A Comparison of Machine Learning-Based Models and a Simple Clinical Bedside Tool to Predict Morbidity and Mortality After Gastrointestinal Cancer Surgery in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Data Sources
2.3. Patients’ Characteristics, Preoperative Variables, and Objectives of This Study
2.4. Statistical Analysis
3. Results
3.1. Study Population
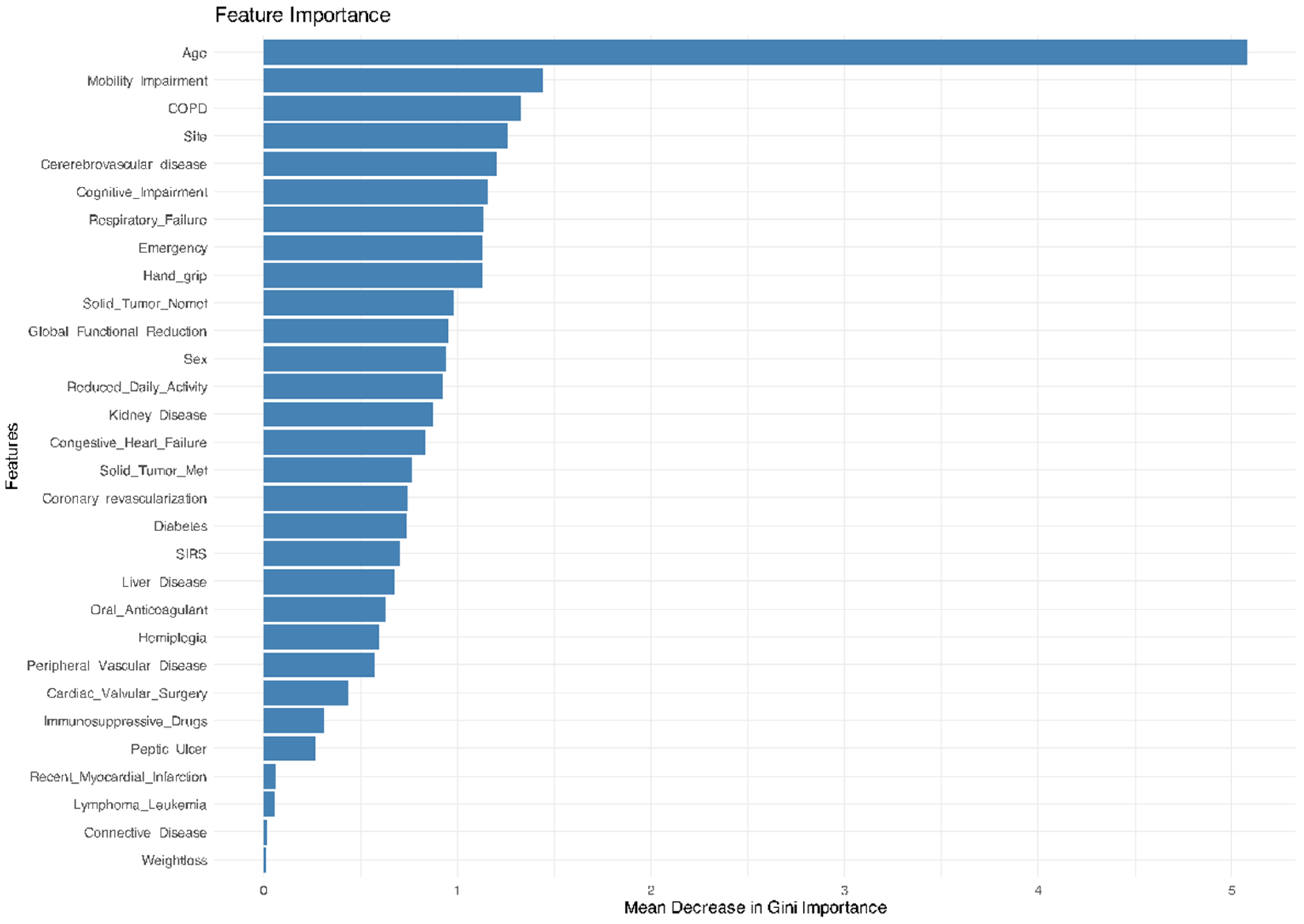
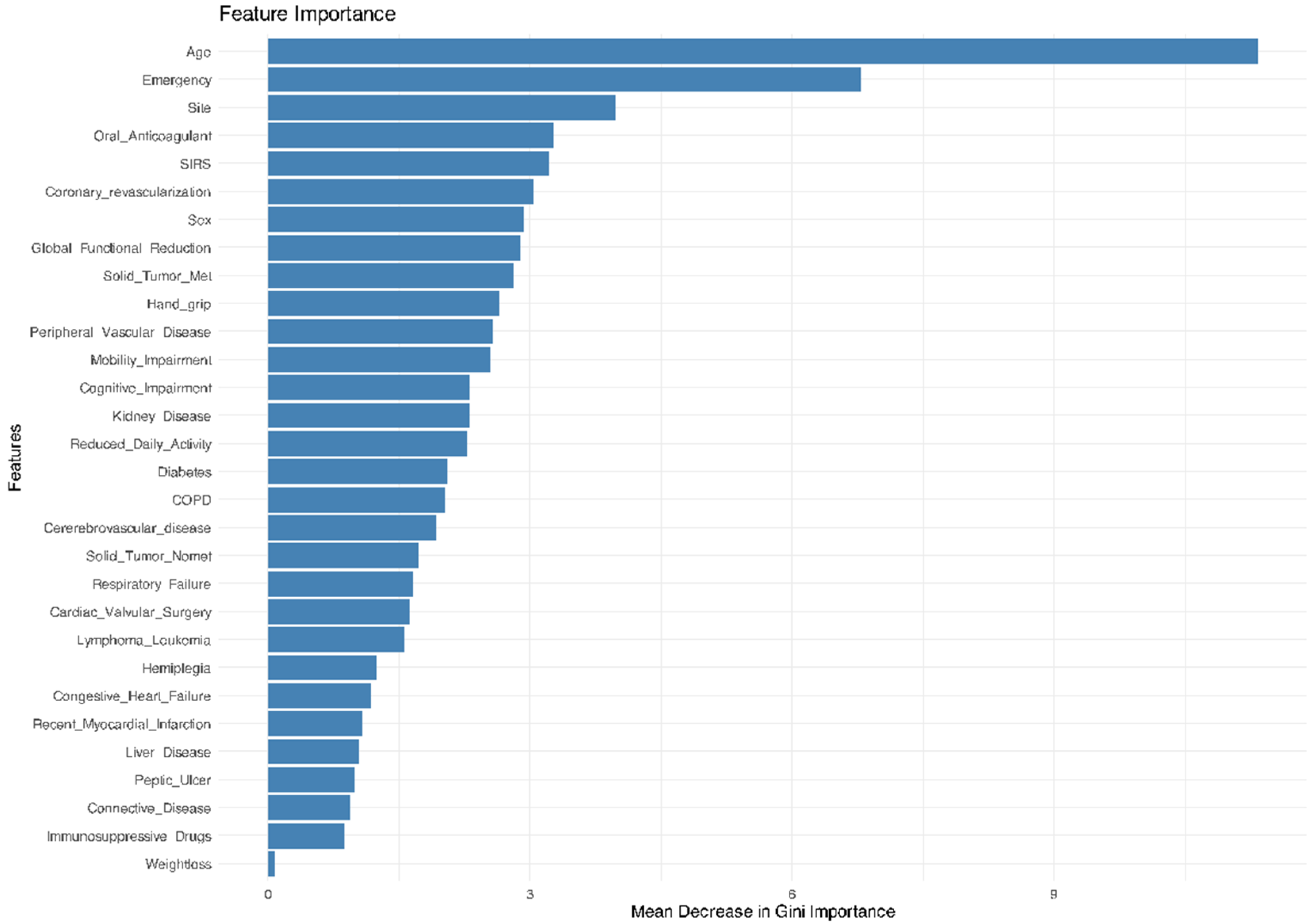
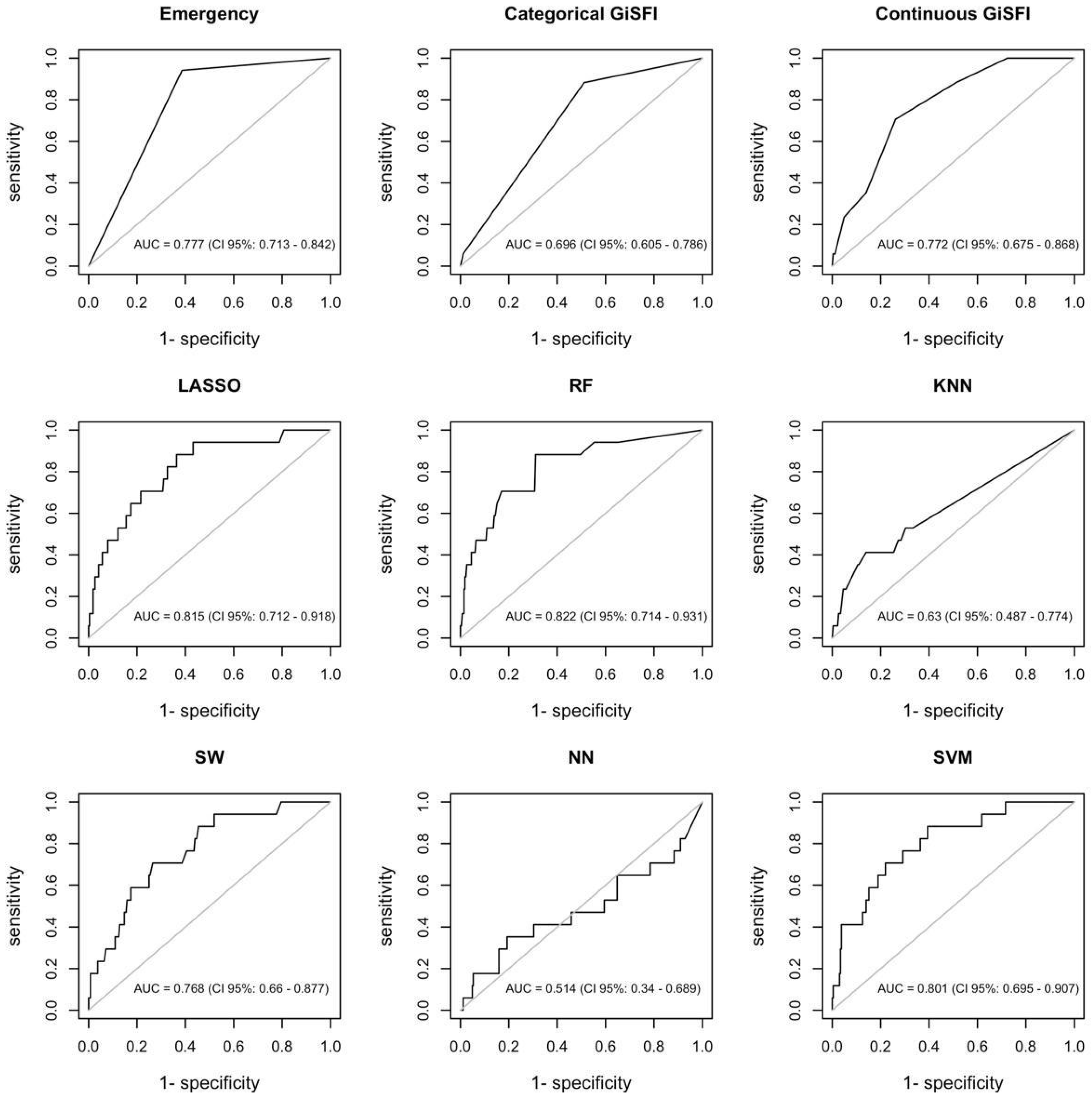
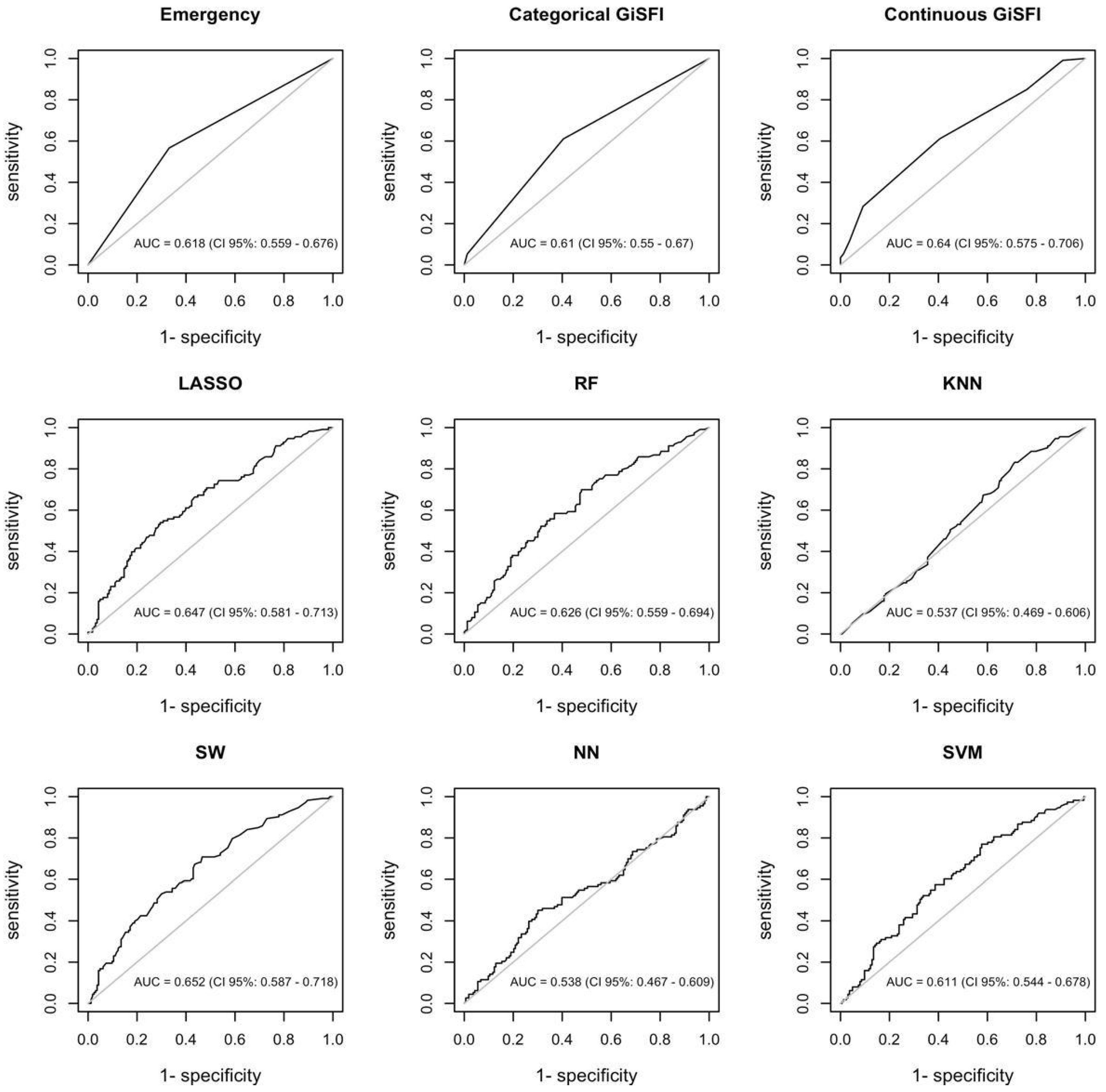
3.2. Machine Learning Algorithms’ Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurostat. Number of Surgical Operations and Procedures; European Union: Bruxelles, Belgium, 2024. [Google Scholar]
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical Risk Factors, Morbidity, and Mortality in Elderly Patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef]
- Al Saedi, A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. CIA 2019, 14, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Taljaard, M.; Bryson, G.L.; Beaulé, P.E.; Gagné, S.; Hamilton, G.; Hladkowicz, E.; Huang, A.; Joanisse, J.A.; Lavallée, L.T.; et al. Frailty as a Predictor of Death or New Disability After Surgery: A Prospective Cohort Study. Ann. Surg. 2020, 271, 283–289. [Google Scholar] [CrossRef]
- Lisk, R.; Uddin, M.; Parbhoo, A.; Yeong, K.; Fluck, D.; Sharma, P.; Lean, M.E.J.; Han, T.S. Predictive model of length of stay in hospital among older patients. Aging Clin. Exp. Res. 2019, 31, 993–999. [Google Scholar] [CrossRef]
- Costa, G.; Bersigotti, L.; Massa, G.; Lepre, L.; Fransvea, P.; Lucarini, A.; Mercantini, P.; Balducci, G.; Sganga, G.; Crucitti, A.; et al. The Emergency Surgery Frailty Index (EmSFI): Development and internal validation of a novel simple bedside risk score for elderly patients undergoing emergency surgery. Aging Clin. Exp. Res. 2021, 33, 2191–2201. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, E.-H.; Choi, I.-C. An ensemble machine learning approach to predict postoperative mortality in older patients undergoing emergency surgery. BMC Geriatr. 2023, 23, 262. [Google Scholar] [CrossRef]
- Dai, A.; Liu, H.; Shen, P.; Feng, Y.; Zhong, Y.; Ma, M.; Hu, Y.; Huang, K.; Chen, C.; Xia, H.; et al. Incorporating preoperative frailty to assist in early prediction of postoperative pneumonia in elderly patients with hip fractures: An externally validated online interpretable machine learning model. BMC Geriatr. 2024, 24, 472. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Mazzotta, A.D.; Burti, E.; Causio, F.A.; Orlandi, A.; Martinelli, S.; Longaroni, M.; Pinciroli, T.; Debs, T.; Costa, G.; Miccini, M.; et al. Machine Learning Approaches for the Prediction of Postoperative Major Complications in Patients Undergoing Surgery for Bowel Obstruction. JPM 2024, 14, 1043. [Google Scholar] [CrossRef] [PubMed]
- Fransvea, P.; Fransvea, G.; Liuzzi, P.; Sganga, G.; Mannini, A.; Costa, G. Study and validation of an explainable machine learning-based mortality prediction following emergency surgery in the elderly: A prospective observational study. Int. J. Surg. 2022, 107, 106954. [Google Scholar] [CrossRef]
- ERASO (Elderly Risk Assessment for Surgical Outcome) Collaborative Study Group; Costa, G.; Massa, G. Frailty and emergency surgery in the elderly: Protocol of a prospective, multicenter study in Italy for evaluating perioperative outcome (The FRAILESEL Study). Updates Surg. 2018, 70, 97–104. [Google Scholar] [CrossRef]
- Costa, G.; Fransvea, P.; Puccioni, C.; Giovinazzo, F.; Carannante, F.; Bianco, G.; Catamero, A.; Masciana, G.; Miacci, V.; Caricato, M.; et al. Gastro-intestinal emergency surgery: Evaluation of morbidity and mortality. Protocol of a prospective, multicenter study in Italy for evaluating the burden of abdominal emergency surgery in different age groups. (The GESEMM study). Front. Surg. 2022, 9, 927044. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hocking, R.R. A Biometrics Invited Paper. The Analysis and Selection of Variables in Linear Regression. Biometrics 1976, 32, 1–49. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Mucherino, A.; Papajorgji, P.J.; Pardalos, P.M. k-Nearest Neighbor Classification. In Data Mining in Agriculture; Springer Optimization and Its Applications; Springer: New York, NY, USA, 2009; Volume 34, pp. 83–106. ISBN 978-0-387-88614-5. [Google Scholar]
- McCulloch, W.S.; Pitts, W. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys 1943, 5, 115–133. [Google Scholar] [CrossRef]
- Hearst, M.A.; Dumais, S.T.; Osuna, E.; Platt, J.; Scholkopf, B. Support vector machines. IEEE Intell. Syst. Their Appl. 1998, 13, 18–28. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Klar, J. Goodness-of-Fit Testing for the Logistic Regression Model when the Estimated Probabilities are Small. Biom. J. 1988, 30, 911–924. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Dlima, S.D.; Hall, A.; Aminu, A.Q.; Akpan, A.; Todd, C.; Vardy, E.R.L.C. Frailty: A global health challenge in need of local action. BMJ Glob. Health 2024, 9, e015173. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.L.; Law, J.; Carter, B.; Hewitt, J.; Boyle, J.M.; Casey, P.; Maitra, I.; Farrell, I.S.; Pearce, L.; Moug, S.J. Frailty in Older Patients Undergoing Emergency Laparotomy: Results From the UK Observational Emergency Laparotomy and Frailty (ELF) Study. Ann. Surg. 2021, 273, 709–718. [Google Scholar] [CrossRef]
- Tan, H.L.; Chia, S.T.X.; Nadkarni, N.V.; Ang, S.Y.; Seow, D.C.C.; Wong, T.H. Frailty and functional decline after emergency abdominal surgery in the elderly: A prospective cohort study. World J. Emerg. Surg. 2019, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Fehlmann, C.A.; Patel, D.; McCallum, J.; Perry, J.J.; Eagles, D. Association between mortality and frailty in emergency general surgery: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2022, 48, 141–151. [Google Scholar] [CrossRef]
- Gao, J.; Merchant, A.M. A Machine Learning Approach in Predicting Mortality Following Emergency General Surgery. Am. Surg. 2021, 87, 1379–1385. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, M.; Dang, Y.; Lei, M.; Zhang, D. Machine Learning-Based Prediction of Postoperative Pneumonia Among Super-Aged Patients With Hip Fracture. CIA 2025, 20, 217–230. [Google Scholar] [CrossRef]
- Pean, C.A.; Buddhiraju, A.; Shimizu, M.R.; Chen, T.L.-W.; Esposito, J.G.; Kwon, Y.-M. Prediction of 30-Day Mortality Following Revision Total Hip and Knee Arthroplasty: Machine Learning Algorithms Outperform CARDE-B, 5-Item, and 6-Item Modified Frailty Index Risk Scores. J. Arthroplast. 2024, 39, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tozzi, F.; Ashraf Ganjouei, A.; Romero-Hernandez, F.; Feng, J.; Calthorpe, L.; Castro, M.; Davis, G.; Withers, J.; Zhou, C.; et al. Machine learning improves prediction of postoperative outcomes after gastrointestinal surgery: A systematic review and meta-analysis. J. Gastrointest. Surg. 2024, 28, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lei, Y.; Li, X.; Li, N.; Si, X.; Chen, C. A dynamic barycenter bridging network for federated transfer fault diagnosis in machine groups. Mech. Syst. Signal Process. 2025, 230, 112605. [Google Scholar] [CrossRef]
- Yang, B.; Lei, Y.; Li, N.; Li, X.; Si, X.; Chen, C. Balance recovery and collaborative adaptation approach for federated fault diagnosis of inconsistent machine groups. Knowl.-Based Syst. 2025, 317, 113480. [Google Scholar] [CrossRef]

| All | Elective Surgery | Emergency Surgery | |
|---|---|---|---|
| 937 | 559 | 378 | |
| Age | |||
| years, mean (SD) | 77.5 (7.1) | 77.1 (6.9) | 78.0 (7.5) |
| years, median (IQR) | 78 (72–83) | 77 (72–82) | 78 (72–84) |
| Gender, male, N (%) | 486 (51.9) | 293 (52.4) | 193 (51.1) |
| GiSFI, mean (SD) | 3.76 (1.70) | 3.59 (1.66) | 4.03 (1.71) |
| GiSFI, median (IQR) | 3 (3–5) | 3 (2–5) | 4 (3–5) |
| GiSFI 0–3, N (%) | 474 (50.6) | 297 (53.1) | 177 (46.8) |
| GiSFI 4–7, N (%) | 436 (46.5) | 250 (44.7) | 186 (49.2) |
| GiSFI ≥ 8, N (%) | 27 (2.9) | 12 (2.1) | 15 (4.0) |
| SIRS | 90 (9.6) | 0 (0.0) | 90 (23.8) |
| Recent myocardial infarction (>30 days) | 20 (2.1) | 12 (2.1) | 8 (2.1) |
| Congestive heart failure | 26 (2.8) | 10 (1.8) | 16 (4.2) |
| Coronary revascularization (PCI or CABG) | 169 (18.0) | 78 (14.0) | 91 (24.1) |
| Cardiac valvular surgery | 53 (5.7) | 26 (4.7) | 27 (7.1) |
| Arterial hypertension | 535 (57.1) | 306 (54.7) | 229 (60.6) |
| Peripheral vascular diseases | 94 (10.0) | 40 (7.2) | 54 (14.3) |
| Cerebrovascular diseases | 52 (5.5) | 18 (3.2) | 34 (9.0) |
| Oral anticoagulant | 124 (13.2) | 56 (10.0) | 68 (18.0) |
| COPD | 128 (13.7) | 65 (11.6) | 63 (16.7) |
| Chronic respiratory failure with home oxygen delivery | 37 (3.9) | 10 (1.8) | 27 (7.1) |
| Other non-metastatic solid tumors | 98 (10.5) | 7 (1.3) | 91 (24.1) |
| Other solid metastatic tumors | 128 (13.7) | 31 (5.5) | 97 (25.7) |
| Lymphoma/Leukemia | 20 (2.1) | 8 (1.4) | 12 (3.2) |
| Liver disease | 27 (2.9) | 10 (1.8) | 17 (4.5) |
| Kidney disease | 58 (6.2) | 15 (2.7) | 43 (11.4) |
| Diabetes | 199 (21.2) | 125 (22.4) | 74 (19.6) |
| Peptic ulcer | 23 (2.5) | 2 (0.4) | 21 (5.6) |
| Connective disease | 10 (1.1) | 8 (1.4) | 2 (0.5) |
| Immunosuppressive drugs | 13 (1.4) | 6 (1.1) | 7 (1.9) |
| Hemiplegia or other post-stroke sequelae | 26 (2.8) | 4 (0.7) | 22 (5.8) |
| Cognitive impairment | 75 (8.0) | 25 (4.5) | 50 (13.2) |
| Weight loss | 2 (0.2) | 2 (0.4) | 0 (0.0) |
| Reduced daily activity | 180 (19.2) | 66 (11.8) | 114 (30.2) |
| Mobility impairment (gait speed reduced or wheelchair) | 118 (12.6) | 41 (7.3) | 77 (20.4) |
| Impaired hand grip | 154 (16.4) | 9 (1.6) | 145 (38.4) |
| Reduction in global functional status | 152 (16.2) | 62 (11.1) | 90 (23.8) |
| Morbidity, N (%) | 335 (35.7) | 175 (31.3) | 160 (42.3) |
| Clavien-Dindo I | 104 (11.1) | 58 (10.4) | 46 (12.2) |
| Clavien-Dindo II | 151 (16.1) | 74 (13.2) | 77 (20.4) |
| Clavien-Dindo III | 57 (6.1) | 29 (5.2) | 28 (4.4) |
| Clavien-Dindo IV | 23 (2.4) | 14 (2.5) | 9 (2.4) |
| Mortality, N (%) | 57 (6.1) | 13 (2.3) | 44 (11.6) |
| Site | |||
| Large bowel | 600 (64.0) | 313 (56.0) | 287 (75.9) |
| Stomach | 266 (28.4) | 231 (41.3) | 35 (9.3) |
| Other | 71 (7.6) | 15 (2.7) | 56 (14.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezza, B.; Nurchis, M.C.; Capolupo, G.T.; Carannante, F.; De Prizio, M.; Rondelli, F.; Alunni Fegatelli, D.; Gili, A.; Lepre, L.; Costa, G. A Comparison of Machine Learning-Based Models and a Simple Clinical Bedside Tool to Predict Morbidity and Mortality After Gastrointestinal Cancer Surgery in the Elderly. Bioengineering 2025, 12, 544. https://doi.org/10.3390/bioengineering12050544
Frezza B, Nurchis MC, Capolupo GT, Carannante F, De Prizio M, Rondelli F, Alunni Fegatelli D, Gili A, Lepre L, Costa G. A Comparison of Machine Learning-Based Models and a Simple Clinical Bedside Tool to Predict Morbidity and Mortality After Gastrointestinal Cancer Surgery in the Elderly. Bioengineering. 2025; 12(5):544. https://doi.org/10.3390/bioengineering12050544
Chicago/Turabian StyleFrezza, Barbara, Mario Cesare Nurchis, Gabriella Teresa Capolupo, Filippo Carannante, Marco De Prizio, Fabio Rondelli, Danilo Alunni Fegatelli, Alessio Gili, Luca Lepre, and Gianluca Costa. 2025. "A Comparison of Machine Learning-Based Models and a Simple Clinical Bedside Tool to Predict Morbidity and Mortality After Gastrointestinal Cancer Surgery in the Elderly" Bioengineering 12, no. 5: 544. https://doi.org/10.3390/bioengineering12050544
APA StyleFrezza, B., Nurchis, M. C., Capolupo, G. T., Carannante, F., De Prizio, M., Rondelli, F., Alunni Fegatelli, D., Gili, A., Lepre, L., & Costa, G. (2025). A Comparison of Machine Learning-Based Models and a Simple Clinical Bedside Tool to Predict Morbidity and Mortality After Gastrointestinal Cancer Surgery in the Elderly. Bioengineering, 12(5), 544. https://doi.org/10.3390/bioengineering12050544







