1. Introduction
Parkinson’s disease (PD) causes both motor and non-motor symptoms. Visible early motor manifestations include slowness and loss of quality of movement reflected in an altered and more variable gait pattern [
1,
2,
3]. Motor deficits affect walking, posture, balance, and functioning in everyday activities. Parkinsons impacts on movement automaticity, increasing both physical and mental effort during walking, particularly when navigating more complex environments [
4,
5,
6], placing people with PD at high risk of falls [
7,
8] and diminished quality of life [
9].
Rehabilitation for people with PD mainly focuses on the motor effects of PD as they affect most aspects of function [
10]. Motor focused rehabilitation interventions are strongly task oriented with improvement in walking the most common target [
11]. This most recent systematic review [
11] also identified that walking practice was the most common modality used in rehabilitation, although research recognizes that gait impairments underpin walking [
12]. Walking competency, defined as the ability to competently and safely navigate in the community [
13], requires a safe effective gait pattern, balance, core and peripheral strength, movement speed and endurance, and environmental awareness [
14]. Walking and balance are highly interdependent [
15,
16,
17]. Both are required for safe and independent capacity for activities of daily living [
18,
19], while improving balance improves walking and walking can improve balance [
20,
21,
22]. Focusing on training a heel-to-toe gait pattern has been recommended to improve gait in people with PD [
23], which is a way of changing posture and stride length.
The non-motor effects of PD, the invisible disabilities, share a common pathological pathway [
24,
25], and are known to have a direct effect on activity [
26] and quality of life [
9,
27]. Importantly there is also evidence that these non-motor symptoms are linked to temporo–spatial gait metrics [
28]. Kim et al., [
29] demonstrated an association between cognitive impairment and stride length. Depression in people with PD has been linked to magnitude and variability in temporo–spatial gait parameters [
30,
31,
32]. Muscle fatigue has also been implicated in gait and falls [
33], and perception of fatigue has been linked to axial/postural/gait impairment [
34]. However, the effect of non-motor symptoms on kinematic gait parameters that underlie these temporo–spatial parameters [
35,
36] remains understudied.
This study aimed to estimate both the direct and indirect effects of non-motor symp-toms, specifically depressive symptoms, fatigue, and concentration on kinematic gait parameters and physical function. Direct effects refer to the immediate influence of these symptoms on kinematic gait parameters, while indirect effects involve pathways medi-ated by other variables, such as temporo–spatial gait metrics. A path analysis model isemployed to disentangle these relationships, quantifying the direct and indirect effects within a structural framework. This approach aligns with the hypothesis that non-motor symptoms impact physical function both directly and indirectly through kinematic gait parameters, which in turn influence indicators of physical function.
Furthermore, this study has the potential to support the qualification of specific kinematic metrics as digital clinical outcome assessments (dCOA) for PD. These metrics will be evaluated for their reliability, sensitivity, and specificity to assess PD-related changes using established psychometric validation processes, including repeatability analyses and responsiveness to clinical change. By elucidating these relationships, the study aims to inform targeted interventions to improve physical function in individuals with PD.
2. Materials and Methods
This study is a secondary analysis of baseline data from a randomized pilot and feasibility study. The aim of the pilot trial was to estimate the extent to which training over a three-month period with a commercially available therapeutic wearable, the Heel2ToeTM sensor, was feasible and acceptable to participants and to estimate changes in and gait pattern and walking capacity when using the sensor to provide feedback for an optimal stepping pattern [
37,
38,
39]. The trial was prospectively registered on 3 April 2020 under the title “Improving Walking With Heel-To-Toe Device” on
ClinicalTrials.gov (NCT04300348). The project was approved by the Research Ethics Board of the McGill University Health Center. All participants provided written informed consent [
40].
The Heel2ToeTM sensor, a small device that clips to the side of shoe, uses inertial measurement units (IMU), gyroscope, and accelerometer, to measure angular velocity (AV) of the ankle during the gait cycle. For the trial, two groups were formed: one group trained with the Heel2Toe sensor and one group did not. For this re-analysis, the baseline data for the sample were analyzed.
2.1. Population
The sample comprised people with PD manifesting gait impairments and meeting the criterion that usual walking is without a walking aid [
41], corresponding to the Hoehn and Yahr Scale of 2 to 3, and they were recruited from the Movement Disorders Clinics at McGill sites and the Quebec Parkinson Network. People with a Montreal Cognitive Assessment (MOCA) [
42] score indicating cognitive impairment (≤25/30) were excluded. Participants were assessed during the “on” period of their medication schedule [
43].
2.2. Measures
Balance, functional walking capacity, and self-reported physical function were one aspect of the motor effects of PD. Functional walking capacity was measured by the 6 Minute Walk Test (6MWT) [
44]. Self-reported physical function was measured with the Neuro-QoL [
45] comprising 8 items, measured on a 5-point ordinal scale, related to changing body position against gravity from lying, sitting, standing, or the floor (5 items), pushing with arms (1 item), walking (1 item), and running errands or shopping (1 item). Balance was measured with the Mini-BESTest (Balance Evaluation Systems Test) which comprises 14 static and dynamic balance tests performed by the participant and graded by a trained evaluator from score 0 to 2. Higher values on these three tests indicate better physical function [
46].
Three constructs were used to represent the non-motor effects of PD: ratings of depression, fatigue, and concentration. Symptoms of depression and fatigue were measured using Visual Analogue Health States [
47] on a 0 to 100 scale with higher values indicating poorer states. Values greater than 40 are considered to reflect a clinical situation where treatment might be indicated [
48,
49]. There was no specific measure of concentration but there was one item on the 8-item Parkinson Deficit Questionnaire (PDQ) [
50] querying problems with concentration when reading or watching television, measured on a 5-point ordinal scale from never to always.
Other motor effects of PD were indicators of gait quality obtained directly from the IMUs in the Heel2Toe™ sensor worn without feedback during the baseline assessment of the 6MWT. These indicators are angular velocity (AV) of the ankle joint during heel strike, push-off, and foot swing and their associated coefficients of variation (CV). AV of heel strike and push-off have a negative sign and AV of foot swing has a positive sign, thus, the magnitude of the path parameters is of relevance, not the sign [
51].
2.3. Analysis
We hypothesized that non-motor symptoms would affect gait kinematics which would affect physical function requiring mediation analysis [
52]. A path analysis was conducted to estimate both direct and indirect effects [
53]. The path analysis parameters included direct effects between variables, expressed as regression coefficients (ß), with associated standard errors (SE),
p-values, and the ratio of ß/SE, which is the equivalent to a
t-test. As each of the path variables has a different measurement scale, the regression parameters need to be standardized to compare path strengths. This process also accounts for the dual roles of some variables that are both an explanatory (left hand side) variable and an outcome (right hand side) variable. To this end the parameter StdYX is presented, and it is interpreted as the effect of a left-hand side path variable on a right-hand side path variable such that for every SD difference on a left-hand variable, the right-hand variable differs by ßSD units. As the sample size was restricted, we wanted to test a path model that was as parsimonious as possible. Correlations of all variables were carried out and variables with weak values were not included.
2.4. Sample Size
This analysis used an existing dataset with 27 participants. We used a sample size calculator for structural equation modeling (SEM) to identify what effect size was detectable with the sample size [
54]. We considered that the multi-item Mini-BESTest and the Neuro-QoL measures as latent variables. Our model therefore comprised 2 latents and 7 observed variables. The minimum sample size for effect estimation of 0.5 or greater was 23. The sample size to test the model structure would be much greater (
n = 138).
3. Results
Table 1 presents the characteristics of the 27 participants with PD (mean age 70 years; 70% men) contributing data for this analysis (
Table 1). On the non-motor effects of depressed mood and fatigue, participants scored in the concerning range (>40/100), and 25.9% reported often having trouble concentrating (18.5% sometimes).
The 27 participants contributed a total of 1354 steps, with the number of steps during baseline assessment ranging from 10 to 170. AVs and AVCVs for heel strike, push-off, and foot swing were averaged across all steps taken by each participant during the baseline 6MWT assessment [
55]. The mean AV during heel strike was −141.0 (SD: 96.5). During push-off it was −105.8 (SD: 66.7), and during foot swing it was 196.4 (SD: 69.3). CVs for these kinematic gait parameters were over 75% for heel strike and push-off, and 32.0% for foot swing [
51,
56,
57].
For balance, the average score on the Mini Best test was 19.4 (SD: 6.6), whereas the age normative value is estimated at 23. The average 6MWT was 391.2 m (SD: 153.9), approximately 72% of that predicted for age. The average value on the Neuro-QoL lower extremity measure was 34.0 (SD = 4.7), whereas the optimal value is 40.
While both AVs and AVCVs for heel strike, push-off, and foot swing were potentially relevant for the path analysis, the correlations between AVCVs and the physical function outcomes were low, so we only included AVs.
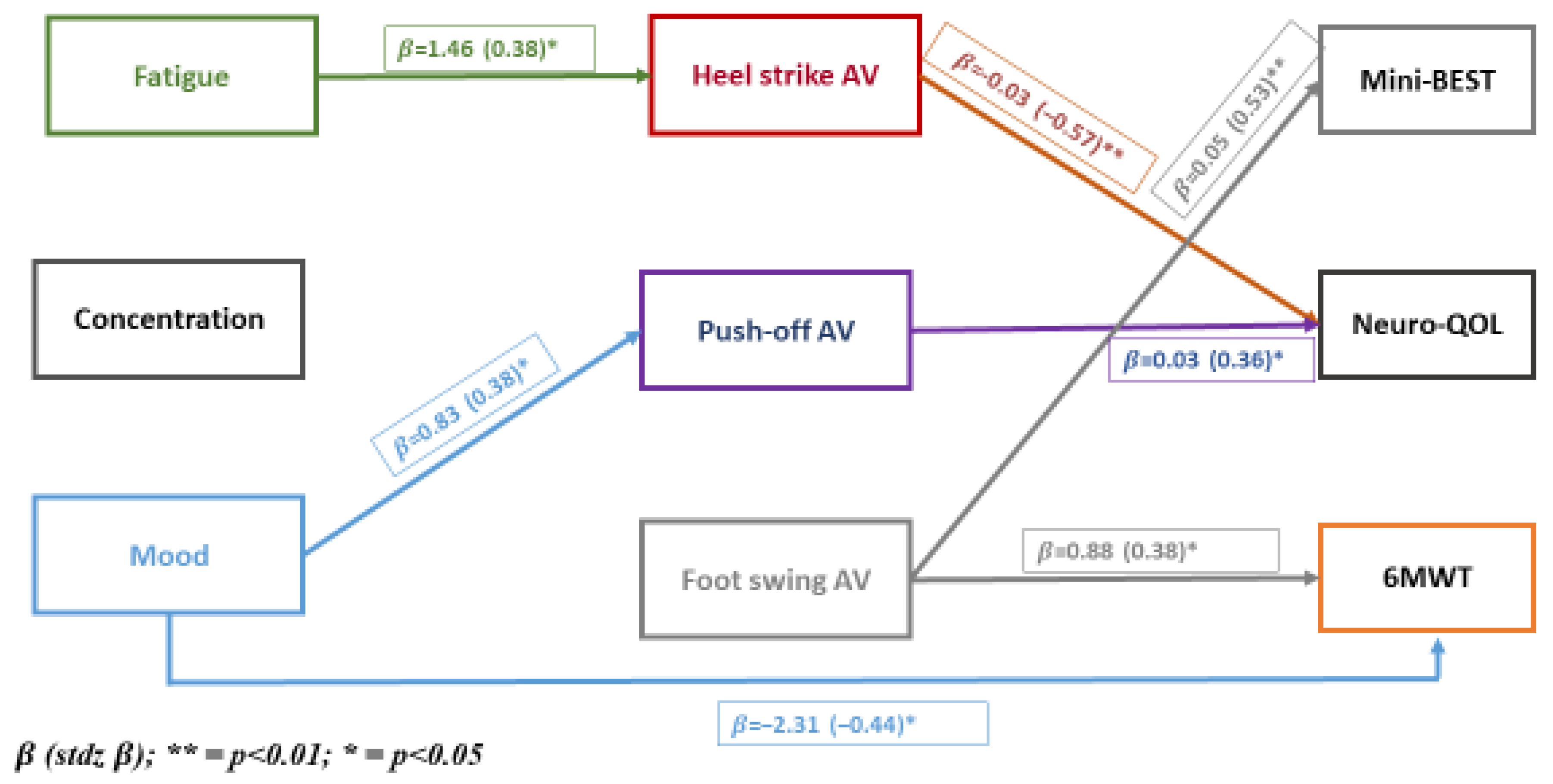
The results of the path analysis are presented in
Figure 1. All 27 direct and indirect paths are hypothesized. The three non-motor symptoms are on the left-hand side of the model, termed exogenous variables and are allowed to have paths to motor symptoms directly as well as through paths to kinematic parameters, which are hypothesized to have paths to motor symptoms.
Figure 1 shows that fatigue was associated with heel strike directly and with Neuro-QoL indirectly through heel strike. Mood was associated with push-off and 6MWT directly and with Neuro-QoL indirectly through push-off. Direct effects were observed for heel strike and push-off with Neuro-QoL, and foot swing was associated with balance and 6MWT. Concentration was not associated with any path variable.
To complement the path parameters presented in
Figure 1 showing the connections across levels, we present in
Table 2 the extent to which the variables at each level are correlated (
Figure 1,
Table 2). At the level of non-motor symptoms, correlations were low; kinematic parameters were moderately correlated; for physical function measures, balance (Mini-BEST) and 6MWT were highly correlated, and self-reported mobility of the lower extremity (Neuro-QoL) showed low correlation with these performance measures.
Table 3 summarizes the direct path parameters (
Table 3). The ones that reached significance are presented in bold font. The parameter labeled StdYX allows a comparison to be made for each pair-wise path. The three strongest direct effects were for heel strike AV and Neuro-QoL (−0.572), foot-swing AV and Mini-BEST (0.529), and mood and 6MWT (0.440). As there were indirect effects of fatigue on Neuro-QoL through heel strike AV, the total effect of fatigue on Neuro-QoL was 0.382 + |0.127|, totaling 0.509. The total effects of mood on Neuro-QoL are given by |0.232 + 0.003|, where |r| represents the absolute value of the path parameter. Since AV for heel strike and push-off are negative, the absolute value notation is used.
4. Discussion
Our study investigated the relationships between non-motor symptoms and the motor effects of Parkinson’s disease (PD), focusing on their effects on kinematic gait parameters and physical function [
58,
59]. We found that fatigue and mood significantly affected kinematic gait parameters. Fatigue was directly linked to heel strike parameters, affecting the initial phase of gait, while mood was associated with push-off parameters and the 6-Minute Walk Test (6MWT), influencing the terminal stance phase and walking endurance. These findings are interesting as parameters relating to fatigue may predispose to less stable gait, and factors associated with mood may reduce walking propulsion. Non-motor symptoms also exert indirect effects on physical function through kinematic gait parameters. Fatigue indirectly affected Neuro-QoL via heel strike, suggesting that addressing fatigue could improve gait kinematics and physical function. Similarly, the indirect effect of mood on Neuro-QoL through push-off highlighted the connection between emotional well-being and motor function. Kinematic parameters had direct effects on physical function measures. Heel strike and push-off were directly linked to Neuro-QoL, underscoring their importance in functional mobility. Foot swing was associated with balance (Mini-BEST) and 6MWT, emphasizing its role in stability and endurance. Significant direct path parameters included heel strike AV on Neuro-QoL (−0.572), foot swing AV on Mini-BEST (0.529), and mood on 6MWT (0.440). The total effects showed the impact of non-motor symptoms on physical function, with the total effect of fatigue on Neuro-QoL being 0.509 and mood on Neuro-QoL being 0.235.
4.1. Fatigue and Its Impact on Gait and Physical Function
Our results indicate that fatigue significantly affects motor symptoms and physical function, particularly impacting heel strike parameters. This aligns with the study by Hagell et al. [
34], which found fatigue to be a prominent symptom in PD, associated with increased Hoehn and Yahr stages and various non-motor symptoms like anxiety, depression, and lack of motivation. The association of fatigue with axial/postural/gait impairment but not with other motor symptoms such as tremor or rigidity further supports our findings that fatigue influences specific aspects of gait. In the review by Ghani et al. [
33], lower limb muscle fatigue was shown to alter gait performance, increasing stride length and reducing stride duration, which could potentially increase the risk of falls. This supports our findings that fatigue impacts the initial phase of gait (heel strike), suggesting that interventions to reduce fatigue could improve gait stability and reduce fall risk. The work by Terra et al. [
60], found fatigue did not have a direct relationship with balance but they did not investigate indirect effects.
4.2. Mood and Emotional Well-Being and Their Impact on Gait and Physical Function
Mood significantly affected push-off parameters and the 6-Minute Walk Test (6MWT, a measure of walking endurance. This is consistent with the findings by Kincses et al. [
30], who reported that depressive symptoms in PD patients were associated with altered gait characteristics, including reduced velocity and shorter stride length, which in turn affected health-related quality of life. Our study further demonstrates that mood has indirect effects on physical function through its impact on gait parameters, underscoring the interconnectedness of emotional well-being and motor function. The influence of mood on push-off and 6MWT is consistent with research by Liguori et al. [
28], who found moderate correlations between non-motor symptoms like pain and fatigue with motor performance in PD—specifically gait duration and values on the Timed-Up-and-Go test (TUG) under single and dual tasks conditions.
4.3. Indirect Effects of Non-Motor Symptoms on Physical Function
Our path analysis revealed that non-motor symptoms exert indirect effects on physical function mediated through kinematic gait parameters. For instance, fatigue had an indirect effect on Neuro-QoL via heel strike, suggesting that addressing fatigue could enhance gait kinematics and quality of life. This mediation pathway was also noted in the study by Kincses et al. [
30], which highlighted the association between gait characteristics and depression in PD patients, reinforcing the importance of considering emotional health in gait and physical function assessments [
60].
4.4. Kinematic Parameters and Effects on Physical Function
Kinematic parameters, including heel strike and push-off, directly influence various aspects of physical function such as Neuro-QoL, Mini-BEST, and 6MWT. Significant direct path parameters were heel strike AV on Neuro-QoL (−0.572), foot swing AV on Mini-BEST (0.529), and mood on 6MWT (0.440). This is corroborated by the research of Lord et al. [
32,
61], who found that gait speed and step variability were crucial determinants of balance and functional mobility in PD patients. Our findings emphasize that the precise timing and force of these gait phases are critical for maintaining physical function.
4.5. Strength and Limitations
A major strength of this study is the use of path analysis to explore the direct and indirect relationships between non-motor symptoms, kinematic gait parameters, and physical function. This methodological approach allowed us to elucidate complex interactions and mediation effects, providing a nuanced understanding of how non-motor symptoms impact physical function in PD. Our study emphasizes a multi-factorial perspective, considering both motor and non-motor symptoms. This holistic approach highlights the importance of addressing the full spectrum of PD symptoms, which is crucial for developing more effective and comprehensive treatment strategies.
A notable limitation of this study is the relatively small sample size. While the results provide valuable insights, the generalizability of our findings may be limited. We also had a limited portfolio of measures which was necessary to reduce the respondent burden so that the focus could be on the processes of the trial. Thus, our measure of cognition was restricted to a single item for concentration. The observation that concentration did not have any impact may be due to under-specification of this important construct. A richer set of measures for mood and fatigue would have been optimal allowing for a structural equation model to be fit to the data and permit a focus to be on constructs rather than individual measures, which less accurately reflect the constructs of interest.
The cross-sectional nature of the study limits our ability to infer causality. Future studies with larger cohorts are necessary to confirm these relationships and ensure the robustness of the conclusions drawn.
The potential of wearable technology to transform PD management is promising but faces challenges in under-resourced areas, including high costs, limited access, and insufficient training. Future research should focus on subsidizing costs, integrating wearables into existing systems, and designing affordable, user-friendly devices. Collaborations with policymakers and industry stakeholders will be key to ensuring equitable access and scalability.
5. Conclusions
The findings of this study provide important insights for the current management of PD. The clear relationships identified between non-motor symptoms, kinematic gait parameters, and physical function suggest that a comprehensive approach to treatment is essential. Clinicians should consider not only the direct motor symptoms but also the significant impact of non-motor symptoms such as fatigue and mood on patients’ gait and overall physical function. This implies that current therapeutic strategies should be broadened to include interventions targeting non-motor symptoms, gait mechanics, as well as physical function. For now, these results advocate for the integration of multi-disciplinary teams in the care of PD patients. Neurologists, physical therapists, and mental health professionals need to work collaboratively to address both motor and non-motor symptoms. For example, targeted therapies to alleviate fatigue could indirectly benefit gait parameters, while interventions aimed at improving mood might enhance endurance and physical function. The incorporation of these comprehensive treatment plans can lead to more effective management of PD, reducing the burden of the disease on patients.
This study lays the groundwork for further exploration into the complex interplay between non-motor and motor symptoms in PD. Future research should investigate the mechanisms by which non-motor symptoms influence gait kinematics and physical function. Longitudinal studies could provide more definitive evidence on the causal relationships and the long-term effects of integrated treatment approaches.
The advancement of wearable technology and mobile health applications presents an exciting opportunity for monitoring and managing PD motor effects in real-time. Future research could explore how these technologies can be used to track gait parameters, providing personalized feedback, and enabling timely interventions. The development of such tools could revolutionize the management of PD, making it possible to tailor treatments to individual patients’ needs more precisely. The findings highlight the potential of personalized medicine in treating PD. By recognizing the unique constellation of symptoms each patient experiences, healthcare providers can develop more targeted and effective treatment plans. Future studies should focus on identifying biomarkers that predict individual responses to different therapies, facilitating a more personalized approach to managing both motor and non-motor symptoms. On a broader scale, these findings should inform healthcare policies aimed at improving the standard of care for PD patients. Policies that encourage the integration of mental health support into PD management plans and the funding of multi-disciplinary care teams could enhance patient outcomes. Additionally, investment in research and development of new technologies for monitoring and treating PD will be crucial in addressing the growing prevalence of this condition.
In conclusion, the insights gained from this study highlight the potential of using gait parameters as dCOAs for non-motor symptoms and suggest areas for intervention.
Author Contributions
E.T. carried out the analyses for this project, reviewed the literature, drafted the tables and results sections, and participated in the writing of the manuscript. A.-A.S. conducted the original trial that produced the data for this secondary analysis as part of his PhD. He reviewed the results of this paper and made edits to the manuscript. K.K.V.M. was involved in the trial that produced the data for this secondary analysis. He made suggestions on this current manuscript. H.D. contributed expertise on the mechanism of action and on the interpretation of the results. She made comments on the manuscript. N.E.M. is the inventor of the Heel2Toe sensor. She was the principal investigator on the trial that produced the data for this secondary analysis. She supervised the analysis of the data and contributed to all components of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by Health Brains for Healthy Lives (HBHL), Innovative Ideas Competition 2, Project A56. H.D. is funded by NIHR Exeter BRC. The Scientific and Technological Research Council of Turkey (TÜBİTAK) provided a scholarship to E.T. under the project number 59B192203150.
Institutional Review Board Statement
The project was approved by the Research Ethics Board of the McGill University Health Center on Feb 17, 020 (File # 2020-5842).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
Data could be made available for inclusion in meta-analysis.
Acknowledgments
The study team wishes to thank the participants for their patience in testing a new technology. The team also acknowledges the contribution of Stanley Hum for the design and management of the REDCap 14.3.13 database and Lyne Nadeau for conducting the analysis.
Conflicts of Interest
A.-A.S.; discloses relationship with PhysioBiometrics Inc. for this work. K.K.V.M.; discloses relationship with PhysioBiometrics Inc. for this work. E.H.; discloses relationship with PhysioBiometrics Inc. for this work. H.D.; discloses relationship with PhysioBiometrics Inc. for this work. N.E.M.; discloses relationship with PhysioBiometrics Inc. for this work.
References
- Rangel-Barajas, C.; Coronel, I.; Florán, B. Dopamine receptors and neurodegeneration. Aging Dis. 2015, 6, 349. [Google Scholar] [PubMed]
- Wu, T.; Liu, J.; Zhang, H.; Hallett, M.; Zheng, Z.; Chan, P. Attention to automatic movements in Parkinson’s disease: Modified automatic mode in the striatum. Cereb. Cortex 2015, 25, 3330–3342. [Google Scholar] [CrossRef]
- Gepshtein, S.; Li, X.; Snider, J.; Plank, M.; Lee, D.; Poizner, H. Dopamine function and the efficiency of human movement. J. Cogn. Neurosci. 2014, 26, 645–657. [Google Scholar] [CrossRef]
- Maidan, I.; Rosenberg-Katz, K.; Jacob, Y.; Giladi, N.; Deutsch, J.; Hausdorff, J.; Mirelman, A. Altered brain activation in complex walking conditions in patients with Parkinson’s disease. Park. Relat. Disord. 2016, 25, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.; Buttelli, O.; Riff, J.; Sellam, N.; Vidailhet, M.; Welter, M.-L.; Lalo, E. “The whole perimeter is difficult”: Parkinson’s disease and the conscious experience of walking in everyday environments. Disabil. Rehabil. 2019, 41, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Orcioli-Silva, D.; Barbieri, F.A.; Simieli, L.; Vitorio, R.; Dos Santos, P.C.R.; Beretta, V.S.; Gobbi, L.T.B. Walking behavior over multiple obstacles in people with Parkinson’s disease. Gait Posture 2017, 58, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef]
- Tosin, M.H.; Goetz, C.G.; Stebbins, G.T. Patient With Parkinson Disease and Care Partner Perceptions of Key Domains Affecting Health-Related Quality of Life: Systematic Review. Neurology 2024, 102, e208028. [Google Scholar] [CrossRef]
- He, L.; Lee, E.-Y.; Sterling, N.W.; Kong, L.; Lewis, M.M.; Du, G.; Eslinger, P.J.; Huang, X. The key determinants to quality of life in Parkinson’s disease patients: Results from the Parkinson’s Disease Biomarker Program (PDBP). J. Park. Dis. 2016, 6, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Folkerts, A.-K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2024, 4, CD013856. [Google Scholar]
- Kaneko, M.; Morimoto, Y.; Kimura, M.; Fuchimoto, K.; Fuchimoto, T. A kinematic analysis of walking and physical fitness testing in elderly women. Can. J. Sport Sci. J. Can. Sci. Sport 1991, 16, 223–228. [Google Scholar]
- Salbach, N.M.; Mayo, N.E.; Robichaud-Ekstrand, S.; Hanley, J.A.; Richards, C.L.; Wood-Dauphinee, S. The effect of a task-oriented walking intervention on improving balance self-efficacy poststroke: A randomized, controlled trial. J. Am. Geriatr. Soc. 2005, 53, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Abou-Sharkh, A.; Mate, K.K.; Inceer, M.; Morais, J.A.; Morin, S.N.; Mayo, N.E. What do older Canadians think they need to walk well? Physiother. Can. 2022, 75, 198–205. [Google Scholar] [CrossRef]
- Spagnuolo, D.L.; Jürgensen, S.P.; Iwama, Â.M.; Dourado, V.Z. Walking for the assessment of balance in healthy subjects older than 40 years. Gerontology 2010, 56, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, R.L.; Newton, R.A. Relationship between balance and gait stability in healthy older adults. J. Aging Phys. Act. 2004, 12, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ringsberg, K.; Gerdhem, P.; Johansson, J.; Obrant, K.J. Is there a relationship between balance, gait performance and muscular strength in 75-year-old women? Age Ageing 1999, 28, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.M.; Evans, A.L.; Duncan, G. Gait speed and activities of daily living function in geriatric patients. Arch. Phys. Med. Rehabil. 1995, 76, 997–999. [Google Scholar] [CrossRef]
- Van Kan, G.A.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Howe, T.E.; Rochester, L.; Neil, F.; Skelton, D.A.; Ballinger, C. Exercise for improving balance in older people. Cochrane Database Syst. Rev. 2011, 11, CD004963. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.; Wong-Yu, I.S. Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: A randomized controlled trial. J. Park. Dis. 2021, 11, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Ginis, P.; Pirani, R.; Basaia, S.; Ferrari, A.; Chiari, L.; Heremans, E.; Canning, C.G.; Nieuwboer, A. Focusing on heel strike improves toe clearance in people with Parkinson’s disease: An observational pilot study. Physiotherapy 2017, 103, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Modugno, N.; Lena, F.; Di Biasio, F.; Cerrone, G.; Ruggieri, S.; Fornai, F. A clinical overview of non-motor symptoms in Parkinson’s Disease. Arch. Ital. Biol. 2013, 151, 148–168. [Google Scholar]
- Khan, A.; Ezeugwa, J.; Ezeugwu, V.E. A systematic review of the associations between sedentary behavior, physical inactivity, and non-motor symptoms of Parkinson’s disease. PLoS ONE 2024, 19, e0293382. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Schönenberg, A.; Santos-García, D.; Mir, P.; Group, C.S.; Prell, T. The Impact of Nonmotor Symptoms on Health-Related Quality of Life in Parkinson’s Disease: A Network Analysis Approach. J. Clin. Med. 2023, 12, 2573. [Google Scholar] [CrossRef]
- Liguori, S.; Moretti, A.; Palomba, A.; Paoletta, M.; Gimigliano, F.; De Micco, R.; Siciliano, M.; Tessitore, A.; Iolascon, G. Non-motor impairments affect walking kinematics in Parkinson disease patients: A cross-sectional study. NeuroRehabilitation 2021, 49, 481–489. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, D.H.; Yang, Y.; Ha, S.W.; Han, J.H. Gait patterns in Parkinson’s disease with or without cognitive impairment. Dement. Neurocogn. Disord. 2018, 17, 57–65. [Google Scholar] [CrossRef]
- Kincses, P.; Kovács, N.; Karádi, K.; Feldmann, Á.; Dorn, K.; Aschermann, Z.; Komoly, S.; Szolcsányi, T.; Csathó, Á.; Kállai, J. Association of Gait Characteristics and Depression in Patients with Parkinson’s Disease Assessed in Goal-Directed Locomotion Task. Park. Dis. 2017, 2017, 6434689. [Google Scholar] [CrossRef] [PubMed]
- Dragašević-Mišković, N.T.; Bobić, V.; Kostić, M.; Stanković, I.; Radovanović, S.; Dimitrijević, K.; Svetel, M.; Petrović, I.; Đurić-Jovičić, M. Impact of depression on gait variability in Parkinson’s disease. Clin. Neurol. Neurosurg. 2021, 200, 106324. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Coleman, S.; Burn, D.; Rochester, L. Mild depressive symptoms are associated with gait impairment in early Parkinson’s disease. Mov. Disord. 2013, 28, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Ghani, H.A.; Justine, M.; Manaf, H. Effects of lower limb muscle fatigue on gait performance and postural control among individuals with Parkinson’s disease: A review of literature. Phys. Ther. Rev. 2019, 24, 274–279. [Google Scholar] [CrossRef]
- Hagell, P.; Brundin, L. Towards an understanding of fatigue in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 489–492. [Google Scholar] [CrossRef]
- de Campos, D.d.S.F.; Shokur, S.; de Lima-Pardini, A.C.; Runfeng, M.; Bouri, M.; Coelho, D.B. Kinematics predictors of spatiotemporal parameters during gait differ by age in healthy individuals. Gait Posture 2022, 96, 216–220. [Google Scholar] [CrossRef]
- Mate, K.; Abou-Sharkh, A.; Morais, J.; Mayo, N. Putting the best foot forward: Relationships between indicators of step quality and cadence in three gait vulnerable populations. NeuroRehabilitation 2019, 44, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mayo, N.E.; Mate, K.K.; Fellows, L.K.; Morais, J.A.; Sharp, M.; Lafontaine, A.-L.; Hill, E.T.; Dawes, H.; Sharkh, A.-A. Real-time auditory feedback for improving gait and walking in people with Parkinson’s disease: A pilot and feasibility trial. Pilot Feasibility Stud. 2024, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.P.; Mate, K.K.; Cinar, E.; Abou-Sharkh, A.; Lafontaine, A.-L.; Mayo, N.E. A new approach toward gait training in patients with Parkinson’s disease. Gait Posture 2020, 81, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vadnerkar, A.; Figueiredo, S.; Mayo, N.E.; Kearney, R.E. Design and validation of a biofeedback device to improve heel-to-toe gait in seniors. IEEE J. Biomed. Health Inform. 2017, 22, 140–146. [Google Scholar] [CrossRef]
- Vadnerkar, A.; Figueiredo, S.; Mayo, N.E.; Kearney, R.E. Classification of gait quality for biofeedback to improve heel-to-toe gait. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3626–3629. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lessig, S.; Nie, D.; Xu, R.; Corey-Bloom, J. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov. Disord. 2012, 27, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- PL, E. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003, 123, 325–327. [Google Scholar]
- Kozlowski, A.J.; Cella, D.; Nitsch, K.P.; Heinemann, A.W. Evaluating individual change with the quality of life in neurological disorders (Neuro-QoL) short forms. Arch. Phys. Med. Rehabil. 2016, 97, 650–654.e8. [Google Scholar] [CrossRef]
- Gonzalez-Robles, C.; Weil, R.S.; van Wamelen, D.; Bartlett, M.; Burnell, M.; Clarke, C.S.; Hu, M.T.; Huxford, B.; Jha, A.; Lambert, C. Outcome measures for disease-modifying trials in Parkinson’s disease: Consensus paper by the EJS ACT-PD multi-arm multi-stage trial initiative. J. Park. Dis. 2023, 13, 1011–1033. [Google Scholar] [CrossRef] [PubMed]
- Rosenzveig, A.; Kuspinar, A.; Daskalopoulou, S.S.; Mayo, N.E. Toward patient-centered care: A systematic review of how to ask questions that matter to patients. Medicine 2014, 93, e120. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [PubMed]
- Jensen, M.P.; Chen, C.; Brugger, A.M. Interpretation of visual analog scale ratings and change scores: A reanalysis of two clinical trials of postoperative pain. J. Pain 2003, 4, 407–414. [Google Scholar] [CrossRef]
- Franchignoni, F.; Giordano, A.; Ferriero, G. Rasch analysis of the short form 8-item Parkinson’s Disease Questionnaire (PDQ-8). Qual. Life Res. 2008, 17, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Palermo, E.; Rossi, S.; Cappa, P. Gait partitioning methods: A systematic review. Sensors 2016, 16, 66. [Google Scholar] [CrossRef]
- Gunzler, D.; Chen, T.; Wu, P.; Zhang, H. Introduction to mediation analysis with structural equation modeling. Shanghai Arch. Psychiatry 2013, 25, 390. [Google Scholar] [PubMed]
- Streiner, D.L. Finding our way: An introduction to path analysis. Can. J. Psychiatry 2005, 50, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Soper, D. A-Priori Sample Size Calculator for Structural Equation Models. Software. 2021. Available online: https://www.danielsoper.com/statcalc/ (accessed on 22 November 2020).
- Pappas, I.P.; Popovic, M.R.; Keller, T.; Dietz, V.; Morari, M. A reliable gait phase detection system. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 113–125. [Google Scholar] [CrossRef] [PubMed]
- van der Krogt, M.M.; Bregman, D.J.; Wisse, M.; Doorenbosch, C.A.; Harlaar, J.; Collins, S.H. How crouch gait can dynamically induce stiff-knee gait. Ann. Biomed. Eng. 2010, 38, 1593–1606. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Anderson, F.C.; Pandy, M.G.; Delp, S.L. Muscles that influence knee flexion velocity in double support: Implications for stiff-knee gait. J. Biomech. 2004, 37, 1189–1196. [Google Scholar] [CrossRef]
- Murri, M.B.; Triolo, F.; Coni, A.; Tacconi, C.; Nerozzi, E.; Escelsior, A.; Respino, M.; Neviani, F.; Bertolotti, M.; Bertakis, K. Instrumental assessment of balance and gait in depression: A systematic review. Psychiatry Res. 2020, 284, 112687. [Google Scholar] [CrossRef] [PubMed]
- Cusso, M.E.; Donald, K.J.; Khoo, T.K. The impact of physical activity on non-motor symptoms in Parkinson’s disease: A systematic review. Front. Med. 2016, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.B.; Caramaschi, I.K.F.; Araújo, H.A.G.d.O.; Souza, R.J.d.; Silva, T.C.O.d.; Nascimento, T.S.; Probst, V.S.; Smaili, S.M. Is fatigue associated with balance in Parkinson’s disease? Mot. Rev. Educ. Fís. 2022, 28, e10220013921. [Google Scholar] [CrossRef]
- Lord, S.; Baker, K.; Nieuwboer, A.; Burn, D.; Rochester, L. Gait variability in Parkinson’s disease: An indicator of non-dopaminergic contributors to gait dysfunction? J. Neurol. 2011, 258, 566–572. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).