Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Candy Waste Used as a Carbon Source
2.2. Microorganism Used and Growth Conditions
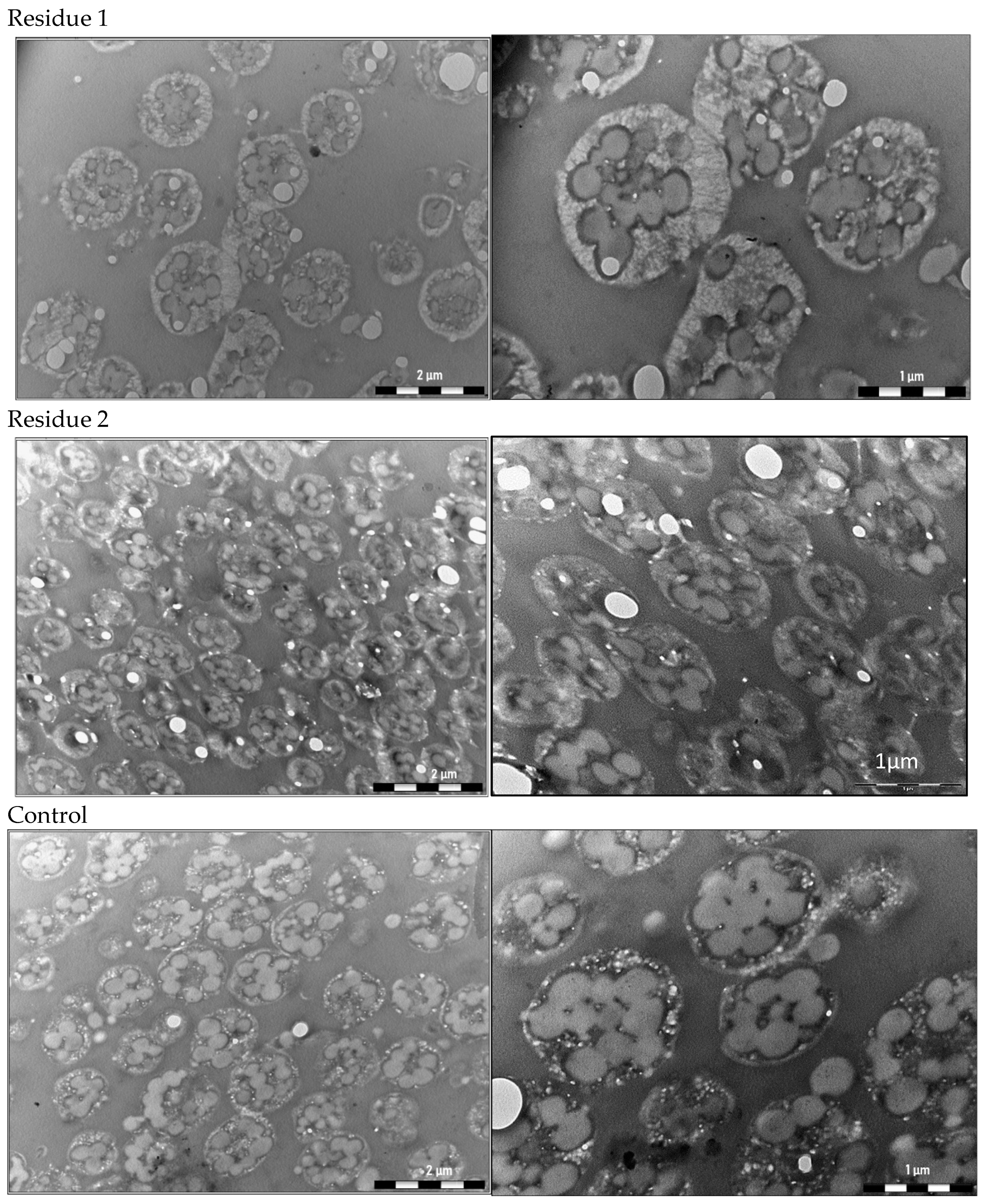
2.3. Analysis of the PHA Granules within the Cells by Transmission Electron Microscopy (TEM)
2.4. PHA Extraction Method
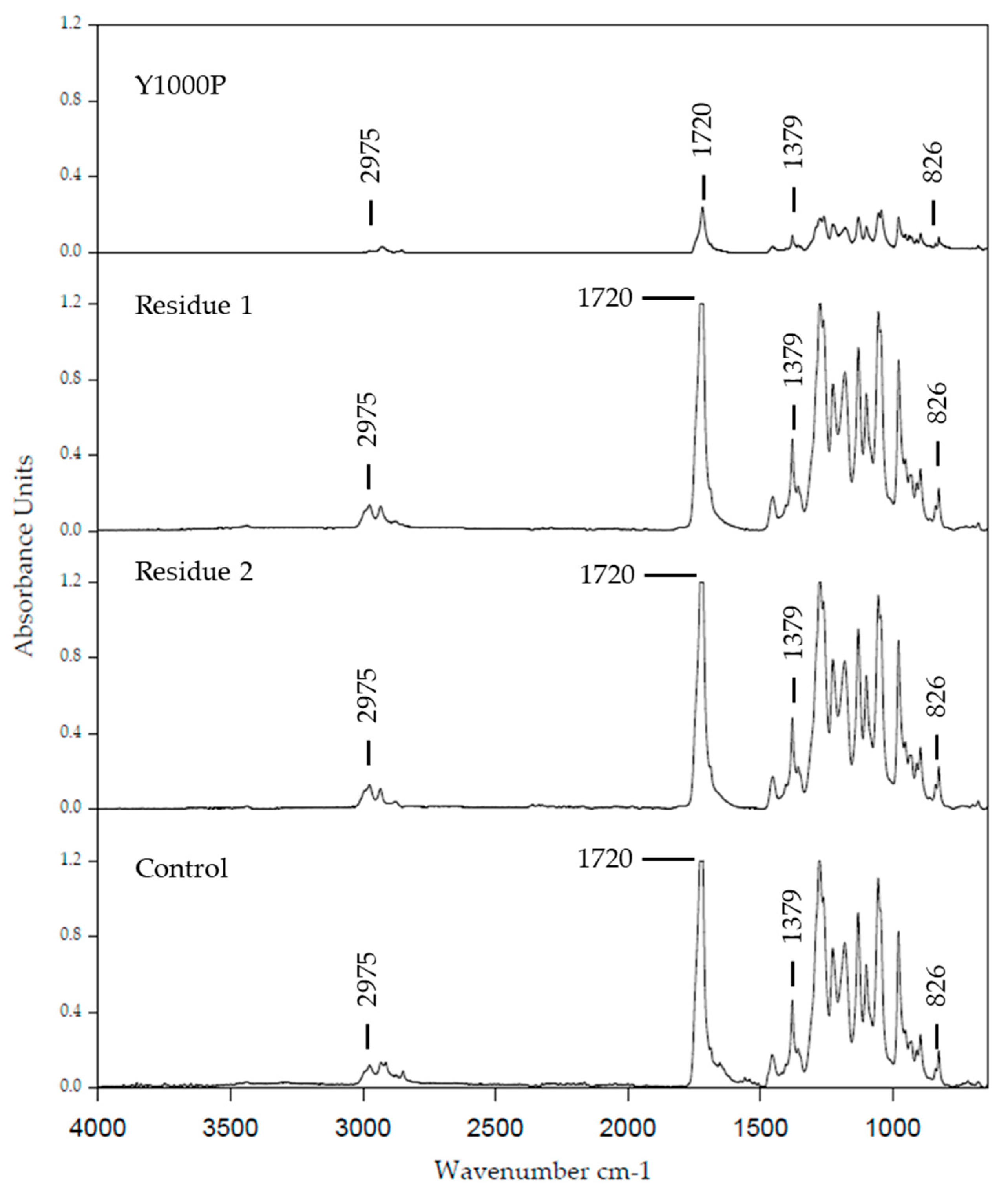
2.5. Characterization by Attenuated Total Reflect and Fourier-Transform Infrared Spectroscopy (ATR–FTIR)
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Methanolysis and PHA Quantification by Gas Chromatography (GC)
2.8. Analysis of the Biopolymer by Differential Scanning Calorimetry (DSC)
2.9. Thermogravimetric Analysis (TGA)
3. Results
3.1. Monitorization of the Cultures Growth
3.2. Analysis of the PHA Granules within the Cells by Transmission Electron Microscopy (TEM)
3.3. PHA Quantification
3.4. Characterization of the Biopolymer Produced by H. mediterranei
3.4.1. Analysis of the Biopolymer by FTIR
3.4.2. NMR and GC
3.4.3. Thermal Characterization of PHBV: Differential Scanning Calorimetry and Thermogravimetric Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gianico, A.; Gallipoli, A.; Gazzola, G.; Pastore, C.; Tonanzi, B.; Braguglia, C.M. A novel cascade biorefinery approach to transform food waste into valuable chemicals and biogas through thermal pretreatment integration. Bioresour. Technol. 2021, 338, 125517. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Whasington, DC, USA, 2018. [Google Scholar]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Menicagli, V.; Balestri, E.; Lardicci, C. Exposure of coastal dune vegetation to plastic bagleachates: A neglected impact of plastic litter. Sci. Total Environ. 2019, 683, 737–748. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ibarra, J.D.; Robles, A.; Montemayor, A.; Iñiguez, A.; Blanco, A.; Torrecillas, A. A lean six sigma project to reduce waste and variability in a confectionery manufacturing. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Toronto, ON, Canada, 23–25 October 2019. [Google Scholar]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Lin, K.-Y.A.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Ehman, N.; De León, A.P.; Felissia, F.; Vallejos, M.; Area, M.C.; Chinga-Carrasco, G. Biocomposites of Polyhydroxyalkanoates and Lignocellulosic Components: A Focus on Biodegradation and 3D Printing. In Bioplastics for Sustainable Development; Springer: Singapore, 2021; pp. 325–345. [Google Scholar] [CrossRef]
- Poltronieri, P.; Kumar, P. Polyhydroxyalkanoates (PHAs) in industrial applications. In Handbook of Ecomaterials; Springer International Publishing: Cham, Switzerland, 2017; Volume 4, pp. 2843–2872. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef]
- Nagarajan, D.; Aristya, G.R.; Lin, Y.J.; Chang, J.J.; Yen, H.W.; Chang, J.S. Microbial cell factories for the production of polyhydroxyalkanoates. Essays Biochem. 2021, 65, 337–353. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Rao, Z.M.; Xue, Y.F.; Gong, P.; Ji, Y.Z.; Ma, Y.H. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Haloarchaeon Halogranum amylolyticum. Appl. Microbiol. Biotechnol. 2015, 99, 7639–7649. [Google Scholar] [CrossRef]
- Simó-Cabrera, L.; García-Chumillas, S.; Hagagy, N.; Saddiq, A.; Tag, H.; Selim, S.; AbdElgawad, H.; Arribas Agüero, A.; Monzó Sánchez, F.; Cánovas, V.; et al. Haloarchaea as cell factories to produce bioplastics. Mar. Drugs 2021, 19, 159. [Google Scholar] [CrossRef]
- Hagagy, N.; Saddiq, A.A.; Tag, H.M.; Selim, S.; AbdElgawad, H.; Martínez-Espinosa, R.M. Characterization of polyhydroxybutyrate, PHB, synthesized by newly isolated haloarchaea Halolamina spp. Molecules 2022, 27, 7366. [Google Scholar] [CrossRef]
- Han, J.; Wu, L.P.; Hou, J.; Zhao, D.; Xiang, H. Biosynthesis, characterization, and hemostasis potential of tailor-made poly(3-hydroxybutyrate-co-3-hydroxyvalerate) produced by Haloferax mediterranei. Biomacromolecules 2015, 16, 578–588. [Google Scholar] [CrossRef]
- Mitra, R.; Xu, T.; Xiang, H.; Han, J. Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb. Cell Factories 2020, 19, 86. [Google Scholar] [CrossRef]
- Ren, Y.; Ling, C.; Hajnal, I.; Wu, Q.; Chen, G.Q. Construction of Halomonas bluephagenesis capable of high cell density growth for efficient PHA production. Appl. Microbiol. Biotechnol. 2018, 102, 4499–4510. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.Y.; Du, H.T.; Zhang, X.; Ma, Y.M.; Chen, J.C.; Ye, J.W.; Jiang, X.R.; Chen, G.Q. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV). Metab. Eng. 2019, 54, 69–82. [Google Scholar] [CrossRef]
- Ye, J.; Hu, D.; Yin, J.; Huang, W.; Xiang, R.; Zhang, L.; Wang, X.; Han, J.; Chen, G.Q. Stimulus response-based fine-tuning of polyhydroxyalkanoate pathway in Halomonas. Metab. Eng. 2020, 57, 85–95. [Google Scholar] [CrossRef]
- Oren, A.; Hallsworth, J.E. Microbial weeds in hypersaline habitats: The enigma of the weed-like Haloferax mediterranei. FEMS Microbiol. Lett. 2014, 359, 134–142. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Xue, Q.; Zhou, J.; Zhao, D.H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an efficient platform for high-level production of lycopene. Front. Microbiol. 2018, 9, 2893. [Google Scholar] [CrossRef]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef]
- Martínez-Espinosa, R.M.; Marhuenda-Egea, F.C.; Bonete, M.J. Assimilatory nitrate reductase from the haloarchaeon Haloferax mediterranei: Purification and characterisation. FEMS Microbiol. Lett. 2001, 204, 381–385. [Google Scholar] [CrossRef]
- Noble, R.T.; Fuhrman, J.A. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 1998, 14, 113–118. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Pire, C.; Martínez-Espinosa, R.M.; Bergaust, L. Denitrifying haloarchaea within the genus Haloferax display divergent respiratory phenotypes, with implications for their release of nitrogenous gases. Environ. Microbiol. 2019, 21, 427–436. [Google Scholar] [CrossRef]
- Tian, J.; Sinskey, A.J.; Stubbe, J.A. Kinetic studies of polyhydroxybutyrate granule formation in Wautersia eutropha H16 by transmission electron microscopy. J. Bacteriol. 2005, 187, 3814–3824. [Google Scholar] [CrossRef]
- Kumar, M.; Singhal, A.; Verma, P.K.; Thakur, I.S. Production and characterization of polyhydroxyalkanoate from lignin derivatives by Pandoraea sp. ISTKB. ACS Omega 2017, 2, 9156–9163. [Google Scholar] [CrossRef]
- Bautista, V.; Esclapez, J.; Pérez-Pomares, F.; Martínez-Espinosa, R.M.; Camacho, M.; Bonete, M.J. Cyclodextrin glycosyltransferase: A key enzyme in the assimilation of starch by the halophilic archaeon Haloferax mediterranei. Extremophiles 2012, 16, 147–159. [Google Scholar] [CrossRef]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the Polyhydroxyalkanoate PHBV from Ricotta Cheese Exhausted Whey by Haloferax mediterranei Fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cai, J.Y.; Liu, Z.; Zheng, Y.; Wang, H.; Li, Q.; He, N. Biosynthesis and thermal properties of PHBV produced from levulinic acid by Ralstonia eutropha. PLoS ONE 2013, 8, e60318. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Biosynthesis and characterization of polyhydroxyalkanoates with controlled composition and microstructure. Biomacromolecules 2018, 19, 996–1005. [Google Scholar] [CrossRef]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Moopantakath, J.; Imchen, M.; Anju, V.T.; Busi, S.; Dyavaiah, M.; Martínez-Espinosa, R.M.; Kumavath, R. Bioactive molecules from haloarchaea: Scope and prospects for industrial and therapeutic applications. Front Microbiol. 2023, 14, 1113540. [Google Scholar] [CrossRef]
- Mojica, F.J.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 2000, 36, 244–246. [Google Scholar] [CrossRef]
- Mojica, F.J.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Oren, A. Industrial and environmental applications of halophilic microorganisms. Environ. Technol. 2010, 31, 825–834. [Google Scholar] [CrossRef]
- Giani, M.; Montoyo-Pujol, Y.G.; Peiró, G.; Martínez-Espinosa, R.M. Haloarchaeal carotenoids exert an in vitro antiproliferative effect on human breast cancer cell lines. Sci. Rep. 2023, 13, 7148. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K. Haloarchaea: Worth exploring for their biotechnological potential. Biotechnol. Lett. 2017, 39, 1793–1800. [Google Scholar] [CrossRef]
- Obulisamy, P.K.; Mehariya, S. Polyhydroxyalkanoates from extremophiles: A review. Bioresour. Technol. 2021, 325, 124653. [Google Scholar] [CrossRef]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef]
- Han, J.; Lu, Q.; Zhou, L.; Zhou, J.; Xiang, H. Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl. Environ. Microbiol. 2007, 73, 6058–6065. [Google Scholar] [CrossRef]
- Han, J.; Hou, J.; Liu, H.; Cai, S.; Feng, B.; Zhou, J.; Xiang, H. Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl. Environ. Microbiol. 2010, 76, 7811–7819. [Google Scholar] [CrossRef]
- Cai, S.; Cai, L.; Liu, H.; Liu, X.; Han, J.; Zhou, J.; Xiang, H. Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl. Environ. Microbiol. 2012, 78, 1946–1952. [Google Scholar] [CrossRef]
- Cai, S.; Cai, L.; Zhao, D.; Liu, G.; Han, J.; Zhou, J.; Xiang, H. A novel DNA-binding protein, PhaR, plays a central role in the regulation of polyhydroxyalkanoate accumulation and granule formation in the haloarchaeon Haloferax mediterranei. Appl. Environ. Microbiol. 2015, 81, 373–385. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill waste water by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Liu, X.B.; Wu, L.P.; Hou, J.; Chen, J.Y.; Han, J.; Xiang, H. Environmental biodegradation of haloarchaea-produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in activated sludge. Appl. Microbiol. Biotechnol. 2016, 100, 6893–6902. [Google Scholar] [CrossRef]
- Cai, S.; Wu, Y.; Li, Y.; Yang, S.; Liu, Z.; Ma, Y.; Lv, J.; Shao, Y.; Jia, H.; Zhao, Y.; et al. Production of polyhydroxyalkanoates in unsterilized hyper-saline medium by halophiles using waste silkworm excrement as carbon source. Molecules 2021, 26, 7122. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate co- and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. In Macromolecular symposia; WILEY-VCH Verlag: Weinheim, Germany, 2007; Volume 253, pp. 33–39. [Google Scholar] [CrossRef]
- Taran, M. Utilization of petrochemical wastewater for the production of poly(3-hydroxybutyrate) by Haloarcula sp. IRU1. J. Hazard. Mater. 2011, 188, 26–28. [Google Scholar] [CrossRef]
- Alsafadi, D.; Ibrahim, M.I.; Alamry, K.A.; Hussein, M.A.; Mansour, A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total Environ. 2020, 737, 139716. [Google Scholar] [CrossRef]
- Khamplod, T.; Wongsirichot, P.; Winterburn, J. Production of polyhydroxyalkanoates from hydrolysed rapeseed meal by Haloferax mediterranei. Bioresour. Technol. 2023, 386, 129541. [Google Scholar] [CrossRef]
- Montemurro, M.; Salvatori, G.; Alfano, S.; Martinelli, A.; Pontonio, E.; Villano, M.; Rizzello, C.G. Exploitation of wasted bread as substrate for polyhydroxyalkanoates production through the use of Haloferax mediterranei and seawater. Front. Microbiol. 2022, 13, 1000962. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Coons, J.; Yeager, C.; Halley, P.; Chemodanov, A.; Belgorodsky, B.; Gozin, M.; Chen, G.-Q.; Golberg, A. Halophyte biorefinery for polyhydroxyalkanoates production from Ulva sp. Hydrolysate with Haloferax mediterranei in pneumatically agitated bioreactors and ultrasound harvesting. Bioresour. Technol. 2022, 344, 125964. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Del Valle, L.J.; Puiggalí, J. Copolymers and blends based on 3-hydroxybutyrate and 3-hydroxyvalerate units. Int. J. Mol. Sci. 2023, 24, 17250. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical applications of the biopolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug encapsulation and scaffold fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef] [PubMed]
- Tarahi, M.; Tahmouzi, S.; Kianiani, M.R.; Ezzati, S.; Hedayati, S.; Niakousari, M. Current innovations in the development of functional gummy candies. Foods 2023, 13, 76. [Google Scholar] [CrossRef]

| Average Values | Residue 1 | Residue 2 |
|---|---|---|
| Total fat (g/100 g) | 0 | 0 |
| Saturated fat (g/100 g) | 0 | 0 |
| Total carbohydrate (g/100 g) | 91 | 83 |
| Sugars (g/100 g) | 90 | 73 |
| Protein (g/100 g) | 0.3 | 4.4 |
| Salt (g/100 g) | 0.01 | 0.16 |
| Energy/100 g | 1558 kJ 374 kcal | 1505 kJ 354 kcal |
| Pictures of each waste |  |  |
| Sample | gCDW/L | gPHBV/L | gPHBV/gCDW | 3HV% by GC a | 3HV% by NMR b | mgPHBV/L |
|---|---|---|---|---|---|---|
| Y100P | - | - | - | - | 1 c | - |
| Residue 1 | 2.840 | 0.256 ± 0.166 | 0.236 ± 0.075 | 8.630 ± 0.350 | 12.129 ± 0.849 | 21.323 ± 3.864 |
| Residue 2 | 2.600 | 0.983 ± 0.330 | 0.378 ± 0.112 | 9.36 0 ± 0.430 | 8.832 ± 0.371 | 66.943 ± 10.912 |
| Control | 1.890 | 0.350 ± 0.095 | 0.111 ± 0.030 | 10.150 ± 0.740 | 11.901 ± 0.853 | 35.018 ± 3.044 |
| TGA | DSC | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | T5% (°C) | Td (°C) | Tg (°C) | Tcc (°C) | Tmp1 (°C) | Tmp2 (°C) | Tm (°C) | Xc (%) |
| Y1000P | 281.2 (1.7) | 298.2 (2.6) | n.d. a | n.d. a | n.d. a | 169.9 (0.11) | 176.20 (0.68) | 64.0 (2.0) |
| Residue 1 | 265.5 (2.4) | 285.6 (3.1) | −0.41 (0.97) | 56.77 (0.99) | 132.17 (0.99) | 145.89 (0.65) | 162.26 (4.77) | 47.67 (2.63) |
| Residue 2 | 265.8 (0.8) | 288.2 (1.2) | −0.10 (1.47) | n.d. a | 134.96 (7.41) | 145.83 (6.13) | 157.55 (0.91) | 51.97 (5.56) |
| Control | 261.3 (2.9) | 283.3 (4.2) | −0.15 (0.89) | 54.46 (10.80) | 131.39 (5.35) | 141.57 (1.88) | 158.66 (1.27) | 48.39 (6.49) |
| Waste | gPHBV/L | HV (%) | References |
|---|---|---|---|
| Residue 1 | 0.256 ± 0.166 | 12.129 ± 0.849 | This study |
| Residue 2 | 0.983 ± 0.330 | 8.832 ± 0.371 | This study |
| Olive mill wastewater | 0.2 | 6.5 | [48] |
| 25% pre-treated vinasse | 19.7 | 12.6 | [51] |
| 50% pre-treated vinasse | 17.4 | 14.09 | [51] |
| Hydrolyzed whey | 12.2 1 | 6 | [52] |
| Date extract | 4.5 | 18 | [54] |
| 75% hydrolyzed rapeseed meal | 0.512 ± 0.164 | 10.000 ± 0.007 | [55] |
| Wasted bread | 1.293 ± 0.216 | 10.78 ± 0.10 | [56] |
| Seaweed hydrolyzed | 2.08 ± 0.34 | 10.6 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simó-Cabrera, L.; García-Chumillas, S.; Benitez-Benitez, S.J.; Cánovas, V.; Monzó, F.; Pire, C.; Martínez-Espinosa, R.M. Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials. Bioengineering 2024, 11, 870. https://doi.org/10.3390/bioengineering11090870
Simó-Cabrera L, García-Chumillas S, Benitez-Benitez SJ, Cánovas V, Monzó F, Pire C, Martínez-Espinosa RM. Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials. Bioengineering. 2024; 11(9):870. https://doi.org/10.3390/bioengineering11090870
Chicago/Turabian StyleSimó-Cabrera, Lorena, Salvador García-Chumillas, Sergio J. Benitez-Benitez, Verónica Cánovas, Fuensanta Monzó, Carmen Pire, and Rosa María Martínez-Espinosa. 2024. "Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials" Bioengineering 11, no. 9: 870. https://doi.org/10.3390/bioengineering11090870
APA StyleSimó-Cabrera, L., García-Chumillas, S., Benitez-Benitez, S. J., Cánovas, V., Monzó, F., Pire, C., & Martínez-Espinosa, R. M. (2024). Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) by Haloferax mediterranei Using Candy Industry Waste as Raw Materials. Bioengineering, 11(9), 870. https://doi.org/10.3390/bioengineering11090870









