Efficient Production of High-Concentration Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 Employing the Recombinant of Cupriavidus necator
Abstract
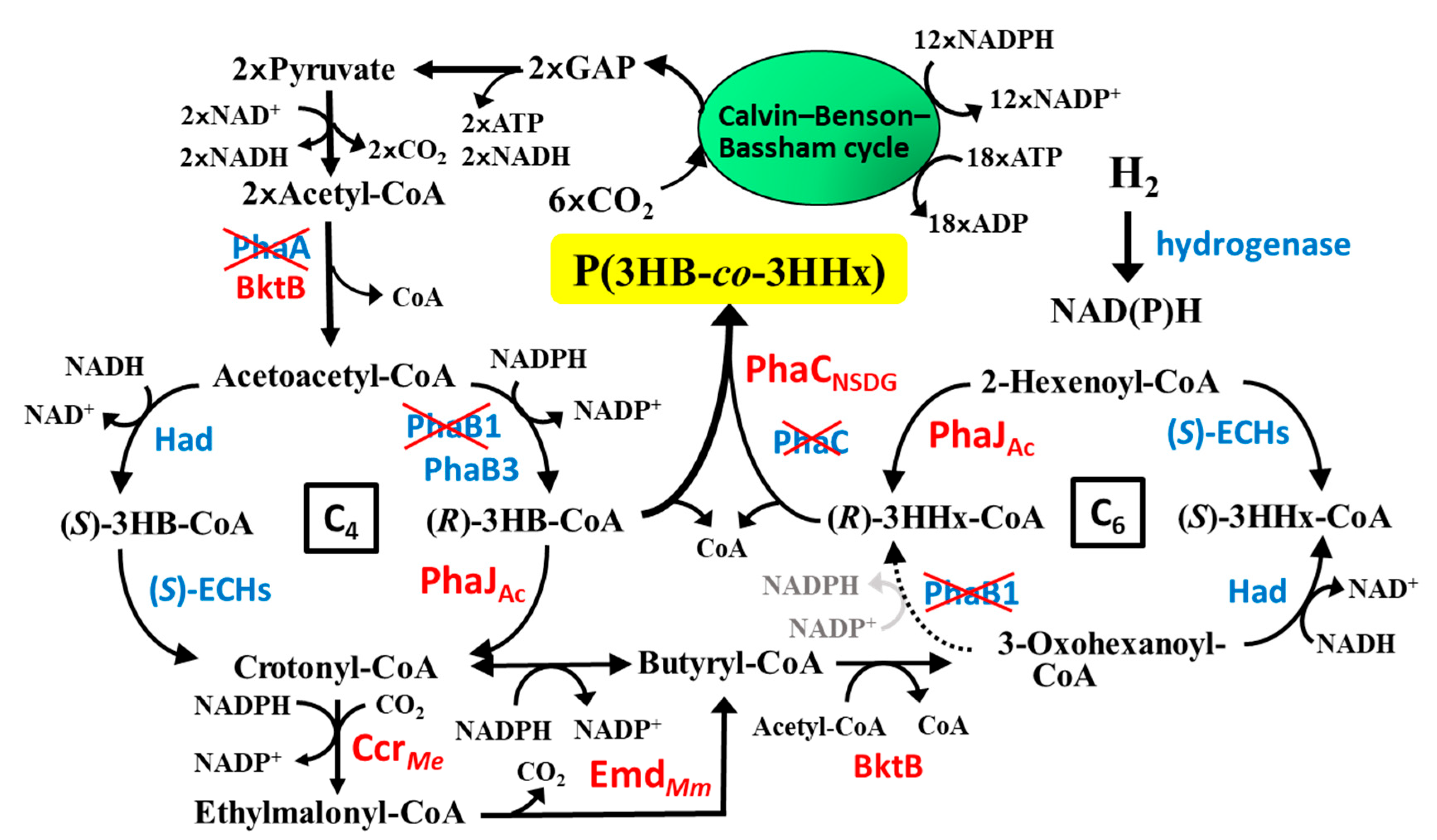
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Culture Medium
2.3. Revival of Recombinant Strain and Preculture
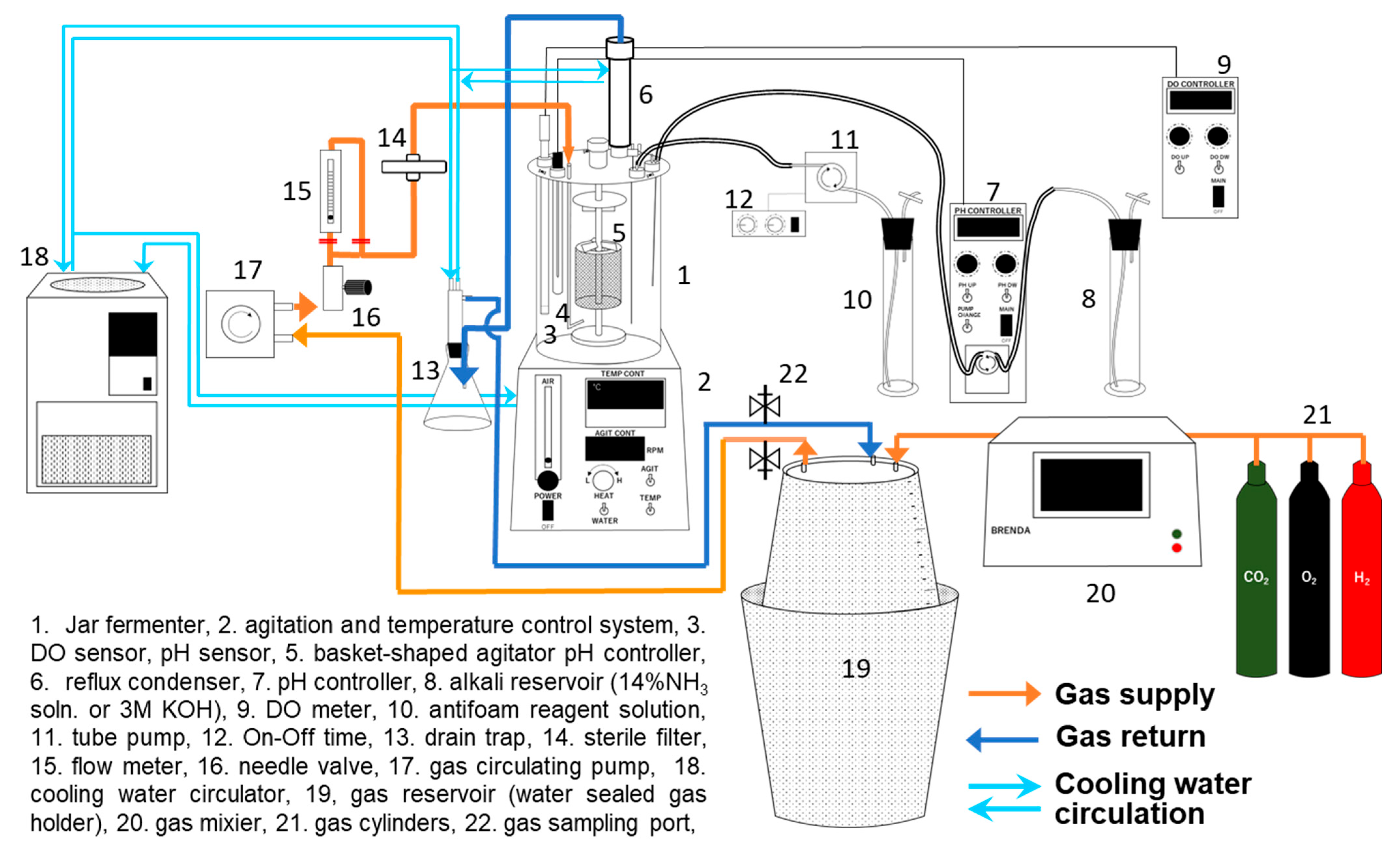
2.4. Culture System
2.5. Conditions for Jar Cultivation
2.6. Analyses
3. Results
3.1. Jar Cultivation Using Synthetic Media with Different Compositions
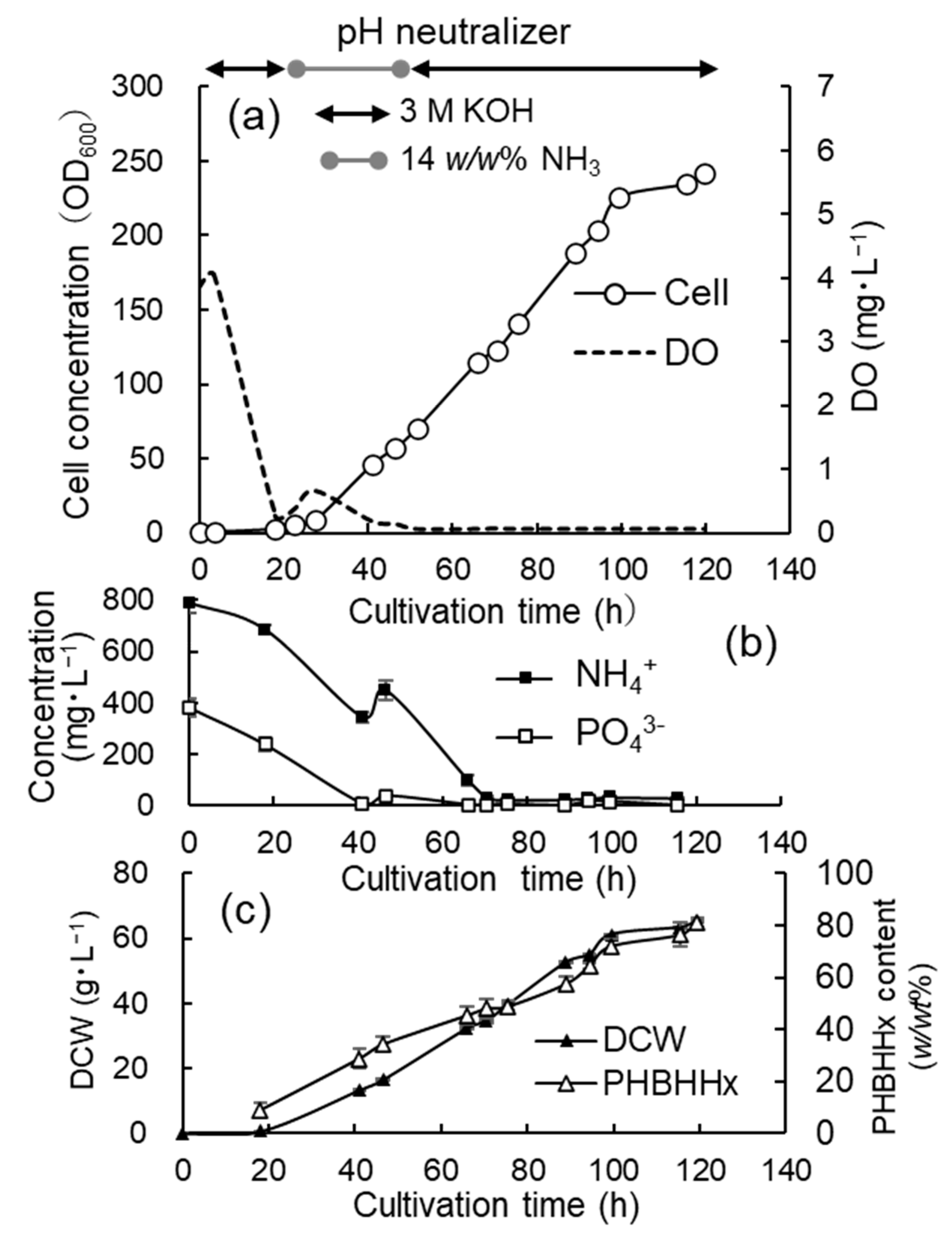
3.2. Jar Cultivation with 3 M KOH, 14 w/w% NH4OH, and Phosphate
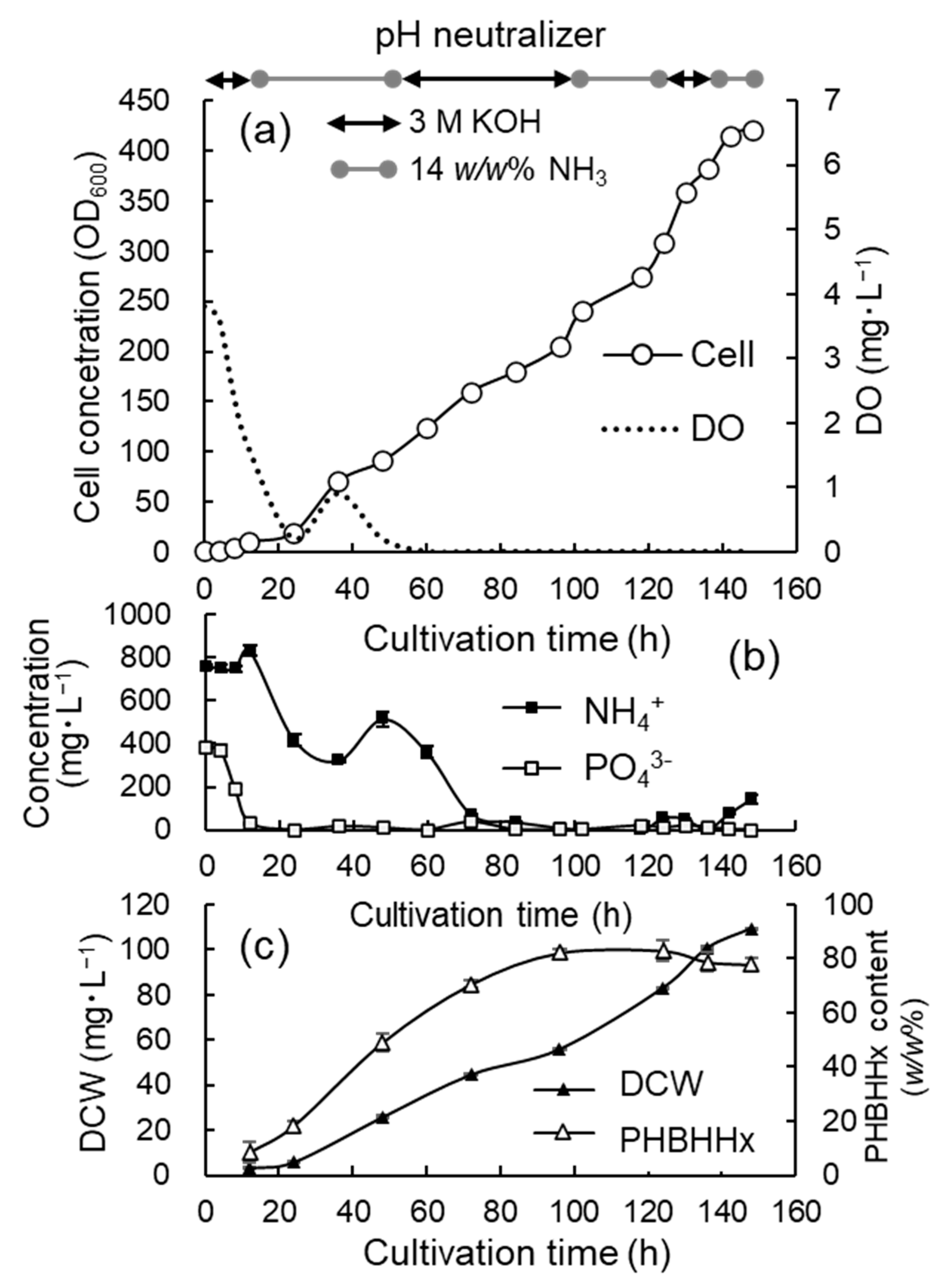
3.3. Jar Cultivation with Limited Addition of Phosphate
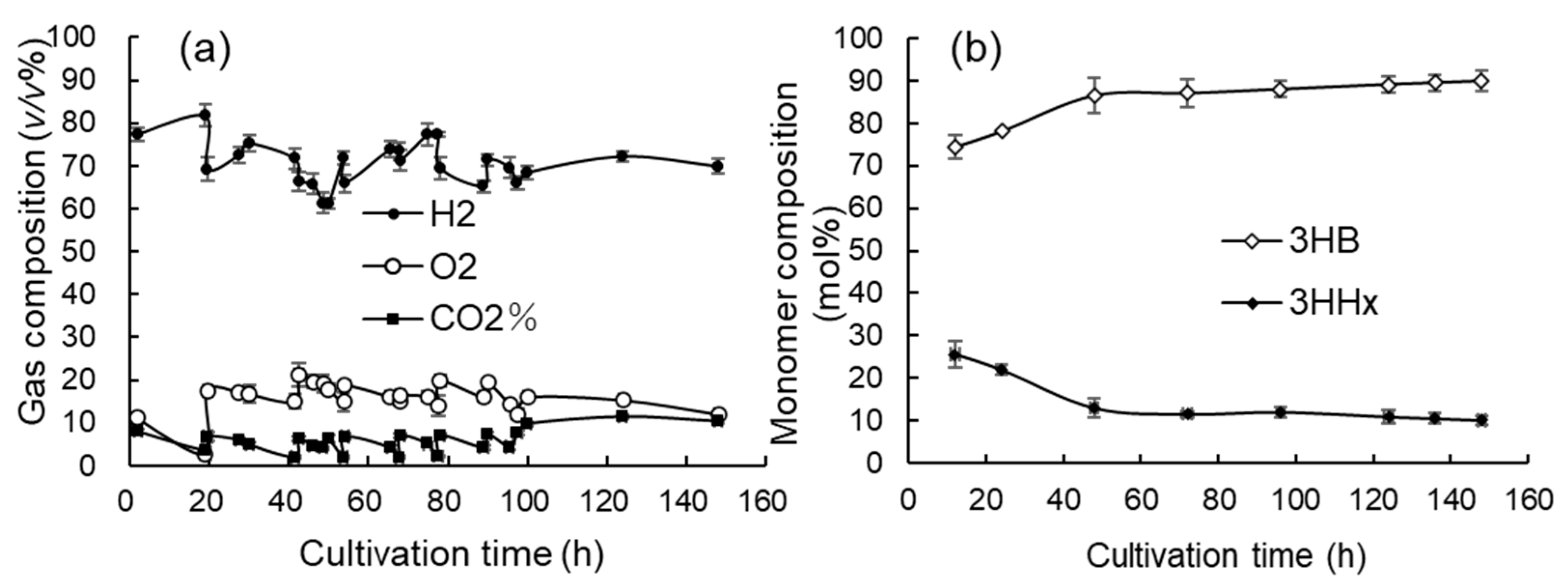
3.4. Jar Cultivation with Continuous Supply of O2-Rich Gas Mixture to Gas Reservoir
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial degradation behavior in seawater of polyester blends containing poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.; Taylor, D.S. Commercial Marine-degradable polymers for flexible packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA Bbopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koller, M. Microbial PolyHydroxyAlkanoate (PHA) biopolymers-Intrinsically natural. Bioengineering 2023, 10, 855. [Google Scholar] [CrossRef]
- Lu, J.; Tappel, R.C.; Nomura, C.T. Mini-Review: Biosynthesis of poly(hydroxyalkanoates). Polym. Rev. 2009, 49, 226–248. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Serio, M.D. In vivo and post-synthesis strategies to enhance the properties of PHB-based materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- Eraslan, k.; Aversa, C.; Nofar, M.; Barletta, M.; Gisario, A.; Salehiyan, R.; Goksu, Y.A. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH): Synthesis, properties, and applications—A review. Eur. Polym. J. 2022, 167, 111044. [Google Scholar] [CrossRef]
- Shimamura, E.; Kasuya, K.; Kobayashi, G.; Shiotani, T.; Shima, Y.; Doi, Y. Physical properties and biodegradability of microbial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1994, 27, 878–880. [Google Scholar] [CrossRef]
- Kaneka Corporation News Release. Completion of Kaneka Biodegradable Polymer PHBH™ Plant with Annual Production of 5,000 Tons. Available online: https://www.kaneka.co.jp/en/topics/news/nr20191219/ (accessed on 19 December 2019).
- Kaneka Corporation News Release. Kaneka to Significantly Increase Its Production Capacity for KANEKA Biodegradable Polymer Green Planet™ in Japan. 7 February 2022. Available online: https://www.kaneka.co.jp/en/topics/news/2022/ennr2202071.html (accessed on 7 February 2022).
- Kalia, V.C.; Patel, S.K.S.; Krishnamurthi, P.; Singh, R.V.; Lee, J.K. Exploiting latent microbial potentials for producing polyhydroxyalkanoates: A holistic approach. Environ. Res. 2025, 269, 120895. [Google Scholar] [CrossRef]
- Insomphun, C.; Xie, H.; Mifune, J.; Kawashima, Y.; Orita, I.; Nakamura, S.; Fukui, T. Improved artificial pathway for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with high C6-monomer composition from fructose in Ralstonia eutropha. Metab. Eng. 2015, 27, 38–45. [Google Scholar] [CrossRef]
- Zhang, M.; Kurita, S.; Orita, I.; Nakamura, S.; Fukui, T. Modification of acetoacetyl-CoA reduction step in Ralstonia eutropha for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from structurally unrelated compounds. Microb. Cell Fact. 2019, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-d-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shishatskaya, E.I.; Zhila, N.O.; Kiselev, E.G. Hydrogen-oxidizing producers of polyhydroxyalkanoates: Synthesis, properties, and applications. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 221–253. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, X.; Yang, J. Problems and corresponding strategies for converting CO2 into value-added products in Cupriavidus necator H16 cell factories. Biotechnol. Adv. 2023, 67, 108183. [Google Scholar] [CrossRef] [PubMed]
- Morlino, M.S.; García, R.S.; Filippo Savio, F.; Zampieri, G.; Morosinotto, T.; Treu, L.; Campanaro, S. Cupriavidus necator as a platform for polyhydroxyalkanoate production: An overview of strains, metabolism, and modeling approaches. Biotechnol. Adv. 2023, 69, 108264. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, L.; Ding, L.; Su, X.; Luo, H.; Huang, H.; Wang, Y.; Yao, B.; Zhang, J.; Wang, X. Unlocking the potential of Cupriavidus necator H16 as a platform for bioproducts production from carbon dioxide. World J. Microb. Biot. 2024, 40, 389. [Google Scholar] [CrossRef]
- Woern, C.; Grossmann, L. Microbial gas fermentation technology for sustainable food protein production. Biotechnol. Adv. 2023, 69, 108240. [Google Scholar] [CrossRef]
- Salinas, A.; McGregor, C.; Irorere, V.; Arenas-López, C.; Bommareddy, R.R.; Winzer, K.; Minton, N.P.; Kovács, K. Metabolic engineering of Cupriavidus necator H16 for heterotrophic and autotrophic production of 3-hydroxypropionic acid. Metab. Eng. 2022, 74, 178–190. [Google Scholar] [CrossRef]
- Sydow, A.; Becker, L.; Lombard, E.; Ulber, R.; Guillouet, S.E.; Holtmann, D. Autotrophic production of the sesquiterpene α-humulene with Cupriavidus necator in a controlled bioreactor. Bioengineering 2023, 10, 1194. [Google Scholar] [CrossRef]
- Garavaglia, M.; McGregor, C.; Bommareddy, R.R.; Irorere, V.; Arenas, C.; Robazza, A.; Minton, N.P.; Kovács, K. Stable platform for mevalonate bioproduction from CO2. ACS Sustain. Chem. Eng. 2024, 12, 13486–13499. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, Y.J.; Gong, G.; Lee, S.-M.; Um, Y.; Kim, K.H.; Ko, J.K. Carbon dioxide valorization into resveratrol via lithoautotrophic fermentation using engineered Cupriavidus necator H16. Microb. Cell Fact. 2024, 23, 122. [Google Scholar] [CrossRef]
- Wang, L.; Yao, J.; Tao, T.; Yao, B.; Zhang, J. Heterotrophic and autotrophic production of l-isoleucine and l-valine by engineered Cupriavidus necator H16. Bioresour. Technol. 2024, 398, 130538. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshida, K.; Orita, I.; Fukui, T. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a recombinant Cupriavidus necator. Bioengineering 2021, 8, 179. [Google Scholar] [CrossRef]
- Tanaka, K.; Orita, I.; Fukui, T. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 via pH-stat jar cultivation of an engineered hydrogen-oxidizing bacterium Cupriavidus necator. Bioengineering 2023, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Tanaka, K. Batch culture of Alcaligenes eutrophus ATCC 17697T using recycled gas closed circuit culture system. J. Ferment. Bioeng. 1990, 69, 170–174. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K. Production of poly-β-hydroxybutyric acid from carbon dioxide by Alcaligenes eutrophus ATCC 17697T. J. Ferment. Bioeng. 1991, 71, 254–257. [Google Scholar] [CrossRef]
- Takeshita, T.; Tanaka, K.; Ishizaki, A.; Stanbury, P.F. Development of a dissolved hydrogen sensor and its application to evaluation of hydrogen mass transfer. J. Ferment. Bioeng. 1993, 76, 148–150. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A.; Kanamaru, T.; Kawano, T. Production of poly(d-3-hydroxybutyrate) from CO2, H2, and CO2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995, 45, 268–275. [Google Scholar] [CrossRef]
- Taga, N.; Tanaka, K.; Ishizaki, A. Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor on poly-d-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium, Alcaligenes eutrophus. Biotechnol. Bioeng. 1997, 53, 529–533. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tsuge, T.; Tanaka, K.; Ishizaki, A. Control of acetic acid concentration by pH-stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of P(3HB) from CO2, H2 and O2 under non-explosive condition. Biotechnol. Bioeng. 1999, 62, 625–631. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyawaki, K.; Yamaguchi, A.; Khosravi-Darani, K.; Matsusaki, H. Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Appl. Microbiol. Biotechnol. 2011, 92, 1161–1169. [Google Scholar] [CrossRef]
- Park, I.; Jho, E.H.; Nam, K. Optimization of carbon dioxide and valeric acid utilization for polyhydroxyalkanoates synthesis by Cupriavidus necator. J. Polym. Environ. 2014, 22, 244–251. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Garcia-Gonzalez, L.; Wever, H.; Volcke, E. Poly(3-hydroxybutyrate) (PHB) production from CO2: Model development and process optimization. Biochem. Eng. J. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Yu, J.; Munasinghe, P. Gas fermentation enhancement for chemolithotrophic growth of Cupriavidus necator on carbon dioxide. Fermentation 2018, 4, 63. [Google Scholar] [CrossRef]
- Ghysels, S.; Mozumder, M.S.; Wever, H.; Volcke, E.; Garcia-Gonzalez, L. Targeted poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic production from carbon dioxide. Bioresour. Technol. 2018, 249, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lu, Y. Carbon dioxide fixation by a hydrogen-oxidizing bacterium: Biomass yield, reversal respiratory quotient, stoichiometric equations and bioenergetics. Biochem. Eng. J. 2019, 152, 107369. [Google Scholar] [CrossRef]
- Nangle, S.N.; Ziesack, M.; Buckley, S.; Trivedi, D.; Loh, D.M.; Nocera, D.G.; Silvera, P.A. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Mozumder, M.S.; Dubreuil, M.; Volcke, E.; Wever, H. Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal. Today 2015, 257, 237–245. [Google Scholar] [CrossRef]
- Miyahara, Y.; Yamamoto, M.; Thorbecke, R.; Mizuno, S.; Tsuge, T. Autotrophic biosynthesis of polyhydroxyalkanoate by Ralstonia eutropha from non-combustible gas mixture with low hydrogen content. Biotechnol. Lett. 2020, 42, 1655–1662. [Google Scholar] [CrossRef]
- Lambauer, V.; Kratzer, R. Lab-Scale Cultivation of Cupriavidus necator on explosive gas mixtures: Carbon dioxide fixation into polyhydroxybutyrate. Bioengineering 2022, 9, 204. [Google Scholar] [CrossRef]
- Lambauer, V.; Permann, A.; Petrášek, Z.; Subotić, V.; Hochenauer, C.; Kratzer, R.; Reichhartinger, M. Automatic control of chemolithotrophic cultivation of Cupriavidus necator: Optimization of oxygen supply for enhanced bioplastic production. Fermentation 2023, 9, 619. [Google Scholar] [CrossRef]
- Vlaeminck, E.; Acuña Lopez, P.A.; Uitterhaegen, E.; Quataert, K.; Delmulle, T.; De Winter, K.D.; Soetaert, W.K.K. Pressure fermentation to boost CO2-based poly(3-hydroxybutyrate) production using Cupriavidus necator. Bioresour. Technol. 2024, 408, 131162. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Reddy, M.V.; Mawatari, Y.; Sarkar, O. Enhanced polyhydroxyalkanoate biosynthesis by Cupriavidus sp. CY-1 utilizing CO2 under controlled non-explosive conditions. Chemosphere 2025, 373, 144181. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Kalacheva, G.S.; Steinbüchel, A. Biosynthesis of multi-component polyhydroxyalkanoates by the bacterium Wautersia eutropha. Macromol. Symp. 2008, 269, 1–7. [Google Scholar] [CrossRef]
- Arikawa, H.; Matsumoto, K. Evaluation of gene expression cassettes and production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) with a fine modulated monomer composition by using it in Cupriavidus necator. Microb. Cell Fact. 2016, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Mifune, J.; Nakamura, S.; Fukui, T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from vegetable oil. Polym. Degrad. Stab. 2010, 95, 1305–1312. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Lafferty, R.M. Novel energy and carbon sources A. The production of biomass from hydrogen and carbon dioxide. Adv. Biochem. Eng. 1971, 143, 143–168. [Google Scholar] [CrossRef]
- Kodama, T.; Igarashi, Y.; Minoda, Y. Isolation and culture conditions of a bacterium grown on hydrogen and carbon dioxide. Agric. Biol. Chem. 1975, 39, 77–82. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, E.I.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Kato, M.; Bao, H.J.; Kang, C.-K.; Fukui, T.; Doi, Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 1996, 45, 363–370. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamane, T.; Shimizu, S. Mass production of poly-β-hydroxybutyric acid by fed-batch culture with controlled carbon/nitrogen feeding. Appl. Microbiol. Biotechnol. 1986, 24, 370–374. [Google Scholar] [CrossRef]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly (3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phosphate limitation. Biotechnol. Bioeng. 1997, 55, 28–32. [Google Scholar] [CrossRef]
- Stadio, G.D.; Izumi Orita, I.; Nakamura, R.; Fukui, T. Gas fermentation combined with water electrolysis for production of polyhydroxyalkanoate copolymer from carbon dioxide by engineered Ralstonia eutropha. Bioresour. Technol. 2024, 394, 130266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Sivashankari, R.M.; Miyahara, Y.; Tsuge, T. Polyhydroxyalkanoate copolymer production by recombinant Ralstonia eutropha strain 1F2 from fructose or carbon dioxide as sole carbon source. Bioengineering 2024, 11, 455. [Google Scholar] [CrossRef] [PubMed]


| Strain | Relevant Marker | References or Resources |
|---|---|---|
| C. necator H16 | Wild type | DSM 428 |
| C. necator MF01 | H16 derivative; ΔphaC::phaCNSDG, ΔphaA::bktB | [48] |
| Plasmid | ||
| pBPP | pBBR1MCS-2 derivative; PphaP1, TrrnB | [48] |
| pBPP-ccrMeJAc-emd | pBPP derivative; ccrMe, phaJAc, emdMm | [13] |
| Initial Concentration in Mineral Medium | Cultivation Time (h) | Cell Concentration (OD600) | PHBHHx Content in Cells (w/w%) | Residual Concentration | ||
|---|---|---|---|---|---|---|
| (NH4)2SO4 (g·L−1) | KH2PO4 (g·L−1) | PO43− (mg·L−1) | NH4+ (mg·L−1) | |||
| 0.5 | 0.3 | 94 | 11.1 | 82.2 ± 1.8 | 273.0 ± 7.2 | 26.0 ± 1.2 |
| 1.0 | 0.5 | 52 | 68.0 | 85.5 ± 2.1 | 14.0 ± 3.5 | 1.2 ± 0.0 |
| 2.0 | 2.5 | 101 | 76.0 | 78.1 ± 1.9 | 1560.0 ± 29.0 | 14.0 ± 0.1 |
| 3.0 | 0.5 | 77 | 102.2 | 83.9 ± 0.9 | 67.0 ± 2.1 | 0.5 ± 0.0 |
| 3.0 | 1.0 | 115 | 96.0 | 82.2 ± 2.6 | 242.0 ± 3.9 | 68.0 ± 1.9 |
| 5.0 | 0.5 | 78 | 117.1 | 80.5 ± 2.9 | 28.2 ± 0.6 | 2.0 ± 0.0 |
| 10.0 | 1.0 | 91 | 94.5 | 39.5 ± 3.1 | 1.0 ± 0.1 | 61.0 ± 2.1 |
| 10.0 | 4.0 | 72 | 70.3 | 48.8 ± 0.7 | 1720.0 ± 36.0 | 30.1 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, K.; Orita, I.; Fukui, T. Efficient Production of High-Concentration Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 Employing the Recombinant of Cupriavidus necator. Bioengineering 2025, 12, 557. https://doi.org/10.3390/bioengineering12060557
Tanaka K, Orita I, Fukui T. Efficient Production of High-Concentration Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 Employing the Recombinant of Cupriavidus necator. Bioengineering. 2025; 12(6):557. https://doi.org/10.3390/bioengineering12060557
Chicago/Turabian StyleTanaka, Kenji, Izumi Orita, and Toshiaki Fukui. 2025. "Efficient Production of High-Concentration Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 Employing the Recombinant of Cupriavidus necator" Bioengineering 12, no. 6: 557. https://doi.org/10.3390/bioengineering12060557
APA StyleTanaka, K., Orita, I., & Fukui, T. (2025). Efficient Production of High-Concentration Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 Employing the Recombinant of Cupriavidus necator. Bioengineering, 12(6), 557. https://doi.org/10.3390/bioengineering12060557






