Zirconia in Dental Implantology: A Review of the Literature with Recent Updates
Abstract
1. Introduction
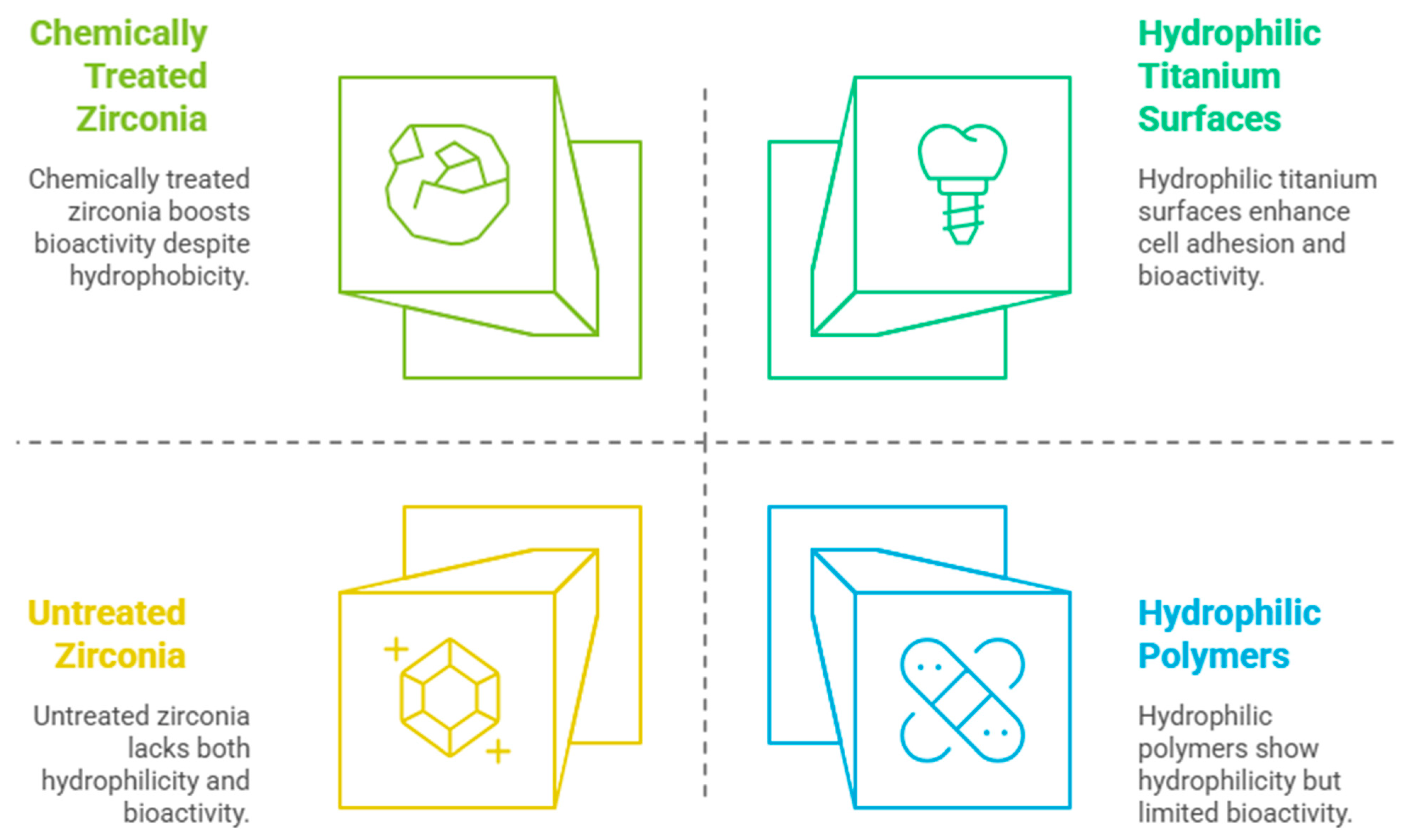
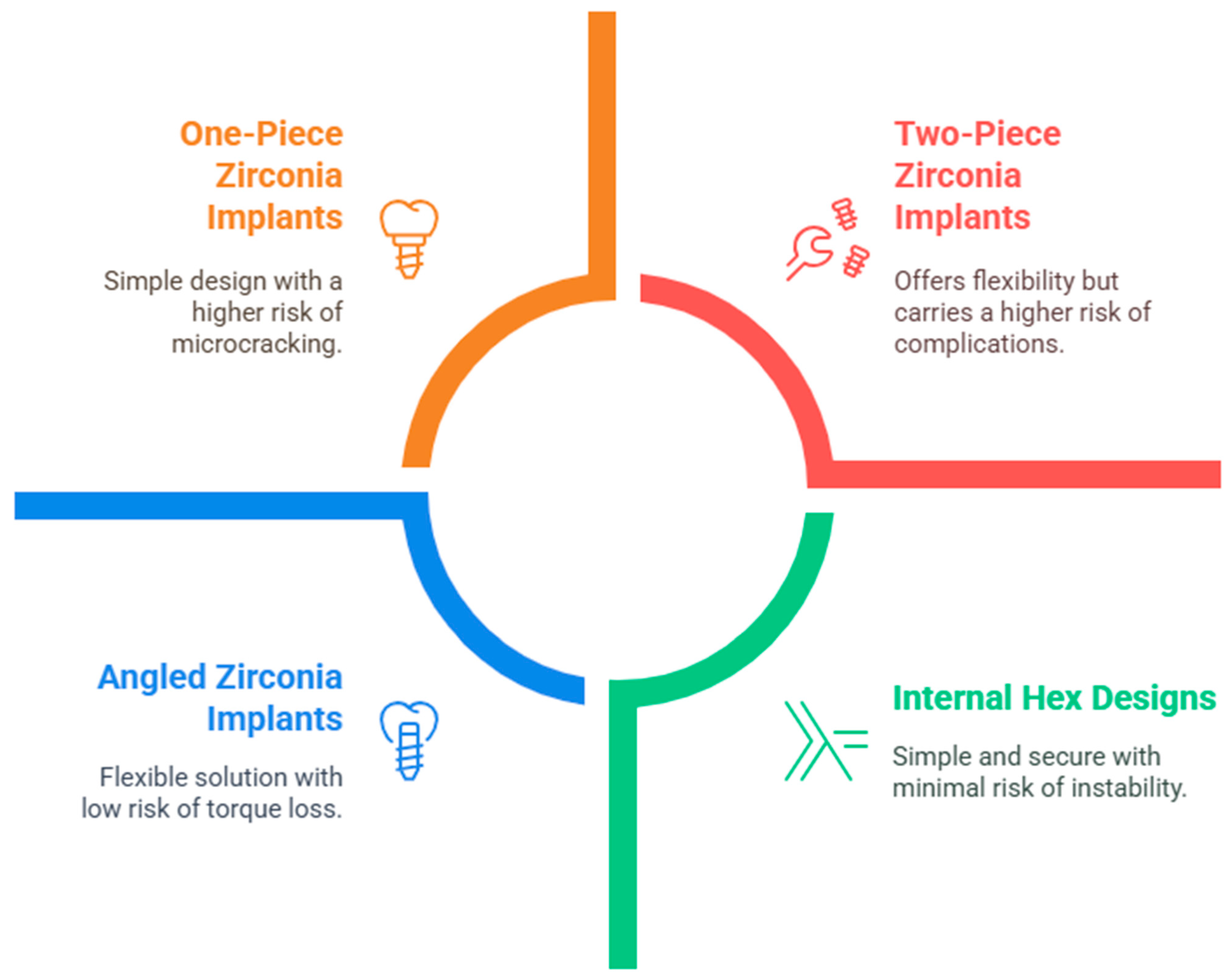
2. Literature Review and Discussion
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, C.; Wang, X. Advancements and challenges in the application of zirconia ceramics for dental restorations. Ceram. Silikáty 2024, 68, 610–623. [Google Scholar] [CrossRef]
- Aramouni, P.; Zebouni, E.; Tashkandi, E.; Dib, S.; Salameh, Z.; Almas, K. Fracture resistance and failure location of zirconium and metallic implant abutments. J. Contemp. Dent. Pract. 2008, 9, 41–48. [Google Scholar] [PubMed]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2022, 13, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Cheng, Z.; Zhang, W.; Tang, Y.; Yang, Z.; Wu, J.; Qiu, S. Temperature sensitivity and transition kinetics of uniform corrosion of zirconium alloys in superheated steam. Heliyon 2024, 10, e33266. [Google Scholar] [CrossRef]
- Saito, M.M.; Onuma, K.; Yamakoshi, Y. Nanoscale osseointegration of zirconia evaluated from the interfacial structure between ceria-stabilized tetragonal zirconia and cell-induced hydroxyapatite. J. Oral Biosci. 2024, 66, 281–287. [Google Scholar] [CrossRef]
- Tang, K.; Luo, M.L.; Zhou, W.; Niu, L.N.; Chen, J.H.; Wang, F. The integration of peri-implant soft tissues around zirconia abutments: Challenges and strategies. Bioact. Mater. 2023, 27, 348–361. [Google Scholar] [CrossRef]
- Abu Al-Faraj, T.M.; Alsubhi, B.M.; Almarhoon, A.N.; A Almarshoud, A.; Alqattan, M.S.; Alqahtani, S.H.; A Al Osaimi, A.; Alshammari, L.S.; I Almakrami, A.; Alwadai, Y.S. Comparison of Peri-Implant Soft Tissue Around Zirconia and Titanium Abutments in the Aesthetic Zone: A Narrative Review. Cureus 2024, 16, e65782. [Google Scholar] [CrossRef]
- Jabber, H.N.; Ali, R.; Al-Delfi, M.N. Monolithic Zirconia in Dentistry: Evolving Aesthetics, Dura-bility, and Cementation Techniques-An In-depth Review. Future 2023, 1, 26–36. [Google Scholar] [CrossRef]
- Jin, H.W.; Noumbissi, S.; Wiedemann, T.G. Comparison of Zirconia Implant Surface Modifications for Optimal Osseointegration. J. Funct. Biomater. 2024, 15, 91. [Google Scholar] [CrossRef]
- Thomas, A.; Sridhar, S.; Aghyarian, S.; Watkins-Curry, P.; Chan, J.Y.; Pozzi, A.; Rodrigues, D.C. Corrosion behavior of zirconia in acidulated phosphate fluoride. J. Appl. Oral. Sci. 2016, 24, 52–60. [Google Scholar] [CrossRef]
- Rohr, N.; Balmer, M.; Jung, R.E.; Kohal, R.J.; Spies, B.C.; Hämmerle, C.H.; Fischer, J. Influence of zirconia implant surface topography on first bone implant contact within a prospective cohort study. Clin. Implant. Dent. Relat. Res. 2021, 23, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 135–153. [Google Scholar] [CrossRef] [PubMed]
- Hafezeqoran, A.; Koodaryan, R. Effect of zirconia dental implant surfaces on bone integration: A systematic review and meta-analysis. BioMed Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.; Aboushelib, M. Bioactive–hybrid–zirconia implant surface for enhancing osseointegration: An in vivo study. Int. J. Implant. Dent. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Corrocher, G.; Dolci, M.; Censi, R.; Vavassori, V.; Maiorana, C. Clinical evaluation of zirconium dental implants placed in esthetic areas: A case series study. Eur. J. Esthet. Dent. 2013, 8, 532–545. [Google Scholar]
- Alshehri, M.; Alghamdi, M.; Alayad, A.S. Anatomical Shaping for Zirconia Custom Implant Abutment to Enhance Anterior Esthetic: A Clinical Technique. Int. J. Dent. 2020, 2020, 8857410. [Google Scholar] [CrossRef]
- Watkin, A.; Kerstein, R.B. Improving darkened anterior peri-implant tissue color with zirconia custom implant abutments. Compendium 2008, 29, 238–242. [Google Scholar]
- Spies, B.C.; Balmer, M.; Patzelt, S.B.M.; Vach, K.; Kohal, R.J. Clinical and patient-reported outcomes of a zirconia oral implant: Three-year results of a prospective cohort investigation. J. Dent. Res. 2015, 94, 1385–1391. [Google Scholar] [CrossRef]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long-lasting zirconia-based composites for dental implants. Part I: Innovative synthesis, microstructural characterization and in vitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef]
- Gutiérrez Robledo, N.; Punset Fuste, M.; Rodríguez-Contreras, A.; García Marro, F.; Manero Planella, J.M.; Figueras-Álvarez, O.; Roig Cayón, M. In Vitro Assessment of a New Block Design for Implant Crowns with Functional Gradient Fabricated with Resin Composite and Zirconia Insert. Materials 2024, 17, 3815. [Google Scholar] [CrossRef]
- Attard, L.; Lee, V.; Le, J.; Lowe, C.; Singh, V.; Zhao, J.; Sharma, D. Mechanical Factors Implicated in Zirconia Implant Fracture Placed within the Anterior Region—A Systematic Review. Dent. J. 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ding, Q.; Li, W.; Gu, R.; Zhang, P.; Zhang, L. Role and mechanism of a micro-/nano-structured porous zirconia surface in regulating the biological behavior of bone marrow mesenchymal stem cells. ACS Appl. Mater. Interfaces 2023, 15, 14019–14032. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, J.; Wu, X.; Li, Y.; Li, X.; Li, R.; Yu, Y. Mechanism of zirconia microgroove surface structure for osseointegration. Mater. Today Adv. 2021, 12, 100159. [Google Scholar] [CrossRef]
- Zhu, L.; Mori, Y.; Song, J.; Kuroda, K.; Okido, M.; Peng, C. Surface modification by pre-adsorption of proteins and polypeptides on Ti substrate with controlled hydrophilicity to improve biocompatibility. Mater. Today Commun. 2023, 37, 107124. [Google Scholar] [CrossRef]
- Illing, B.; Mohammadnejad, L.; Theurer, A.; Schultheiss, J.; Kimmerle-Mueller, E.; Rupp, F.; Krajewski, S. Biological Performance of Titanium Surfaces with Different Hydrophilic and Nanotopographical Features. Materials 2023, 16, 7307. [Google Scholar] [CrossRef]
- Tuna, T.; Wein, M.; Altmann, B.; Steinberg, T.; Fischer, J.; Att, W. Effect of Hydrogen Peroxide on the Surface and Attractiveness of Various Zirconia Implant Materials on Human Osteoblasts: An In Vitro Study. Materials. 2023, 16, 961. [Google Scholar] [CrossRef]
- Rohr, N.; Schönenberger, A.J.; Fischer, J. Influence of Surface Treatment and Accelerated Ageing on Biaxial Flexural Strength and Hardness of Zirconia. Materials 2023, 16, 910. [Google Scholar] [CrossRef]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef]
- Steinherr, T. Implant Materials in Focus: A Comprehensive Review of Zirconia vs. Titanium and Their Impact on Peri-Implant. Health. J. Oral. Med. Dent. Res. 2024, 5, 1–7. [Google Scholar] [CrossRef]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Random, aligned and grassy: Bioactivity and biofilm analysis of Zirconia nanostructures as dental implant modification. Compos. Part. B Eng. 2023, 259, 110725. [Google Scholar] [CrossRef]
- Chiou, L.L.; Panariello, B.H.; Hamada, Y.; Gregory, R.L.; Blanchard, S.; Duarte, S. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. BioMed Res. Int. 2023, 2023, 8728499. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Rosas, E.; de Souza, R.F.; Dias, F.J. Zirconia Implants: A Brief Review and Surface Analysis of a Lost Implant. Coatings 2024, 14, 995. [Google Scholar] [CrossRef]
- Zhang, F.; Monzavi, M.; Li, M.; Čokić, S.; Manesh, A.; Nowzari, H.; Van Meerbeek, B. Fracture analysis of one/two-piece clinically failed zirconia dental implants. Dent. Mater. 2022, 38, 1633–1647. [Google Scholar] [CrossRef]
- Sanon, C.; Chevalier, J.; Douillard, T.; Kohal, R.J.; Coelho, P.G.; Hjerppe, J.; Silva, N.R. Low temperature degradation and reliability of one-piece ceramic oral implants with a porous surface. Dent. Mater. 2013, 29, 389–397. [Google Scholar] [CrossRef]
- Cionca, N.; Müller, N.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: A prospective clinical study. Clin. Oral. Implant. Res. 2015, 26, 413–418. [Google Scholar] [CrossRef]
- AlTarawneh, S.; Limmer, B.; Reside, G.J.; Cooper, L. Dual jaw treatment of edentulism using implant-supported monolithic zirconia fixed prostheses. J. Esthet. Restor. Dent. 2015, 27, 63–70. [Google Scholar] [CrossRef]
- Khaledi, A.A.; Shalileh, S.; Hejazi, M.; Giti, R. Influence of screw channel angulation on reverse torque value and fracture resistance in monolithic zirconia restorations after thermomechanical cycling: An in-vitro study. BMC Oral. Health 2024, 24, 389. [Google Scholar] [CrossRef]
- Öztaş, B.; Kaya, D.I. Marginal Bone Loss and Clinical Evaluation of Angled Implants: A Retrospective Study. NEU Dent J. 2024, 3, 60–69. [Google Scholar] [CrossRef]
- Conejo, J.; Han, S.; Atria, P.J.; Stone-Hirsh, L.; Dubin, J.; Blatz, M.B. Full digital workflow to resolve angled adjacent dental implants: A dental technique. J. Prosthet. Dent. 2024, 132, 306–309. [Google Scholar] [CrossRef]
- Shrivastava, D.; Quadri, S.A.; Alshadidi, A.A.F.; Saini, R.; Dewan, M.; Fernandes, G.V.O.; Srivastava, K.C. Clinical Assessment of the Relationship of Dental Implant Materials (Titanium and Zirconia) and Peri-Implantitis: A Systematic Review. J. Maxillofac. Oral Surg. 2024, 1–19. [Google Scholar] [CrossRef]
- Jaber, M.A.; Jaber, A.M.; Elameen, A.M.; Elameen, F.M. Evaluating the Efficacy, Durability, and Clinical Implications of Zirconia vs. Titanium Dental Implants: A Comprehensive Review. Preprints, 2024. [Google Scholar] [CrossRef]
- Kyung, K.Y.; Park, J.M.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Ahn, J.S.; Yi, Y. Comparative analysis of flexural strength of 3D printed and milled 4Y-TZP and 3Y-TZP zirconia. J. Prosthet. Dent. 2024, 131, 529.e1–529.e9. [Google Scholar] [CrossRef]
- Wiedemann, T. Clinical guideline for zirconia dental implants: A comprehensive and critical review and update. J. Clin. Med. Res. 2024, 5, 1–7. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Nuvvula, S.; Matinllina, J.P.; Yiu, C.K.Y.; King, N.M. Biocompatibility of Various Dental Materials of Contemporary Dentistry: A Narrative Insight. J. Investig. Clin. Dent. 2013, 4, 9–19. [Google Scholar] [CrossRef]
- Beekmans, D.G.; Beekmans, R.M.N.; Cune, M.S. The pink and white aesthetics of a new zirconia implant. Ned. Tijdschr. Voor Tandheelkd. 2018, 125, 389–395. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Alghauli, M.A.; Aljohani, M.H.A.; Zafar, M.S. Advanced additive manufacturing in implant dentistry: 3D printing technologies, printable materials, current applications and future requirements. Bioprinting 2024, 42, e00356. [Google Scholar] [CrossRef]
- Oğuz, E.İ.; Kılıçarslan, M.A. Full-mouth reconstruction with Implant and Tooth-supported Zirconia Restorations in a Digital Workflow. Eur. Ann. Dent. Sci. 2021, 48, 119–123. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant. Dent. Relat. Res. 2005, 7 (Suppl. S1), S13–S20. [Google Scholar] [CrossRef]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral. Implant. Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.; Ramos-Oltra, M.; Negri, B.; Delgado-Ruíz, R.; Ramirez-Fernández, P.; Mate-Sánchez, J.; Abooud, M.; Albiol, J.G.; Nieto, M.S.; Romanos, G. Osseointegration of zirconia dental implants modified by femtosecond laser vs. zirconia implants in healed bone: A histomorphometric study in dogs with three-month follow-up. J. Osseointegr. 2013, 5, 39–44. [Google Scholar] [CrossRef]
- Mihatovic, I.; Golubovic, V.; Becker, J.; Schwarz, F. Bone tissue response to experimental zirconia implants. Clin. Oral. Investig. 2016, 21, 523–532. [Google Scholar] [CrossRef]
- Stübinger, S.; Homann, F.; Etter, C.; Miskiewicz, M.; Wieland, M.; Sader, R. Effect of Er:YAG, CO(2) and diode laser irradiation on surface properties of zirconia endosseous dental implants. Lasers Surg. Med. 2008, 40, 223–228. [Google Scholar] [CrossRef]
- Yasuno, K.; Kakura, K.; Taniguchi, Y.; Yamaguchi, Y.; Kido, H. Zirconia implants with laser surface treatment: Peri-implant bone response and enhancement of osseointegration. J. Hard Tissue Biol. 2014, 23, 93–100. [Google Scholar] [CrossRef]
- Att, W.; Takeuchi, M.; Suzuki, T.; Kubo, K.; Anpo, M.; Ogawa, T. Enhanced osteoblast function on ultraviolet light-treated zirconia. Biomaterials 2009, 30, 1273–1280. [Google Scholar] [CrossRef]
- Brezavšček, M.; Fawzy, A.; Bächle, M.; Tuna, T.; Fischer, J.; Att, W. The effect of UV treatment on the osteoconductive capacity of zirconia-based materials. Materials 2016, 9, 958. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral. Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, M.; Liao, Y.; Zhou, J.; Li, H.; Tan, J. Different behavior of human gingival fibroblasts on surface modified zirconia: A comparison between ultraviolet (UV) light and plasma. Dent. Mater. J. 2019, 38, 756–763. [Google Scholar] [CrossRef]
- Han, A.; Ding, H.; Tsoi, J.K.H.; Imazato, S.; Matinlinna, J.P.; Chen, Z. Prolonged UV-C irradiation is a double-edged sword on the zirconia surface. ACS Omega 2020, 5, 5126–5133. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.-P.; Kübler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head. Face Med. 2008, 4, 29. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.-C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head. Face Med. 2008, 4, 30. [Google Scholar] [CrossRef]
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In vitro biofilm formation on titanium and zirconia implant surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef]
- Stadlinger, B.; Hennig, M.; Eckelt, U.; Kuhlisch, E.; Mai, R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int. J. Oral. Maxillofac. Surg. 2010, 39, 585–592. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Biguetti, C.C.; Natarajan, S.; Arteaga, A.; El Allami, J.; Chandrashekar, B.L.; Garlet, G.P.; Rodrigues, D.C. Cellular and molecular dynamics during early oral osseointegration: A comprehensive characterization in the lewis rat. ACS Biomater. Sci. Eng. 2021, 7, 2392–2407. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; Helms, J.; Brunski, J. Relationship between primary/mechanical and secondary/biological implant stability. Int. J. Oral. Maxillofac. Implant. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Kohal, R.J.; Weng, D.; Bächle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef]
- Yamashita, D.; Machigashira, M.; Miyamoto, M.; Takeuchi, H.; Noguchi, K.; Izumi, Y.; Ban, S. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent. Mater. J. 2009, 28, 461–470. [Google Scholar] [CrossRef]
- Almarghlani, A.; Alshali, S. Survival and fracture rates of onepiece zirconia implants: A systematic review. Cardiometry 2022, 24, 175–181. [Google Scholar]
- Thompson, J.; Schoenbaum, T.R.; Pannu, D.; Knoernschild, K. Survival analysis of zirconia implant-supported, fixed complete dentures: A 5-year retrospective cohort study. J. Prosthet. Dent. 2025, 133, 790–795. [Google Scholar] [CrossRef]
- Kohal, R.J.; Spies, B.C.; Vach, K.; Balmer, M.; Pieralli, S. A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. J. Clin. Med. 2020, 9, 2585. [Google Scholar] [CrossRef]
- Bidra, A.S.; Tischler, M.; Patch, C. Survival of 2039 complete arch fixed implant-supported zirconia prostheses: A retrospective study. J. Prosthet. Dent. 2018, 119, 220–224. [Google Scholar] [CrossRef]
- Shukla, A.K.; Priyadarshi, M.; Kumari, N.; Singh, S.; Goswami, P.; Srivastava, S.B.; Makkad, R.S. Investigating the Long-Term Success and Complication Rates of Zirconia Dental Implants: A Prospective Clinical Study. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 1), S477–S479. [Google Scholar] [CrossRef]
- Bergemann, C.; Duske, K.; Nebe, J.B.; Schöne, A.; Bulnheim, U.; Seitz, H.; Fischer, J. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J. Mater. Sci. Mater. Med. 2015, 26, 5350. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral. Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Spies, B.C.; Stampf, S.; Kohal, R.J. Evaluation of Zirconia-Based All-Ceramic Single Crowns and Fixed Dental Prosthesis on Zirconia Implants: 5-Year Results of a Prospective Cohort Study. Clin. Implant. Dent. Relat. Res. 2015, 17, 1014–1028. [Google Scholar] [CrossRef]
- Kohal, R.J.; Burkhardt, F.; Chevalier, J.; Patzelt, S.B.M.; Butz, F. One-Piece Zirconia Oral Implants for Single Tooth Replacement: Five-Year Results from a Prospective Cohort Study. J. Funct. Biomater. 2023, 14, 116. [Google Scholar] [CrossRef]
- Lolos, D.; Mihali, S.G.; Dinu, S.; Mitariu, M.; Tudor, A.; Oancea, R. Retrospective Long-Term Survival Rate and Clinical Performance of Zirconium Oxide Restorations over the Past 5 Years: A Comparative Study Between Single Crowns and Fixed Dental Prostheses. Medicina 2025, 61, 210. [Google Scholar] [CrossRef]
- Spies, B.C.; Balmer, M.; Jung, R.E.; Sailer, I.; Vach, K.; Kohal, R.J. All-ceramic single crowns supported by zirconia implants: 5-year results of a prospective multicenter study. Clin. Oral. Implant. Res. 2019, 30, 466–475. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhuwayhi, S. Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering 2025, 12, 543. https://doi.org/10.3390/bioengineering12050543
Aldhuwayhi S. Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering. 2025; 12(5):543. https://doi.org/10.3390/bioengineering12050543
Chicago/Turabian StyleAldhuwayhi, Sami. 2025. "Zirconia in Dental Implantology: A Review of the Literature with Recent Updates" Bioengineering 12, no. 5: 543. https://doi.org/10.3390/bioengineering12050543
APA StyleAldhuwayhi, S. (2025). Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering, 12(5), 543. https://doi.org/10.3390/bioengineering12050543





