Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Randomization
2.3. Participants
2.4. Sample Size Calculation
2.5. Rehabilitation Protocol
2.6. Assessment Protocol
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Maximum Effective Angle
3.2.1. Comparison Between Test and Control Groups
3.2.2. Within-Group Comparison (Test Group)
3.3. Hamstring Peak Torque
3.3.1. Comparison Between Test and Control Groups
3.3.2. Within-Group Comparison (Test Group)
3.4. Hamstring-to-Quadriceps Strength Ratio
3.4.1. Comparison Between Test and Control Groups
3.4.2. Within-Group Comparison (Test Group)
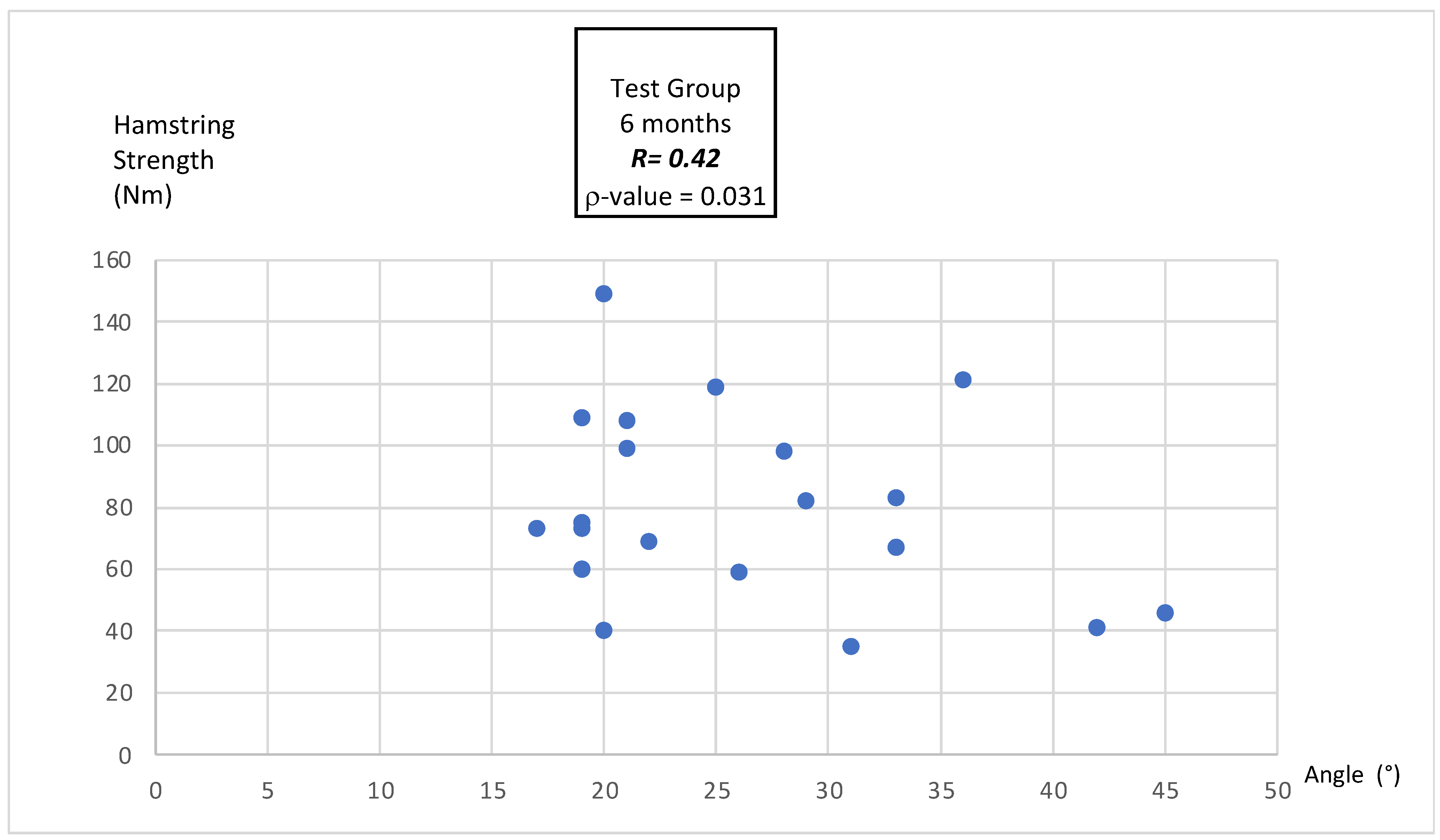
3.4.3. Correlation Between MEA and Hamstring Peak Torque
4. Discussion
4.1. Maximum Effective Angle
4.2. Hamstring Peak Torque Deficits
4.3. Hamstring-to-Quadriceps Strength Ratio
4.4. Correlation Between MEA and Hamstring Peak Torque
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef]
- Forelli, F.; Moiroux-Sahraoui, A.; Nielsen-Le Roux, M.; Miraglia, N.; Gaspar, M.; Stergiou, M.; Bjerregaard, A.; Mazeas, J.; Douryang, M. Stay in the Game: Comprehensive Approaches to Decrease the Risk of Sports Injuries. Cureus 2024, 16, e76461. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.L.; Orofino, A.S.; Morrissey, M.C.; Medeiros, J.M.; Mason, W.J. Assessment of Quadriceps/Hamstring Strength, Knee Ligament Stability, Functional and Sports Activity Levels Five Years after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 1988, 16, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, E.; Goncalves, G.H.; Bricca, A.; Roos, E.M.; Thorlund, J.B.; Juhl, C.B. Knee Osteoarthritis Risk Is Increased 4-6 Fold after Knee Injury–a Systematic Review and Meta-Analysis. Br. J. Sports Med. 2019, 53, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Biały, M.; Kublin, K.; Wilczyński, B.; Forelli, F.; Gnat, R. Does Concomitant Meniscectomy or Meniscus Repair Affect Muscle Strength, Lower Extremity Balance, and Functional Tests after Anterior Cruciate Ligament Reconstruction? J. Clin. Med. 2024, 13, 3310. [Google Scholar] [CrossRef]
- Konrath, J.M.; Vertullo, C.J.; Kennedy, B.A.; Bush, H.S.; Barrett, R.S.; Lloyd, D.G. Morphologic Characteristics and Strength of the Hamstring Muscles Remain Altered at 2 Years After Use of a Hamstring Tendon Graft in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2016, 44, 2589–2598. [Google Scholar] [CrossRef]
- Onishi, H.; Yagi, R.; Oyama, M.; Akasaka, K.; Ihashi, K.; Handa, Y. EMG-Angle Relationship of the Hamstring Muscles during Maximum Knee Flexion. J. Electromyogr. Kinesiol. 2002, 12, 399–406. [Google Scholar] [CrossRef]
- Kellis, E.; Blazevich, A.J. Hamstrings Force-Length Relationships and Their Implications for Angle-Specific Joint Torques: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 166. [Google Scholar] [CrossRef]
- Guex, K.; Gojanovic, B.; Millet, G.P. Influence of Hip-Flexion Angle on Hamstrings Isokinetic Activity in Sprinters. J. Athl. Train. 2012, 47, 390–395. [Google Scholar] [CrossRef]
- Brockett, C.L.; Morgan, D.L.; Proske, U. Human Hamstring Muscles Adapt to Eccentric Exercise by Changing Optimum Length. Med. Sci. Sports Exerc. 2001, 33, 783–790. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle Damage from Eccentric Exercise: Mechanism, Mechanical Signs, Adaptation and Clinical Applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Ivan, Z. Anatomy, Physiology and Biomechanics of Hamstrings Injury in Football and Effective Strength and Flexibility Exercises for Its Prevention. J. Hum. Sport Exerc. 2012, 7, S208–S217. [Google Scholar] [CrossRef]
- Oleksy, Ł.; Mika, A.; Pacana, J.; Markowska, O.; Stolarczyk, A.; Kielnar, R. Why Is Hamstring Strain Injury so Common in Sport Despite Numerous Prevention Methods? Are There Any Missing Pieces to This Puzzle? Front. Physiol. 2021, 12, 586624. [Google Scholar] [CrossRef]
- Kalkhoven, J.T.; Lukauskis-Carvajal, M.; Sides, D.L.; McLean, B.D.; Watsford, M.L. A Conceptual Exploration of Hamstring Muscle-Tendon Functioning during the Late-Swing Phase of Sprinting: The Importance of Evidence-Based Hamstring Training Frameworks. Sports Med. 2023, 53, 2321–2346. [Google Scholar] [CrossRef] [PubMed]
- Opar, D.A.; Williams, M.D.; Shield, A.J. Hamstring Strain Injuries: Factors That Lead to Injury and Re-Injury. Sports Med. 2012, 42, 209–226. [Google Scholar] [CrossRef]
- Kotsifaki, R.; Korakakis, V.; King, E.; Barbosa, O.; Maree, D.; Pantouveris, M.; Bjerregaard, A.; Luomajoki, J.; Wilhelmsen, J.; Whiteley, R. Aspetar Clinical Practice Guideline on Rehabilitation after Anterior Cruciate Ligament Reconstruction. Br. J. Sports Med. 2023, 57, 500–514. [Google Scholar] [CrossRef]
- Van Melick, N.; Van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; Van Tienen, T.; Hullegie, W.; Nijhuis-van Der Sanden, M.W.G. Evidence-Based Clinical Practice Update: Practice Guidelines for Anterior Cruciate Ligament Rehabilitation Based on a Systematic Review and Multidisciplinary Consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef]
- Kakavas, G.; Forelli, F.; Malliaropoulos, N.; Hewett, T.E.; Tsaklis, P. Periodization in Anterior Cruciate Ligament Rehabilitation: New Framework Versus Old Model? A Clinical Commentary. Int. J. Sports Phys. Ther. 2023, 18, 541–546. [Google Scholar] [CrossRef]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-Five per Cent Return to Competitive Sport Following Anterior Cruciate Ligament Reconstruction Surgery: An Updated Systematic Review and Meta-Analysis Including Aspects of Physical Functioning and Contextual Factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef]
- Buckthorpe, M. Optimising the Late-Stage Rehabilitation and Return-to-Sport Training and Testing Process After ACL Reconstruction. Sports Med. 2019, 49, 1043–1058. [Google Scholar] [CrossRef]
- Gobbi, A.; Boldrini, L.; Karnatzikos, G.; Mahajan, V. Clinical Outcomes and Rehabilitation Program After ACL Primary Repair and Bone Marrow Stimulation. In Sports Injuries; Doral, M.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 475–484. ISBN 978-3-642-15629-8. [Google Scholar]
- San Martín-Mohr, C.; Cristi-Sánchez, I.; Pincheira, P.A.; Reyes, A.; Berral, F.J.; Oyarzo, C. Knee Sensorimotor Control Following Anterior Cruciate Ligament Reconstruction: A Comparison between Reconstruction Techniques. PLoS ONE 2018, 13, e0205658. [Google Scholar] [CrossRef] [PubMed]
- Forelli, F.; Le Coroller, N.; Gaspar, M.; Memain, G.; Kakavas, G.; Miraglia, N.; Marine, P.; Maille, P.; Hewett, T.E.; Rambaud, A.J. Ecological and Specific Evidence-Based Safe Return To Play After Anterior Cruciate Ligament Reconstruction In Soccer Players: A New International Paradigm. Int. J. Sports Phys. Ther. 2023, 18, 526–540. [Google Scholar] [CrossRef]
- Traulle, M.; Linard, M.; Vandebrouck, A.; Duffiet, P.; Ratte, L.; Forelli, F. Determination of Predictive Isokinetic Indicators for Return to Sport at 6 Months after ACL Surgery with Semitendinous and Gracilis Tendons. Int. J. Phys. Ther. Rehabil. 2019, 5, 153. [Google Scholar] [CrossRef]
- Croisier, J.-L.; Forthomme, B.; Namurois, M.-H.; Vanderthommen, M.; Crielaard, J.-M. Hamstring Muscle Strain Recurrence and Strength Performance Disorders. Am. J. Sports Med. 2002, 30, 199–203. [Google Scholar] [CrossRef]
- Croisier, J.-L.; Ganteaume, S.; Binet, J.; Genty, M.; Ferret, J.-M. Strength Imbalances and Prevention of Hamstring Injury in Professional Soccer Players: A Prospective Study. Am. J. Sports Med. 2008, 36, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Fink, C.; Herbst, E.; Hoser, C.; Hepperger, C.; Blank, C.; Gföller, P. Higher Hamstring-to-Quadriceps Isokinetic Strength Ratio during the First Post-Operative Months in Patients with Quadriceps Tendon Compared to Hamstring Tendon Graft Following ACL Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 418–425. [Google Scholar] [CrossRef]
- Buckthorpe, M.; Danelon, F.; La Rosa, G.; Nanni, G.; Stride, M.; Della Villa, F. Recommendations for Hamstring Function Recovery after ACL Reconstruction. Sports Med. 2021, 51, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.; Reiman, M. The Role and Implementation of Eccentric Training in Athletic Rehabilitation: Tendinopathy, Hamstring Strains, and Acl Reconstruction. Int. J. Sports Phys. Ther. 2011, 6, 27. [Google Scholar] [PubMed]
- Hiemstra, L.A.; Webber, S.; MacDonald, P.B.; Kriellaars, D.J. Hamstring and Quadriceps Strength Balance in Normal and Hamstring Anterior Cruciate Ligament-Reconstructed Subjects. Clin. J. Sport Med. 2004, 14, 274–280. [Google Scholar] [CrossRef]
- Forelli, F.; Mazeas, J.; Zeghoudi, Y.; Vandebrouck, A.; Duffiet, P.; Ratte, L.; Kakavas, G.; Hewett, T.E.; Korakakis, V.; Rambaud, A.J.M. Intrinsic Graft Laxity Variation with Open Kinetic Chain Exercise after Anterior Cruciate Ligament Reconstruction: A Non-Randomized Controlled Study. Phys. Ther. Sport 2024, 66, 61–66. [Google Scholar] [CrossRef]
- Forelli, F.; Barbar, W.; Kersante, G.; Vandebrouck, A.; Duffiet, P.; Ratte, L.; Hewett, T.E.; Rambaud, A.J.M. Evaluation of Muscle Strength and Graft Laxity With Early Open Kinetic Chain Exercise After ACL Reconstruction: A Cohort Study. Orthop. J. Sports Med. 2023, 11, 23259671231177594. [Google Scholar] [CrossRef]
- Lentz, T.A.; Zeppieri, G.; Tillman, S.M.; Indelicato, P.A.; Moser, M.W.; George, S.Z.; Chmielewski, T.L. Return to Preinjury Sports Participation Following Anterior Cruciate Ligament Reconstruction: Contributions of Demographic, Knee Impairment, and Self-Report Measures. J. Orthop. Sports Phys. Ther. 2012, 42, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Guilhem, G.; Cornu, C.; Guével, A. Neuromuscular and Muscle-Tendon System Adaptations to Isotonic and Isokinetic Eccentric Exercise. Ann. Phys. Rehabil. Med. 2010, 53, 319–341. [Google Scholar] [CrossRef]
- Cristiani, R.; Sarakatsianos, V.; Engström, B.; Samuelsson, K.; Forssblad, M.; Stålman, A. Increased Knee Laxity with Hamstring Tendon Autograft Compared to Patellar Tendon Autograft: A Cohort Study of 5462 Patients with Primary Anterior Cruciate Ligament Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 381–388. [Google Scholar] [CrossRef]
- Collings, T.J.; Diamond, L.E.; Barrett, R.S.; Timmins, R.G.; Hickey, J.T.; Du Moulin, W.S.; Gonçalves, B.A.M.; Cooper, C.; Bourne, M.N. Impact of Prior Anterior Cruciate Ligament, Hamstring or Groin Injury on Lower Limb Strength and Jump Kinetics in Elite Female Footballers. Phys. Ther. Sport 2021, 52, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Beischer, S.; Gustavsson, L.; Senorski, E.H.; Karlsson, J.; Thomeé, C.; Samuelsson, K.; Thomeé, R. Young Athletes Who Return to Sport Before 9 Months After Anterior Cruciate Ligament Reconstruction Have a Rate of New Injury 7 Times That of Those Who Delay Return. J. Orthop. Sports Phys. Ther. 2020, 50, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Forelli, F.; Nguyen, C.; Mazeas, J.; Kakavas, G.; Hewett, T.E.; Bjerregaard, A. The Effect of Blood Flow Restriction Training on Quadriceps Activity After Anterior Cruciate Ligament Reconstruction: A Preliminary Randomized Controlled Trial. Marshall J. Med. 2024, 10, 5. [Google Scholar] [CrossRef]
- Moiroux--Sahraoui, A.; Forelli, F.; Mazeas, J.; Rambaud, A.J.; Bjerregaard, A.; Riera, J. Quadriceps Activation After Anterior Cruciate Ligament Reconstruction: The Early Bird Gets the Worm! Int. J. Sports Phys. Ther. 2024, 19, 1044–1051. [Google Scholar] [CrossRef]
- Forelli, F.; Riera, J.; Marine, P.; Gaspar, M.; Memain, G.; Miraglia, N. Implementing Velocity-Based Training to Optimize Return to Sprint After Anterior Cruciate Ligament Reconstruction in Soccer Players: A Clinical Commentary. Int. J. Sports Phys. Ther. 2024, 19, 355–365. [Google Scholar] [CrossRef]
- Grondin, J.; Crenn, V.; Gernigon, M.; Quinette, Y.; Louguet, B.; Menu, P.; Fouasson-Chailloux, A.; Dauty, M. Relevant Strength Parameters to Allow Return to Running after Primary Anterior Cruciate Ligament Reconstruction with Hamstring Tendon Autograft. Int. J. Environ. Res. Public. Health 2022, 19, 8245. [Google Scholar] [CrossRef]
- Nomura, Y.; Kuramochi, R.; Fukubayashi, T. Evaluation of Hamstring Muscle Strength and Morphology after Anterior Cruciate Ligament Reconstruction. Scand. J. Med. Sci. Sports 2015, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Timmins, R.; Bourne, M.; Shield, A.; Williams, M.; Lorenzen, C.; Opar, D. Biceps Femoris Architecture and Strength in Athletes with a Previous Anterior Cruciate Ligament Reconstruction. Med. Sci. Sports Exerc. 2016, 48, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E. Intra-and Inter-Muscular Variations in Hamstring Architecture and Mechanics and Their Implications for Injury: A Narrative Review. Sports Med. 2018, 48, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.A.; Rush, J.L.; Glaviano, N.R.; Norte, G.E. Hamstrings Muscle Morphology after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1733–1750. [Google Scholar] [CrossRef]
- Makihara, Y.; Nishino, A.; Fukubayashi, T.; Kanamori, A. Decrease of Knee Flexion Torque in Patients with ACL Reconstruction: Combined Analysis of the Architecture and Function of the Knee Flexor Muscles. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 310–317. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Kellis, E.; Galanis, N.; Vasilios, B. Semitendinosus Muscle Architecture during Maximum Isometric Contractions in Individuals with Anterior Cruciate Ligament Reconstruction and Controls. Muscles Ligaments Tendons J. 2017, 7, 147. [Google Scholar] [CrossRef]
- Baumgart, C.; Welling, W.; Hoppe, M.W.; Freiwald, J.; Gokeler, A. Angle-Specific Analysis of Isokinetic Quadriceps and Hamstring Torques and Ratios in Patients after ACL-Reconstruction. BMC Sports Sci. Med. Rehabil. 2018, 10, 23. [Google Scholar] [CrossRef]
- Hart, L.M.; Izri, E.; King, E.; Daniels, K.A. Angle-specific Analysis of Knee Strength Deficits after ACL Reconstruction with Patellar and Hamstring Tendon Autografts. Scand. J. Med. Sci. Sports 2022, 32, 1781–1790. [Google Scholar] [CrossRef]
- Kannus, P.; Järvinen, M.; Lehto, M. Maximal Peak Torque as a Predictor of Angle-Specific Torques of Hamstring and Quadriceps Muscles in Man. Eur. J. Appl. Physiol. 1991, 63, 112–118. [Google Scholar] [CrossRef]
- Read, P.J.; Trama, R.; Racinais, S.; McAuliffe, S.; Klauznicer, J.; Alhammoud, M. Angle Specific Analysis of Hamstrings and Quadriceps Isokinetic Torque Identify Residual Deficits in Soccer Players Following ACL Reconstruction: A Longitudinal Investigation. J. Sports Sci. 2022, 40, 871–877. [Google Scholar] [CrossRef]
- Huang, H.; Guo, J.; Yang, J.; Jiang, Y.; Yu, Y.; Müller, S.; Ren, G.; Ao, Y. Isokinetic Angle-Specific Moments and Ratios Characterizing Hamstring and Quadriceps Strength in Anterior Cruciate Ligament Deficient Knees. Sci. Rep. 2017, 7, 7269. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Control Group (n = 20) | Test Group at 3 Months (n = 20) | Test Group at 6 Months (n = 20) | p-Value |
|---|---|---|---|---|
| Age (years) | 27 (±5.46) | 26.25 (±8.14) | 26.50 (±8.14) | 0.64 |
| BMI (kg/m2) | 24.05 (±3.57) | 23.50 (±3.30) | 23.50 (±3.30) | 0.53 |
| Sex (Male/Female) | Male: 10/Female: 10 | Male: 10/ Female: 10 | Male: 10/ Female: 10 | 0.89 |
| Side Tested (Left/Right) | Left: 9/Right: 11 | Left: 9/Right: 11 | Left: 9/Right: 11 | 0.78 |
| Interval Between Surgery and Assessment (Months) | Ø | 3.37 (±0.42) | 6.21 (±0.40) | Ø |
| Time Point | Control Group (n = 20) | Test Group (n = 20) | p-Value |
|---|---|---|---|
| MEA (°) 3 Months Post-Surgery | 36.4 ± 12.0 | 26.3 ± 8.2 | 0.0019 |
| MEA (°) 6 Months Post-Surgery | 36.4 ± 12.0 | 28.2° ± 9.4 | 0.037 |
| Time Point | Test Group at 3 Months (n = 20) | Test Group at 6 Months (n = 20) | p-Value |
|---|---|---|---|
| MEA (°) | 26.3 ± 8.2 | 28.2 ± 9.4 | 0.089 |
| Time Point | Control Group (n = 20) | Test Group (n = 20) | p-Value |
|---|---|---|---|
| HPT (Nm) 3 Months Post-Surgery | 73.9 ± 36.7 | 55.4 ± 34.5 | 0.081 |
| HPT (Nm) 6 Months Post-Surgery | 73.9 ± 36.7 | 80.3 ± 30.6 | 0.067 |
| Time Point | Test Group at 3 Months (n = 20) | Test Group at 6 Months (n = 20) | p-Value |
|---|---|---|---|
| HPT (Nm) | 55.4 ± 34.5 | 80.3 ± 30.6 | 0.0094 |
| Time Point | Control Group (n = 20) | Test Group (n = 20) | p-Value |
|---|---|---|---|
| H/Q Ratio (%) 3 Months Post-Surgery | 63 ± 14 | 51 ± 12 | 0.027 |
| H/Q Ratio (%) 6 Months Post-Surgery | 63 ± 14 | 56 ± 13 | 0.034 |
| Time Point | Test Group at 3 Months (n = 20) | Test Group at 6 Months (n = 20) | p-Value |
|---|---|---|---|
| H/Q Ratio (%) | 51 ± 12 | 56 ± 13 | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzekraoui Alaoui, I.; Moiroux-Sahraoui, A.; Mazeas, J.; Kakavas, G.; Biały, M.; Douryang, M.; Forelli, F. Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study. Bioengineering 2025, 12, 465. https://doi.org/10.3390/bioengineering12050465
Bouzekraoui Alaoui I, Moiroux-Sahraoui A, Mazeas J, Kakavas G, Biały M, Douryang M, Forelli F. Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study. Bioengineering. 2025; 12(5):465. https://doi.org/10.3390/bioengineering12050465
Chicago/Turabian StyleBouzekraoui Alaoui, Ismail, Ayrton Moiroux-Sahraoui, Jean Mazeas, Georgios Kakavas, Maciej Biały, Maurice Douryang, and Florian Forelli. 2025. "Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study" Bioengineering 12, no. 5: 465. https://doi.org/10.3390/bioengineering12050465
APA StyleBouzekraoui Alaoui, I., Moiroux-Sahraoui, A., Mazeas, J., Kakavas, G., Biały, M., Douryang, M., & Forelli, F. (2025). Impact of Hamstring Graft on Hamstring Peak Torque and Maximum Effective Angle After Anterior Cruciate Ligament Reconstruction: An Exploratory and Preliminary Study. Bioengineering, 12(5), 465. https://doi.org/10.3390/bioengineering12050465








