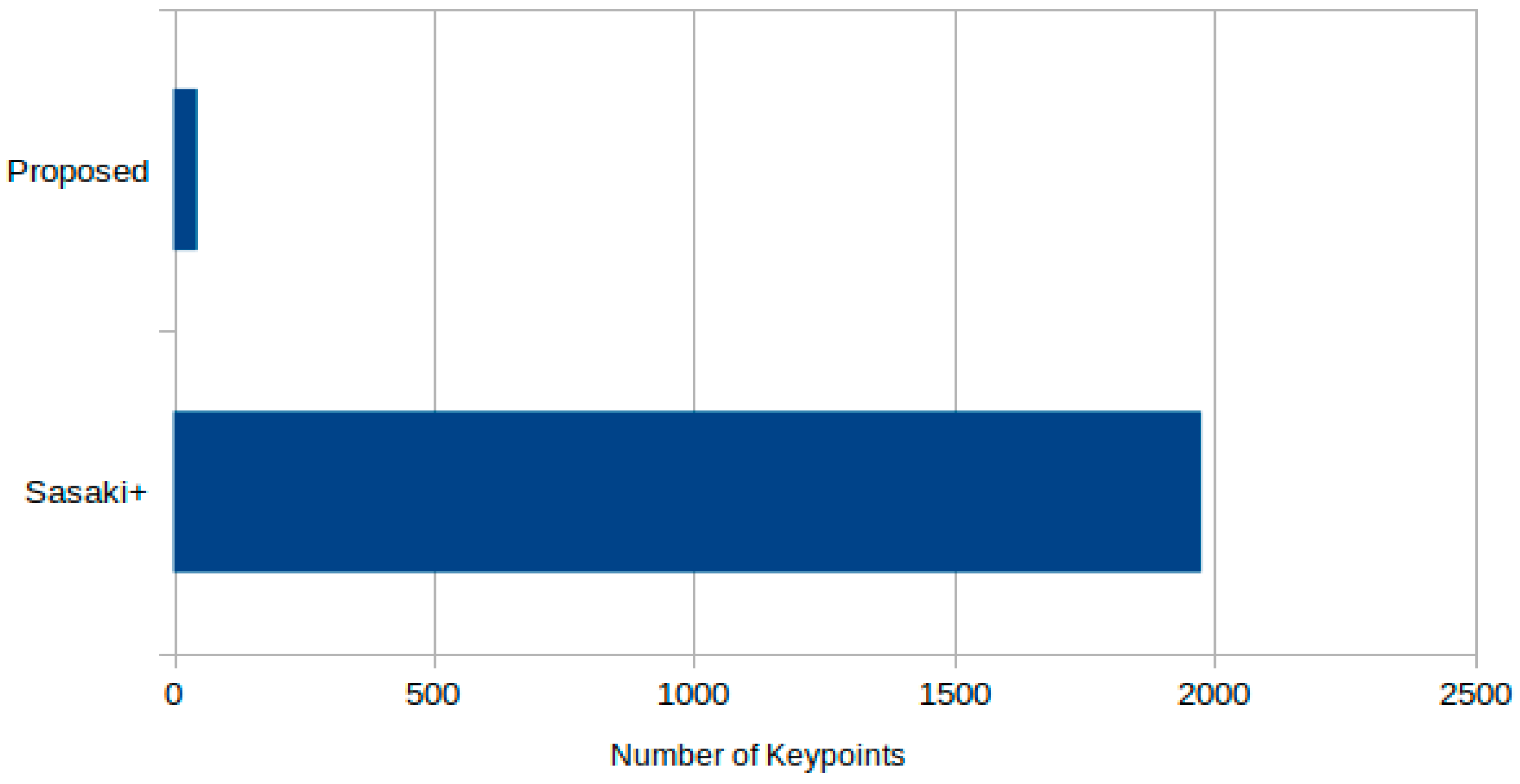
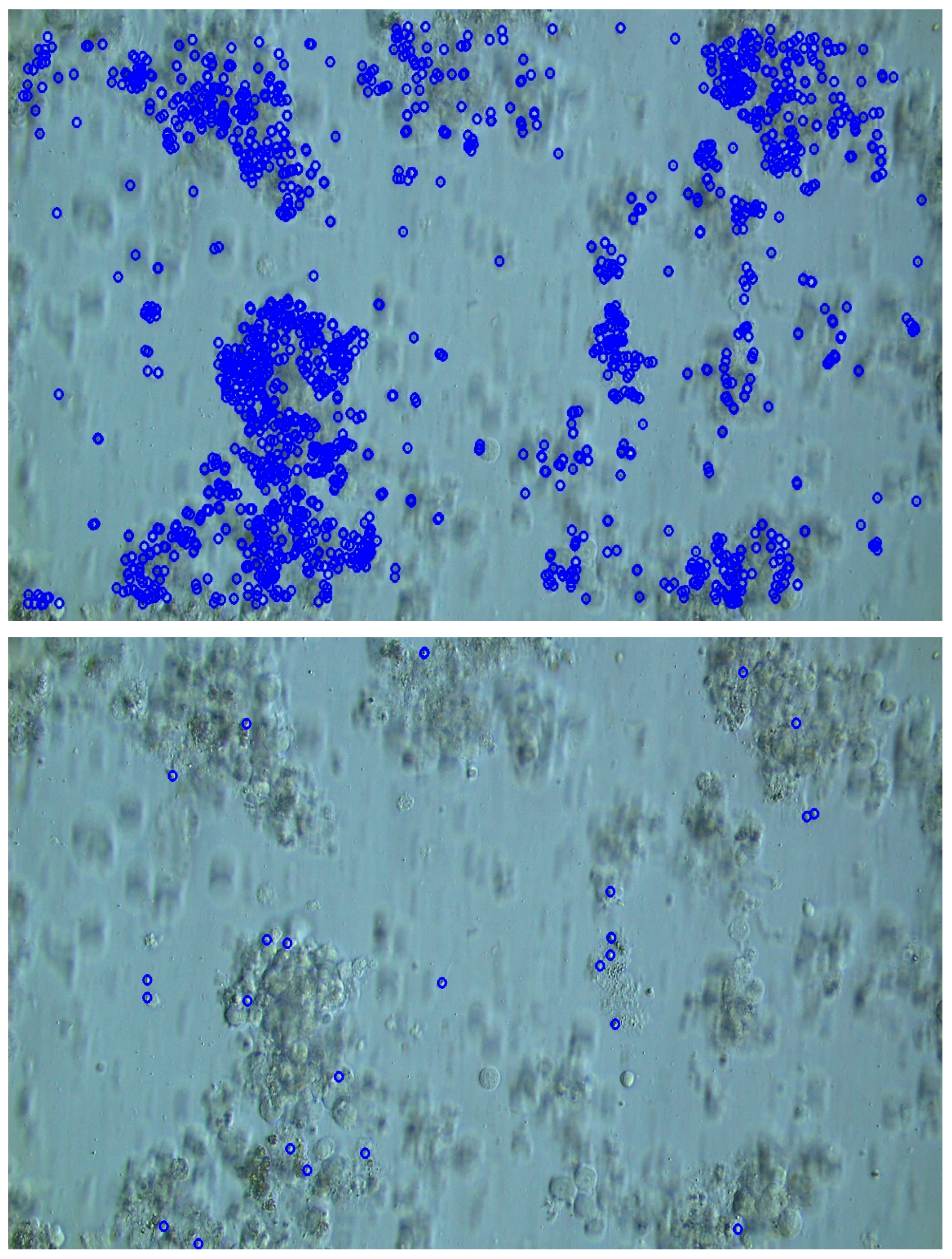
| Proposed Method | Correct Answer Label | Total | Sasaki et al.’s Method | Correct Answer Label | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| With Sperm | No Sperm | With Sperm | No Sperm | ||||||
| Judgment result | With sperm | 83 | 27 | 110 | Judgment result | With sperm | 7544 | 11,879 | 19,423 |
| No sperm | 59 | 486 | 545 | No sperm | 1127 | 27,717 | 28,844 | ||
| Total | 142 | 513 | Total | 8671 | 39,596 | ||||
| True Positive Rate | False Positive Rate | True Negative Rate | False Negative Rate | |
|---|---|---|---|---|
| Proposed Method (SD-CLIP) | 58% | 5% | 95% | 42% |
| Sasaki et al. (MB-LBP) | 87% | 30% | 70% | 13% |
| True Label | |||
|---|---|---|---|
| Sperm | No Sperm | ||
| Prediction | Sperm | 83 | 27 |
| No Sperm | 59 | 486 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.; Kachi, K.; Motoya, K.; Ikeuchi, M. Fast and Accurate Sperm Detection Algorithm for Micro-TESE in NOA Patients. Bioengineering 2025, 12, 601. https://doi.org/10.3390/bioengineering12060601
Mohamed M, Kachi K, Motoya K, Ikeuchi M. Fast and Accurate Sperm Detection Algorithm for Micro-TESE in NOA Patients. Bioengineering. 2025; 12(6):601. https://doi.org/10.3390/bioengineering12060601
Chicago/Turabian StyleMohamed, Mahmoud, Konosuke Kachi, Kohei Motoya, and Masashi Ikeuchi. 2025. "Fast and Accurate Sperm Detection Algorithm for Micro-TESE in NOA Patients" Bioengineering 12, no. 6: 601. https://doi.org/10.3390/bioengineering12060601
APA StyleMohamed, M., Kachi, K., Motoya, K., & Ikeuchi, M. (2025). Fast and Accurate Sperm Detection Algorithm for Micro-TESE in NOA Patients. Bioengineering, 12(6), 601. https://doi.org/10.3390/bioengineering12060601






