In Vivo Evaluation of the Anti-Skin-Ageing Bioactivity of a Recombinant Dual Humanised Collagen and Poly-L-Lactic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Expression and Purification of DuCol
2.3. Physical Characterisation of DuCol
2.4. In Vitro Biocompatibility and Bioactivity Evaluation
2.5. Ageing Model
2.6. Subcutaneous Injection of Implants
2.7. Histological Observation and Quantitative Analysis
2.8. Gel Electrophoresis and Western Blot
2.9. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterisation of DuCol
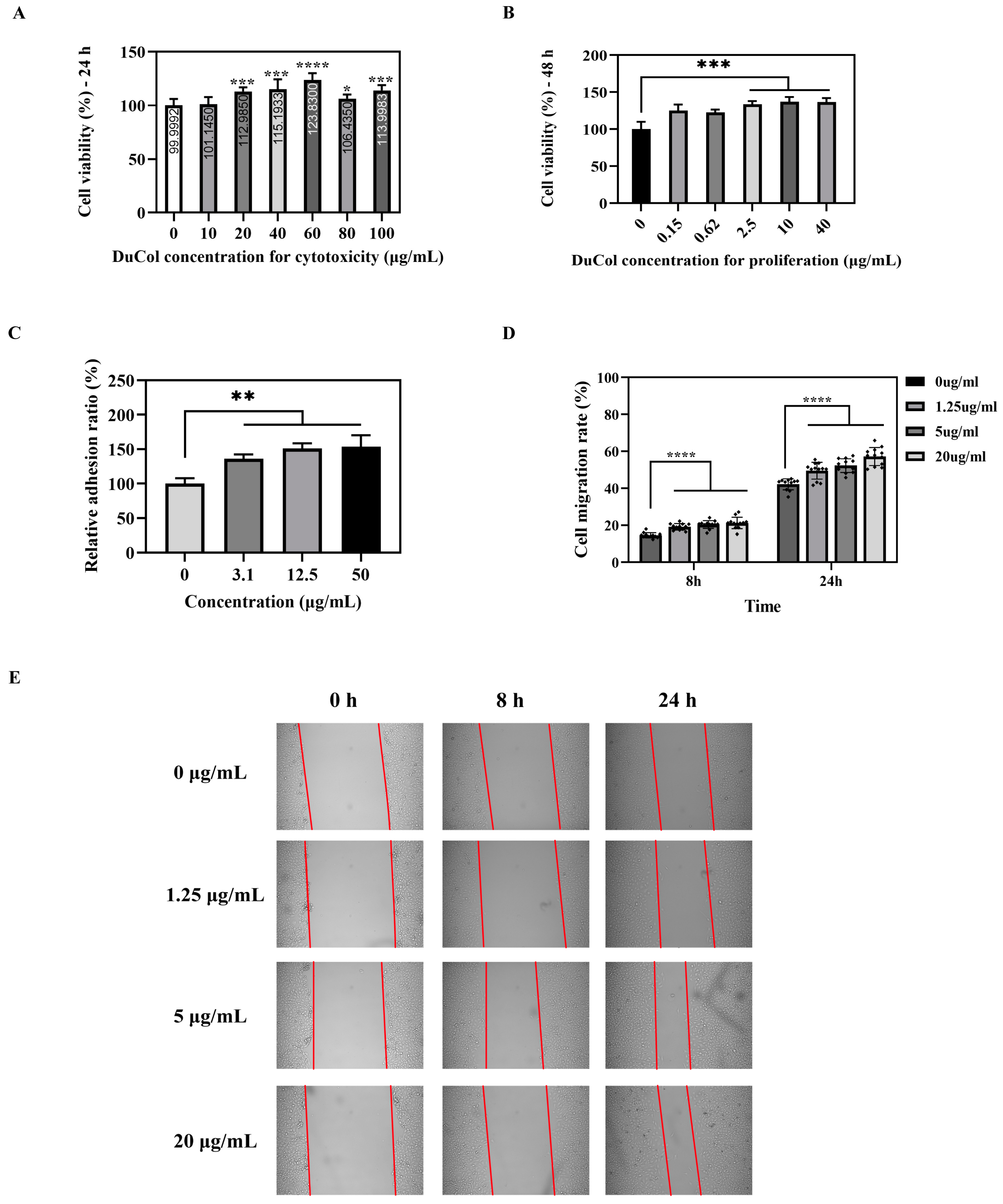
3.2. In Vitro Bioactivity Evaluation of DuCol
3.3. Establishment of the D-Galactose-Induced Ageing Model
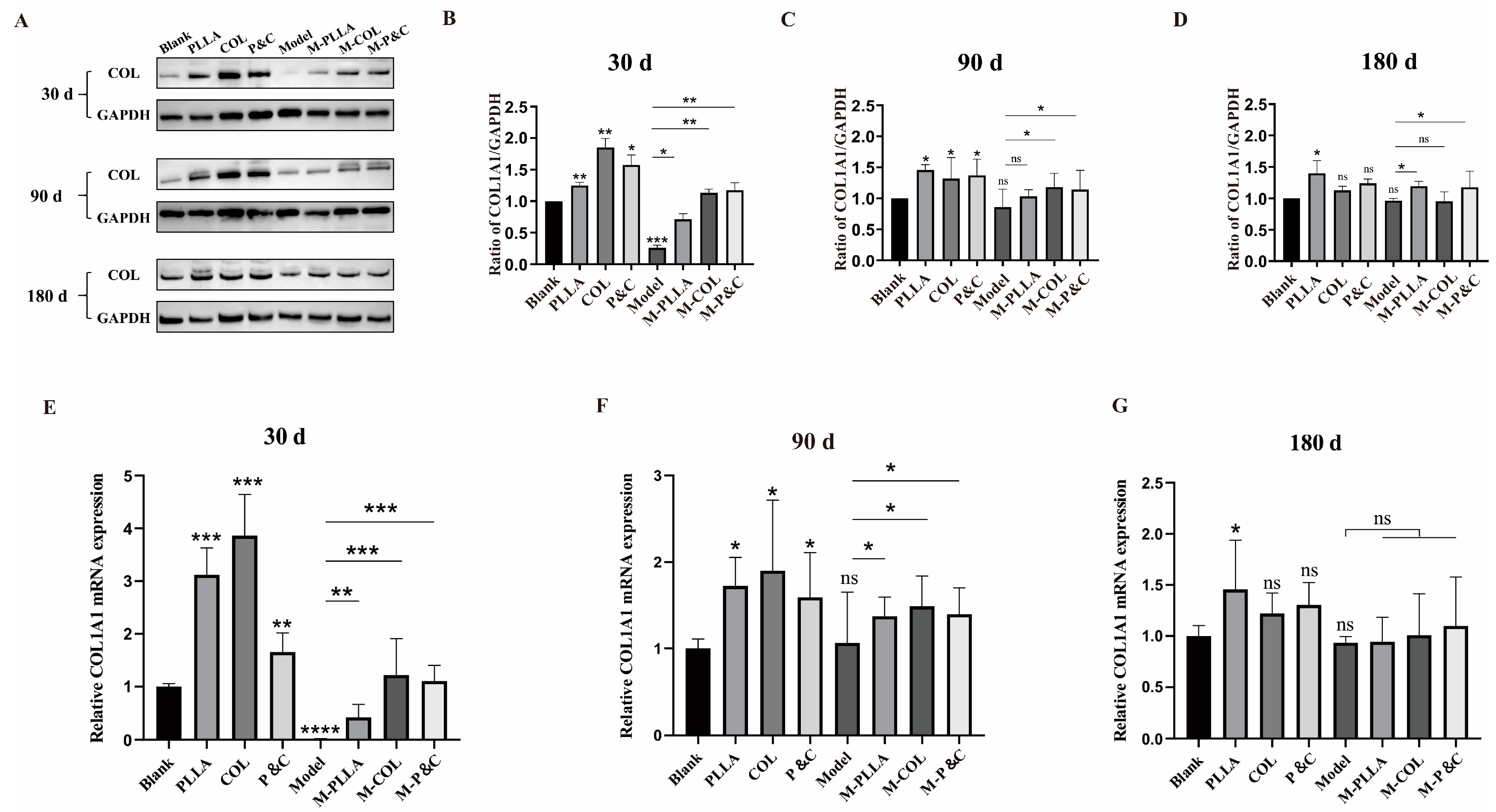
3.4. COL1A1 Expression Level in Skin
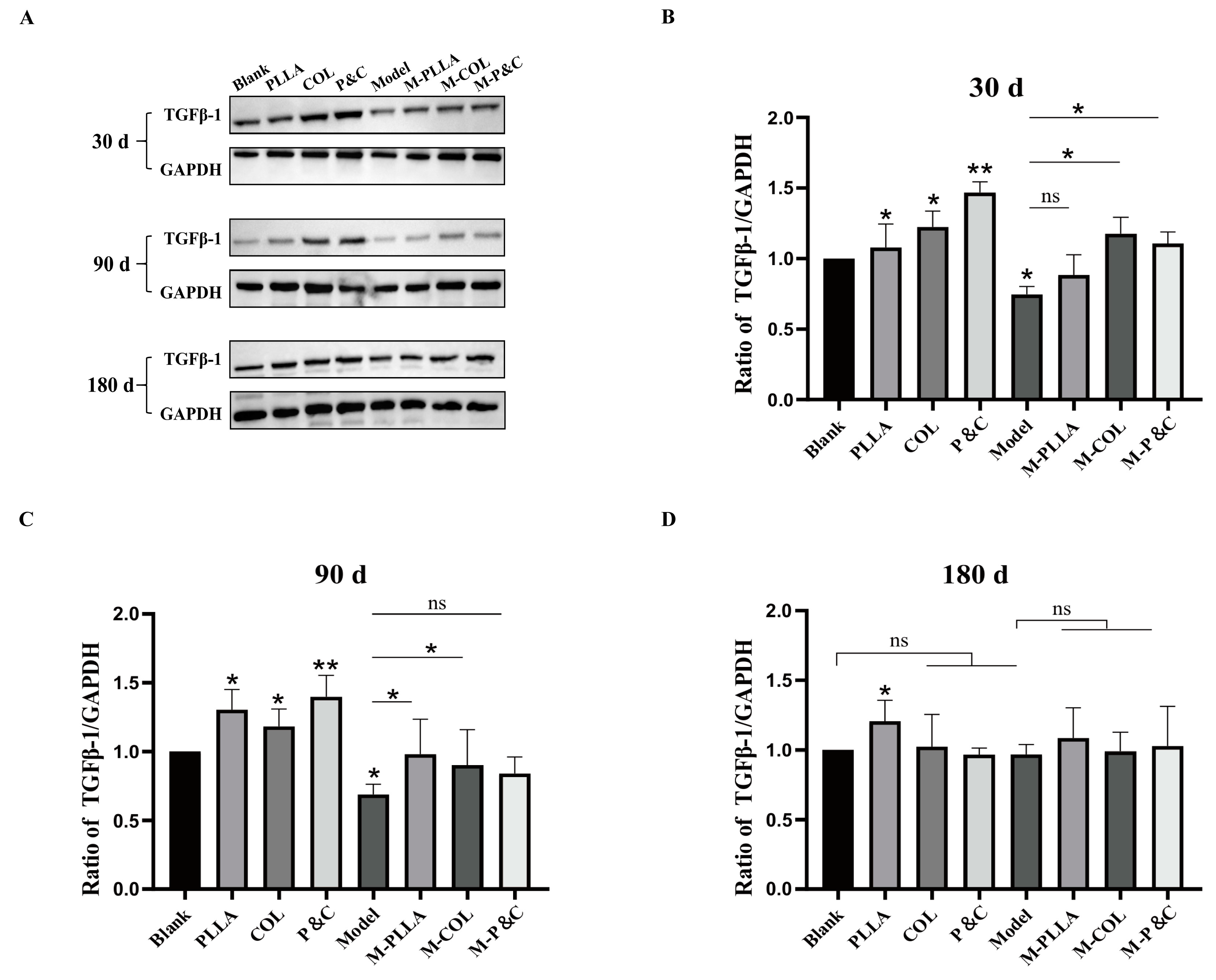
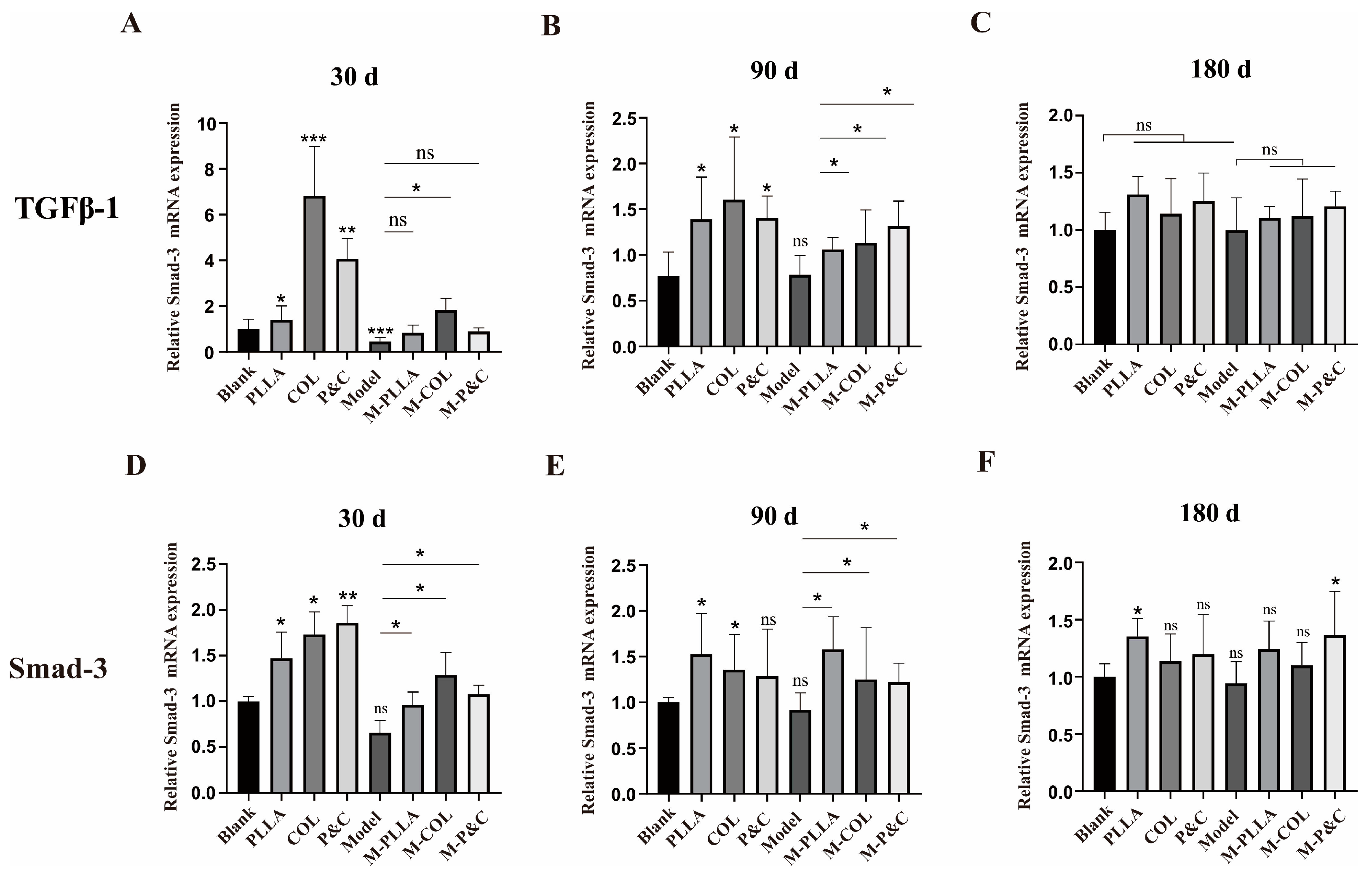
3.5. TGFβ-1 Expression Level in Skin
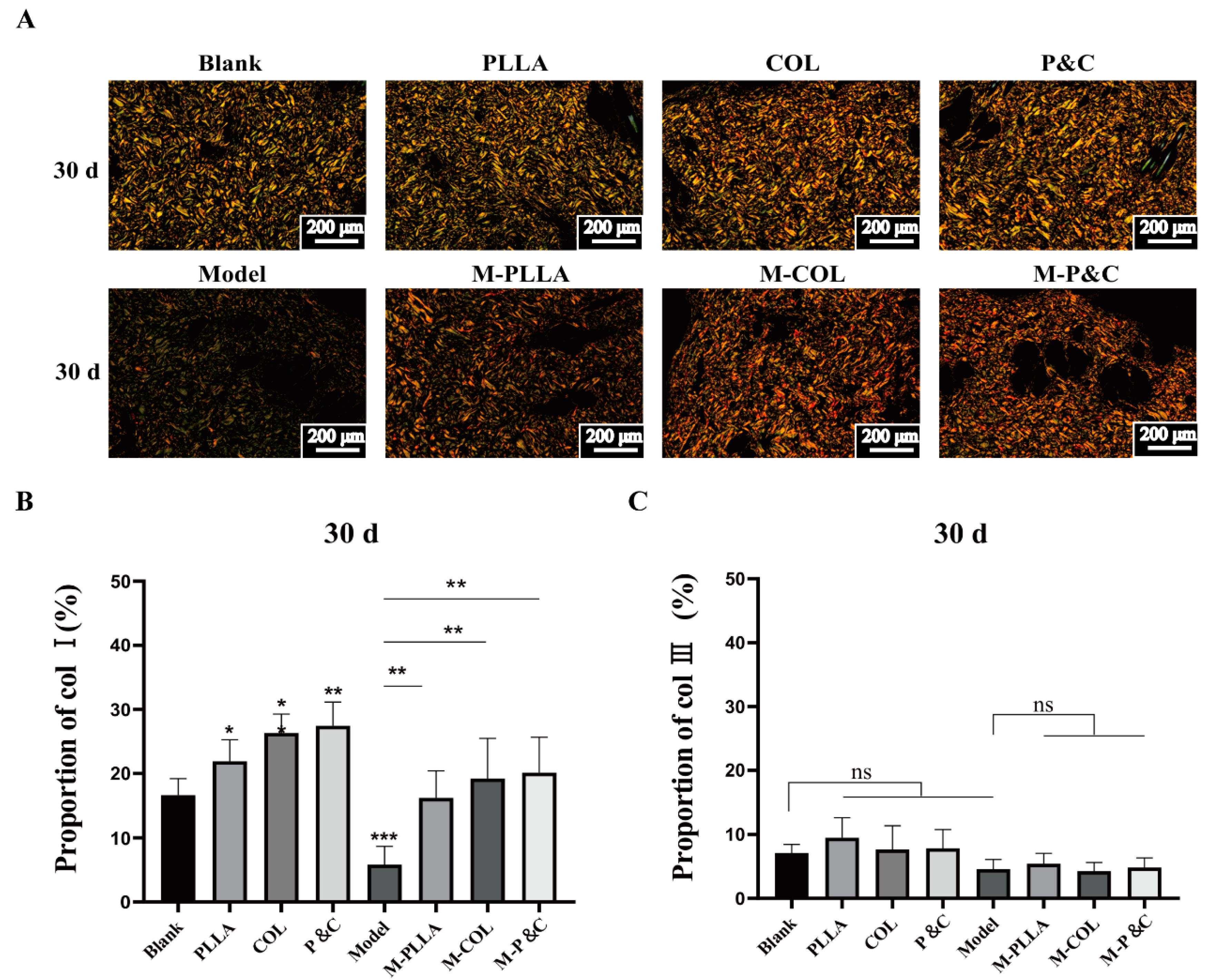
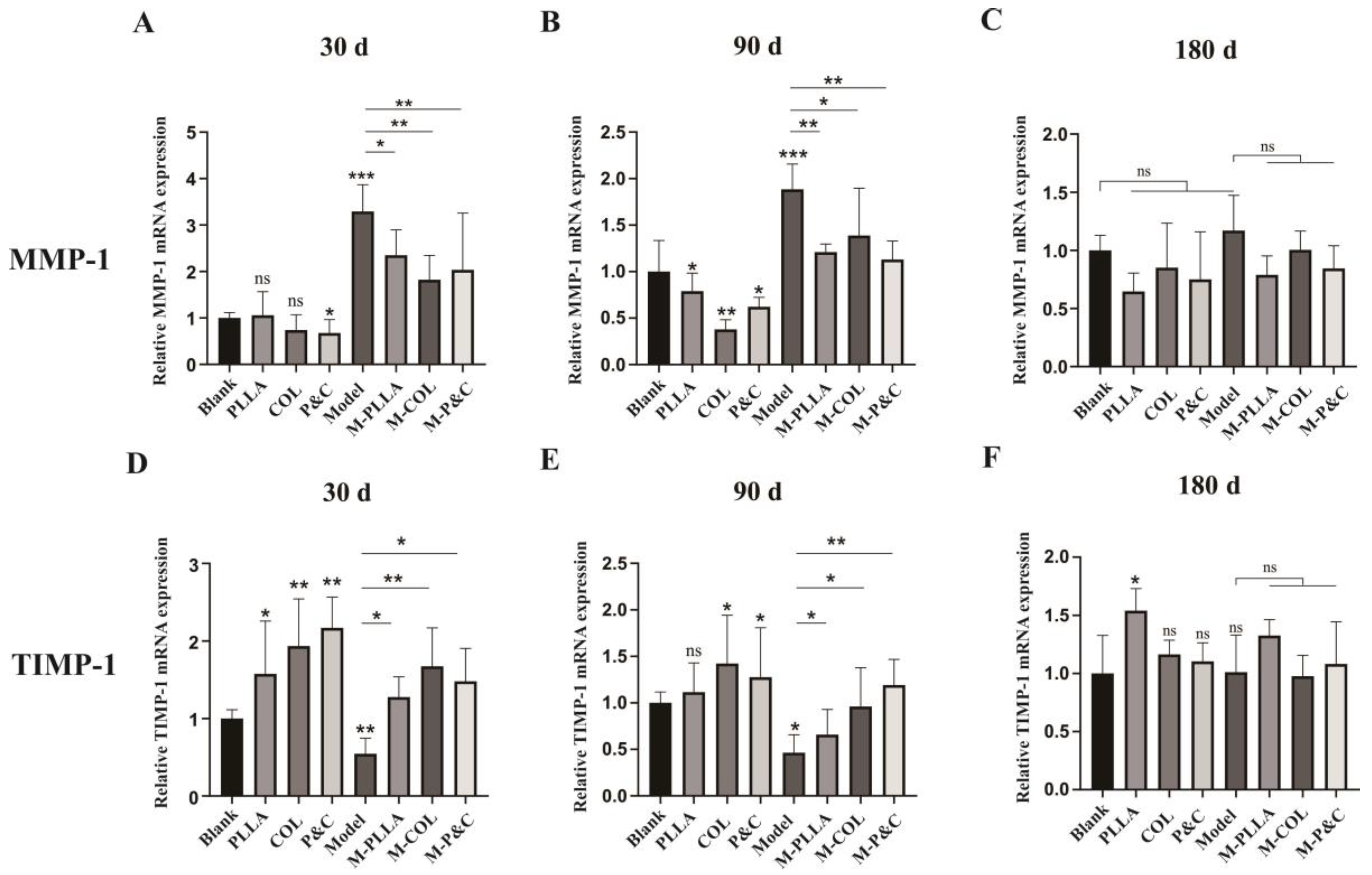
3.6. Collagen Degradation In Vivo
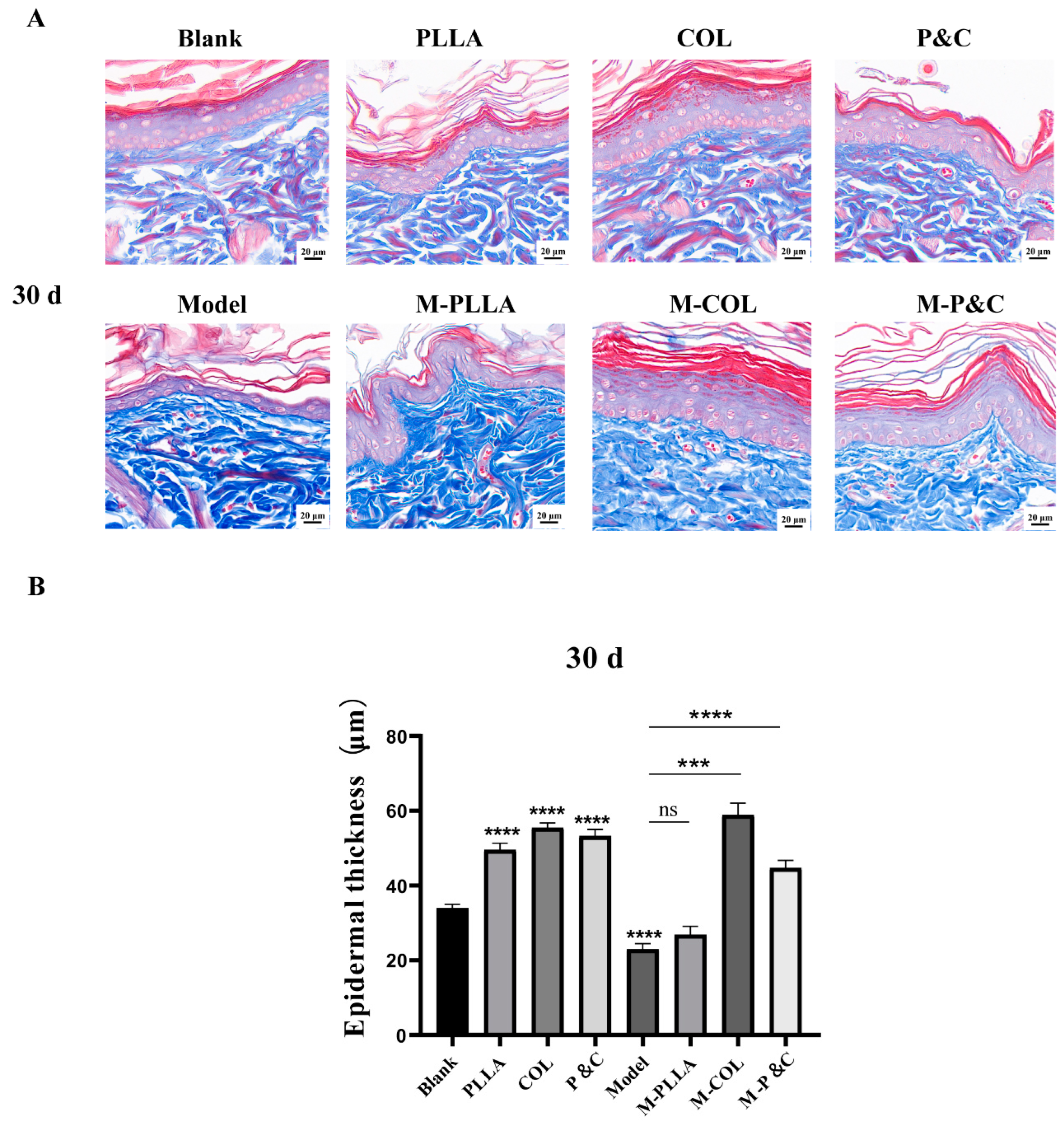
3.7. Epidermal Layer Thickness
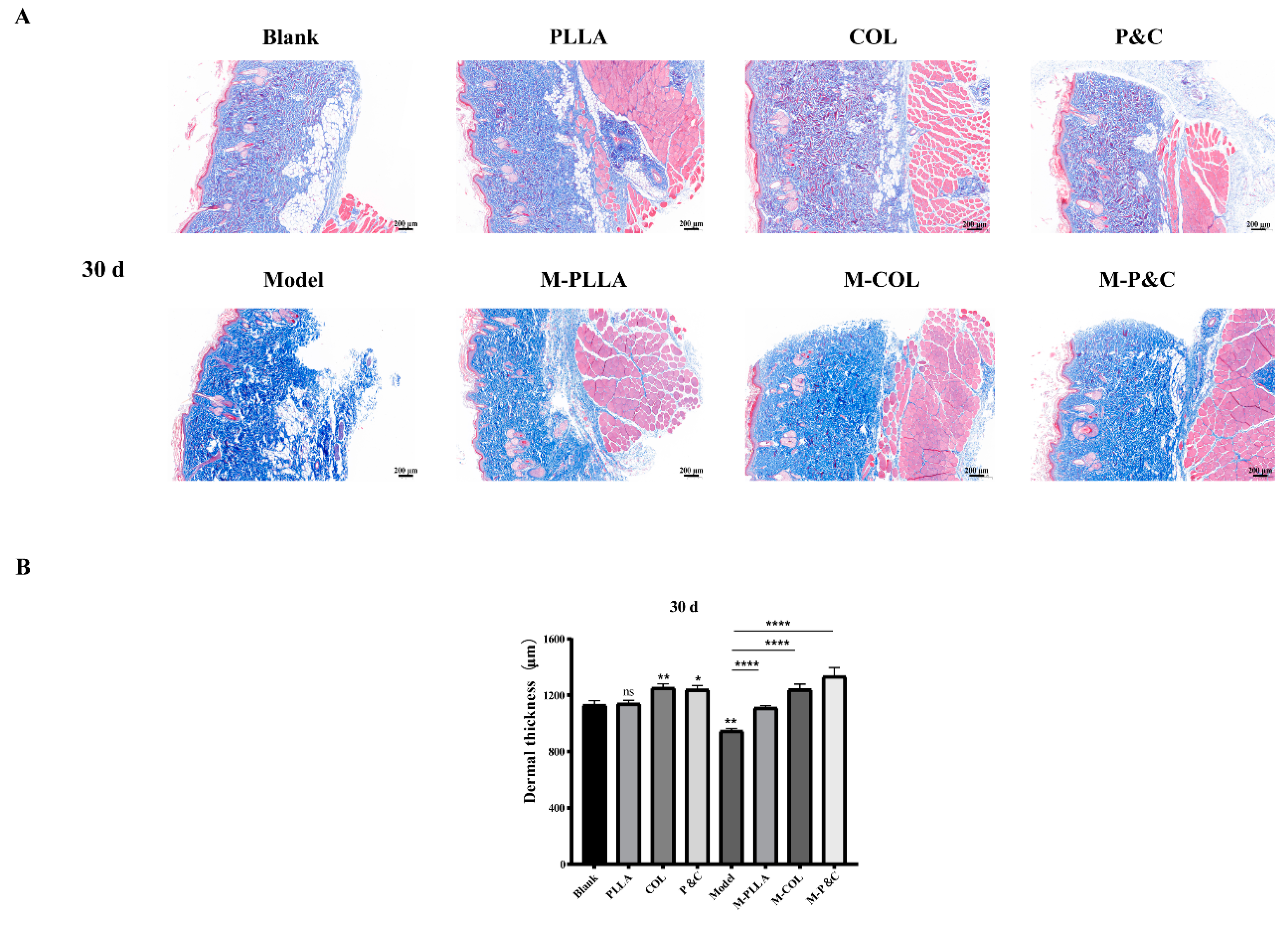
3.8. Dermal Layer Thickness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Qiu, H.; Xu, Y.; Gao, Y.; Tan, P.; Zhao, R.; Liu, Z.; Tang, Y.; Zhu, X.; Bao, C.; et al. The biological effect of recombinant humanized collagen on damaged skin induced by UV-photoaging: An in vivo study. Bioact. Mater. 2022, 11, 154–165. [Google Scholar] [CrossRef]
- Campos, L.D.; Santos Junior, V.A.; Pimentel, J.D.; Carregã, G.L.F.; Cazarin, C.B.B. Collagen supplementation in skin and orthopedic diseases: A review of the literature. Heliyon 2023, 9, e14961. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; G. Sotelo, C. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food chemistry 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Ogura, K.; Taniuchi, Y.; Kataoka, A.; Inoue, N.; Sugihara, F.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline promotes differentiation of MC3T3-E1 osteoblastic cells. Biochem. Biophys. Res. Commun. 2014, 453, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin. Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef]
- Rostan, E. Collagen fillers. Facial Plast. Surg. Clin. North Am. 2007, 15, 55–61. [Google Scholar] [CrossRef]
- Zhao, R.; Qiu, H.; Liu, S.; Cao, L.; Yu, D.; Wang, H. Quantifiable clinical efficacy of injectable porcine collagen for the treatment of structural dark circles. J. Cosmet. Dermatol. 2021, 20, 1520–1528. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34; quiz 35–36. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Castilho, M.; Yue, Z.; Glattauer, V.; Hughes, T.C.; Ramshaw, J.A.; Wallace, G.G. Shaping collagen for engineering hard tissues: Towards a printomics approach. Acta Biomater. 2021, 131, 41–61. [Google Scholar] [CrossRef]
- Fushimi, H.; Hiratsuka, T.; Okamura, A.; Ono, Y.; Ogura, I.; Nishimura, I. Recombinant collagen polypeptide as a versatile bone graft biomaterial. Commun. Mater. 2020, 1, 87. [Google Scholar] [CrossRef]
- Shoseyov, O.; Posen, Y.; Grynspan, F. Human collagen produced in plants: More than just another molecule. Bioengineered 2014, 5, 49–52. [Google Scholar] [CrossRef]
- Tytgat, L.; Dobos, A.; Markovic, M.; Van Damme, L.; Van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A.; et al. High-Resolution 3D Bioprinting of Photo-Cross-linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef]
- Bauman, L. CosmoDerm/CosmoPlast (human bioengineered collagen) for the aging face. Facial Plast. Surg. 2004, 20, 125–128. [Google Scholar] [CrossRef]
- Sharma, S.; Dwivedi, S.; Chandra, S.; Srivastava, A.; Vijay, P. Collagen: A Brief Analysis. Oral Maxillofac. Pathol. J. 2019, 10, 11–17. [Google Scholar] [CrossRef]
- Sadick, N.S. Poly-l-lactic acid: A perspective from my practice. J. Cosmet. Dermatol. 2008, 7, 55–60. [Google Scholar] [CrossRef]
- Moyle, G.J.; Brown, S.; Lysakova, L.; Barton, S.E. Long-term safety and efficacy of poly-l-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006, 7, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.J.; Yi, Y.; Wu, G.H. Application of PLLA (Poly-l-Lactic acid) for rejuvenation and reproduction of facial cutaneous tissue in aesthetics: A review. Medicine 2024, 103, e37506. [Google Scholar] [CrossRef]
- Sztrolovics, R.; van der Rest, M.; Roughley, P.J. Identification of type I collagen gene polymorphisms: Tolerance of sequence variation at an alpha 2(I) helix Y position. Matrix Biol. J. Int. Soc. Matrix Biol. 1994, 14, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Chen, W.; Jin, J.; Gu, W.; Wei, B.; Lei, Y.; Xiong, S.; Zhang, G. Rational design of translational pausing without altering the amino acid sequence dramatically promotes soluble protein expression: A strategic demonstration. J. Biotechnol. 2014, 189, 104–113. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc. Natl. Acad. Sci. USA 1970, 66, 1002–1007. [Google Scholar] [CrossRef]
- Lim, J.J.; Shamos, M.H. Evaluation of kinetic parameters of thermal decomposition of native collagen by thermogravimetric analysis. Biopolymers 1974, 13, 1791–1807. [Google Scholar] [CrossRef]
- Metreveli, N.O.; Jariashvili, K.K.; Namicheishvili, L.O.; Svintradze, D.V.; Chikvaidze, E.N.; Sionkowska, A.; Skopinska, J. UV-vis and FT-IR spectra of ultraviolet irradiated collagen in the presence of antioxidant ascorbic acid. Ecotoxicol. Environ. Saf. 2010, 73, 448–455. [Google Scholar] [CrossRef]
- Fallas, J.A.; O’Leary, L.E.; Hartgerink, J.D. Synthetic collagen mimics: Self-assembly of homotrimers, heterotrimers and higher order structures. Chem. Soc. Rev. 2010, 39, 3510–3527. [Google Scholar] [CrossRef]
- Xia, Z.; Das, P.; Shakhnovich, E.I.; Zhou, R. Collapse of unfolded proteins in a mixture of denaturants. J. Am. Chem. Soc. 2012, 134, 18266–18274. [Google Scholar] [CrossRef]
- Abdul-Gader, A.; Miles, A.J.; Wallace, B.A. A reference dataset for the analyses of membrane protein secondary structures and transmembrane residues using circular dichroism spectroscopy. Bioinformatics 2011, 27, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhu, B.; Liang, Y.; Bi, L.; Hu, Z.; Chen, B.; Zhang, K.; Zhu, J. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, S.; Acharya, K.; Xu, Y.; Guo, H.; Li, T.; Fu, D.; Yang, Z.; Hou, L.; Xing, X.; et al. Decorin attenuates hypertrophic scar fibrosis via TGFβ/Smad signalling. Exp. Dermatol. 2024, 33, e15133. [Google Scholar] [CrossRef]
- Xia, W.; Hammerberg, C.; Li, Y.; He, T.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Expression of catalytically active matrix metalloproteinase-1 in dermal fibroblasts induces collagen fragmentation and functional alterations that resemble aged human skin. Aging Cell 2013, 12, 661–671. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P.R.; Shen, Z.Y.; Chen, X.D. Chemical analysis of Hericium erinaceum polysaccharides and effect of the polysaccharides on derma antioxidant enzymes, MMP-1 and TIMP-1 activities. Int. J. Biol. Macromol. 2010, 47, 33–36. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Mahdavi-Shahri, M. Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen. Biomater. 2017, 4, 309–314. [Google Scholar] [CrossRef]
- Michaud, T. Rheology of hyaluronic acid and dynamic facial rejuvenation: Topographical specificities. J. Cosmet. Dermatol. 2018, 17, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Xie, H.; Wang, G.; An, Y. Risk Comparison of Filler Embolism Between Polymethyl Methacrylate (PMMA) and Hyaluronic Acid (HA). Aesthetic Plast. Surg. 2019, 43, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Ronan, S.J.; Eaton, L.; Lehman, A.; Pilcher, B.; Erickson, C.P. Histologic Characterization of Polymethylmethacrylate Dermal Filler Biostimulatory Properties in Human Skin. Dermatol. Surg. 2019, 45, 1580–1584. [Google Scholar] [CrossRef] [PubMed]

| Groups | Number | D-Gal Injection | Implants (Concentration; Volume) |
|---|---|---|---|
| Blank | 12 | − | Sodium carboxymethyl cellulose solution (1%; 200 μL) |
| PLLA | 12 | − | PLLA in 1% sodium carboxymethyl cellulose solution (68 mg/mL; 200 μL) |
| COL | 12 | − | DuCol in 1% sodium carboxymethyl cellulose solution (4 mg/mL; 200 μL) |
| P&C | 12 | − | PLLA and DuCol in 1% sodium carboxymethyl cellulose solution (68 mg/mL, 4 mg/mL; 200 μL) |
| Model | 12 | + | Sodium carboxymethyl cellulose solution (1%; 200 μL) |
| M-PLLA | 12 | + | PLLA in 1% sodium carboxymethyl cellulose solution (68 mg/mL; 200 μL) |
| M-COL | 12 | + | DuCol in 1% sodium carboxymethyl cellulose solution (4 mg/mL; 200 μL) |
| M-P&C | 12 | + | PLLA and DuCol in 1% sodium carboxymethyl cellulose solution (68 mg/mL, 4 mg/mL; 200 μL) |
| Gene Name | Direction | Sequence (5′–3′) |
|---|---|---|
| GAPDH | Forward | CTCTCTGCTCCTCCCTGTT |
| Reverse | TACGGCCAAATCCGTTCAC | |
| COL1A1 | Forward | GACTGGAAGAGCGGAGAGTA |
| Reverse | TGGGCTGATGTACCAGTTCT | |
| TGFβ-1 | Forward | AGCAACAATTCCTGGCGTTA |
| Reverse | AGCCCTGTATTCCGTCTCC | |
| Smad3 | Forward | AGGAGAAGTGGTGCGAGAA |
| Reverse | CATCCAGTGACCTGGGGAT | |
| TIMP1 | Forward | AGAGACACGCTAGAGCAGATAC |
| Reverse | CCAGGTCCGAGTTGCAGAA | |
| MMP1 | Forward | TCCAGGCTTTATATGGGCCTT |
| Reverse | TCACCTCTCCCCTAAACGTAG |
| Collagen | Sequence |
|---|---|
| DuCol | MGPQGIAGQRGVVGLPGQRGERGFPGLPGPSGEPGKQGPSGAS GERGPPGPMGPPGLAGPPGESGREGAPGAEGSPGRDGSPGAKGD RGETGPAGPPGAPGAPGAPGPVGPAGKSGDRGETGPAGPAGERG GPGGPGPQGPPGKNGETGPQGPPGPTGPGGDKGDTGPPGPQGLQ GLPGTGGPPGENGKPGEPGPKGDAGAPGAPGGKGDAGAPGERG PPGLAGAPGLRGGAGPPGPEGGKGAAGPPGPPGAAGTPGLQGM PGPPGPCCGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, M.; Zhou, X.; Zhong, J.; Qu, D.; Chen, W.; Chen, C.; Wang, Y.; Liu, Y.; Li, S.; Xiao, Y.; et al. In Vivo Evaluation of the Anti-Skin-Ageing Bioactivity of a Recombinant Dual Humanised Collagen and Poly-L-Lactic Acid. Bioengineering 2025, 12, 510. https://doi.org/10.3390/bioengineering12050510
Tong M, Zhou X, Zhong J, Qu D, Chen W, Chen C, Wang Y, Liu Y, Li S, Xiao Y, et al. In Vivo Evaluation of the Anti-Skin-Ageing Bioactivity of a Recombinant Dual Humanised Collagen and Poly-L-Lactic Acid. Bioengineering. 2025; 12(5):510. https://doi.org/10.3390/bioengineering12050510
Chicago/Turabian StyleTong, Mingjie, Xin Zhou, Jiongni Zhong, Dengjian Qu, Wei Chen, Chun Chen, Yiting Wang, Yaoping Liu, Shaochuan Li, Yuan Xiao, and et al. 2025. "In Vivo Evaluation of the Anti-Skin-Ageing Bioactivity of a Recombinant Dual Humanised Collagen and Poly-L-Lactic Acid" Bioengineering 12, no. 5: 510. https://doi.org/10.3390/bioengineering12050510
APA StyleTong, M., Zhou, X., Zhong, J., Qu, D., Chen, W., Chen, C., Wang, Y., Liu, Y., Li, S., Xiao, Y., Wang, N., Guo, C., Xie, Q., & Xiong, S. (2025). In Vivo Evaluation of the Anti-Skin-Ageing Bioactivity of a Recombinant Dual Humanised Collagen and Poly-L-Lactic Acid. Bioengineering, 12(5), 510. https://doi.org/10.3390/bioengineering12050510







