Fluorine-Incorporated Biogenic Hydroxyapatite Enhances Socket Bone Healing via Addressing Macrophage-Mediated Inflammatory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of FBHA and BHA
2.2. In Vitro: Macrophage Responses with BHA and FBHA Extract
2.2.1. Cell Culture and Cell Proliferation
2.2.2. In Vitro Model of Macrophage with FBHA Extract
2.2.3. qRT-PCR of Macrophage Polarization and Inflammation
2.2.4. Nitric Oxide (NO) Synthesis and Phagocytic Function of Macrophages
2.2.5. RNA-Seq Analysis
2.3. In Vitro: Effects of FBHA-Conditioned Macrophage Polarization on Osteogenesis
2.3.1. Macrophage-Conditioned Medium Preparation
2.3.2. qRT-PCR and ALP Staining
2.4. In Vivo: Alveolar Socket Preservation in Canine
2.4.1. Establishment and Surgical Procedures of Animal Model
2.4.2. Sample Acquisition and Analysis Procedure
2.4.3. Micro-CT Analysis
2.4.4. Histomorphometric Analysis
2.4.5. Statistical Analysis
3. Results
3.1. Characterization and Biocompatibility of BHA and FBHA
3.2. FBHA Extract Suppressed M1 Polarization
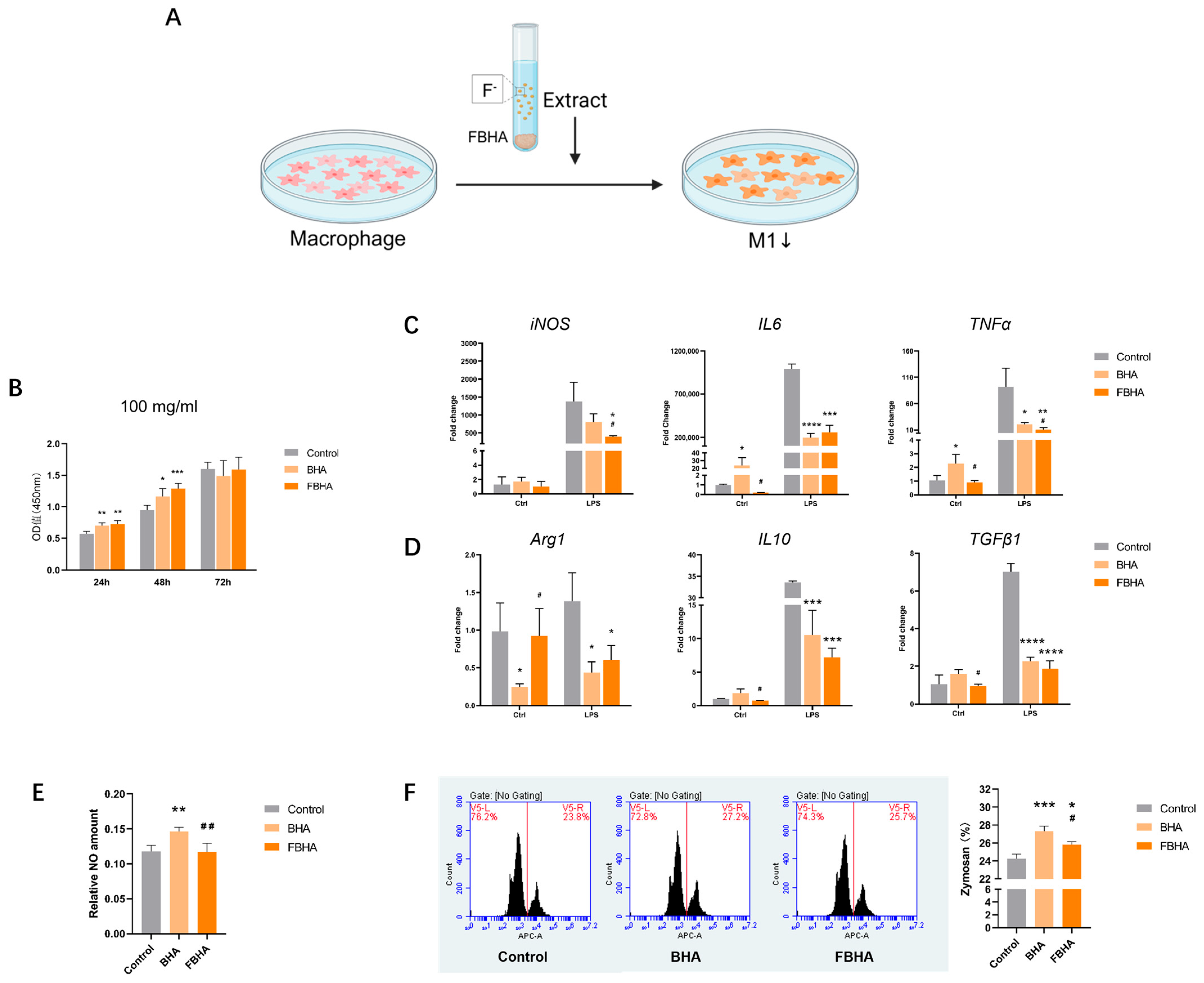
3.3. RNA-Seq Indicated FBHA-Mediated Macrophage Polarization Through Antioxidation
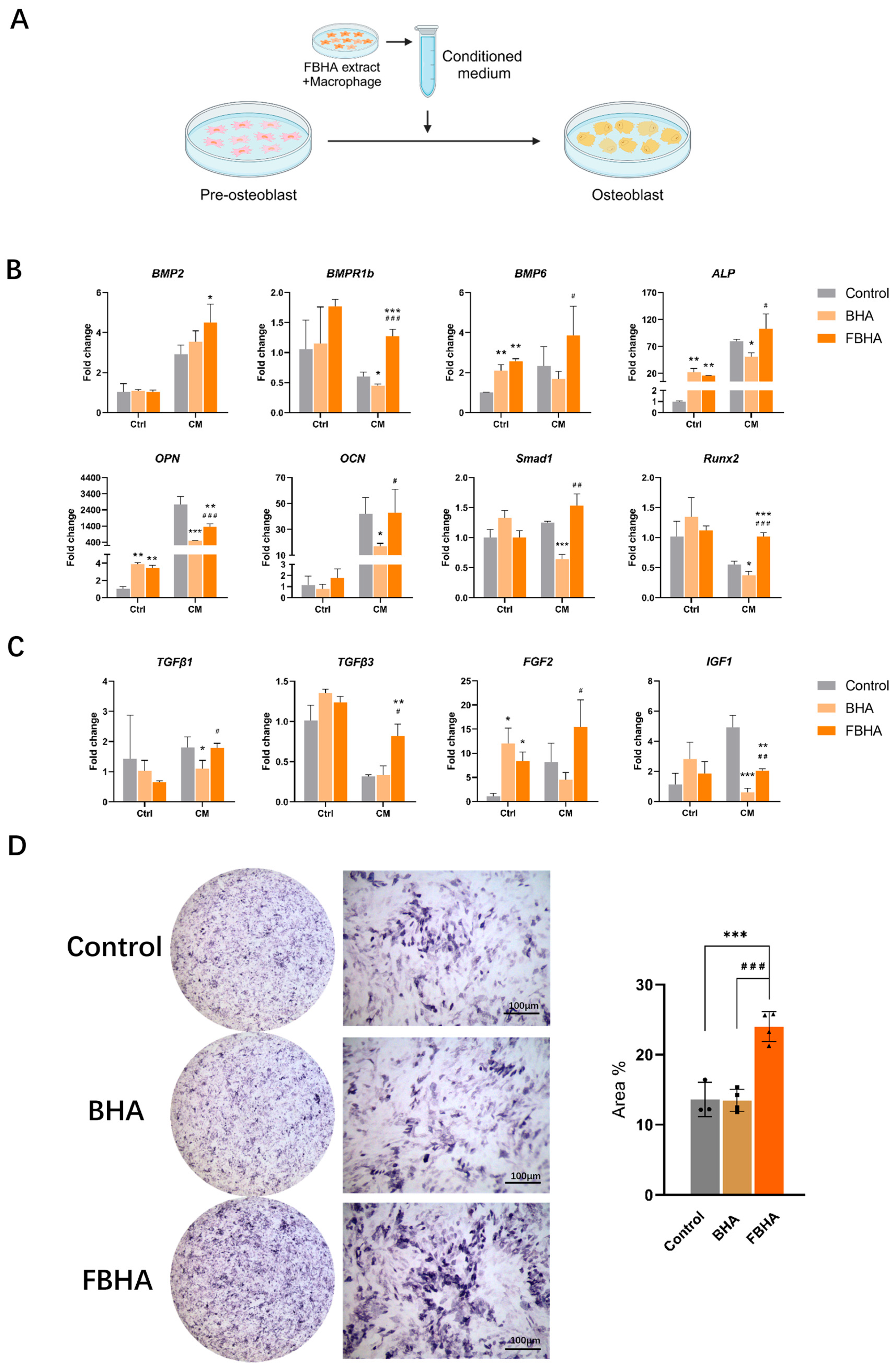
3.4. FBHA Promoted Osteogenic Differentiation via Modulating Macrophage Polarization
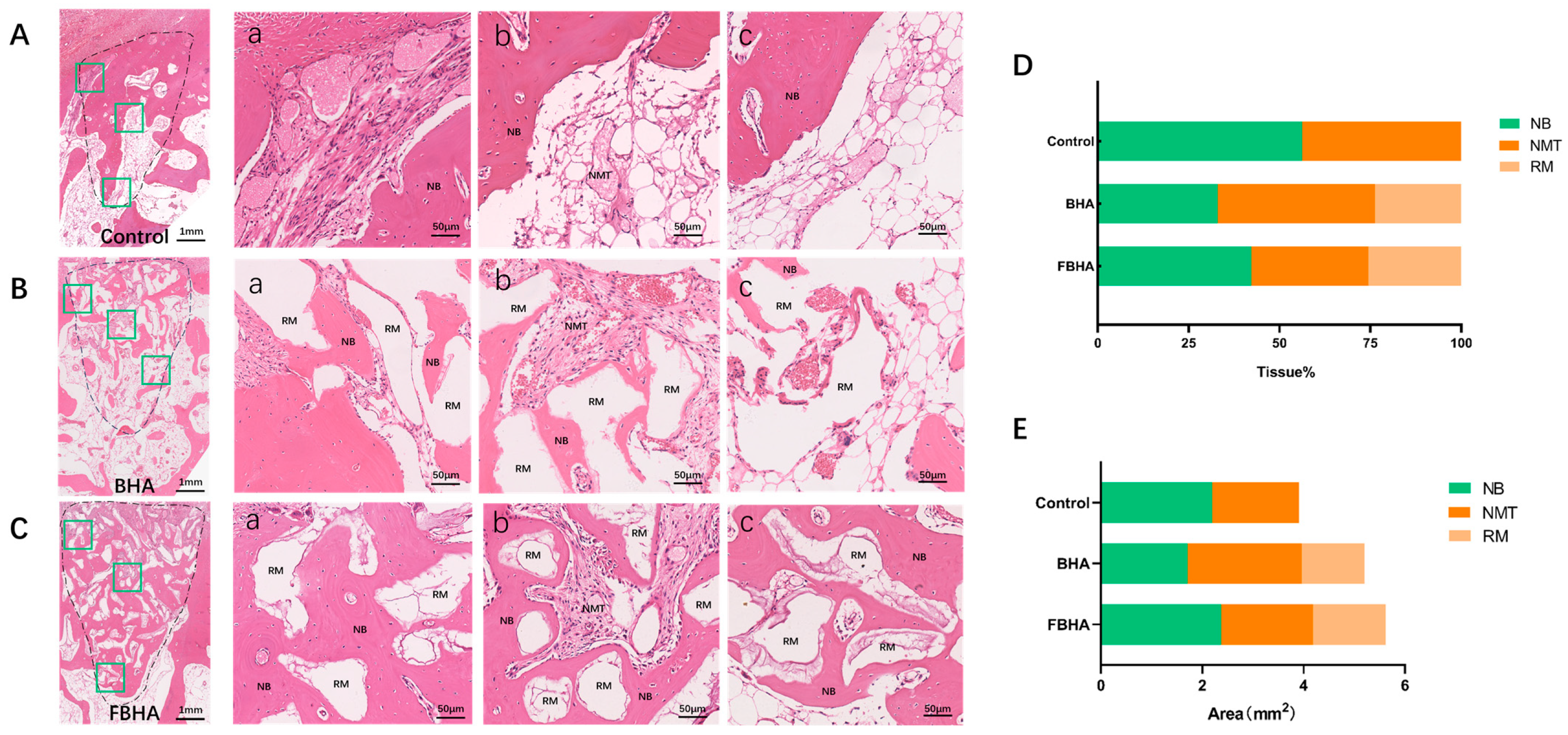
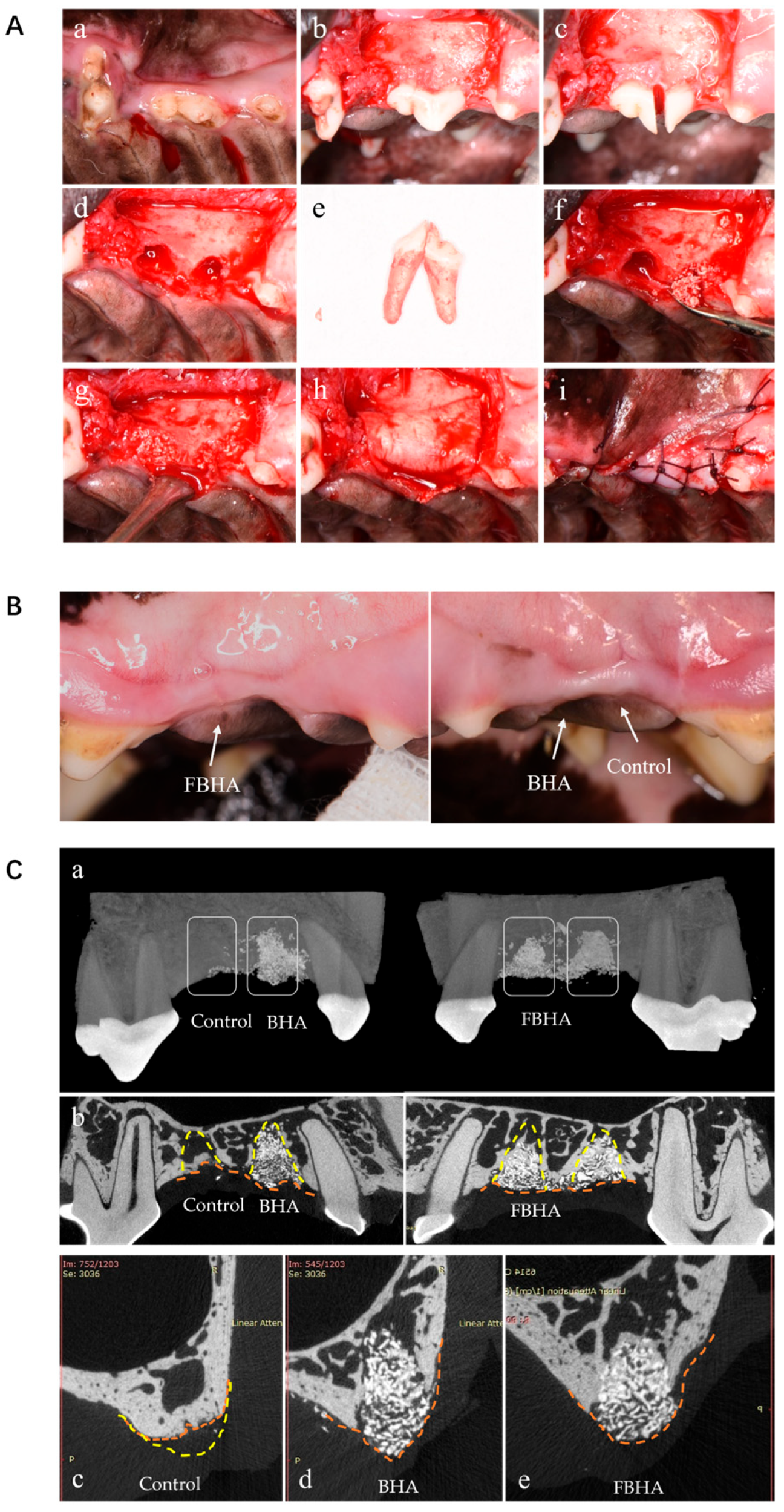
3.5. FBHA Enhanced Bone Regeneration and Biomineralization in Alveolar Sockets
3.5.1. Radiological Evaluation
3.5.2. Histological Observation and Histomorphometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol. 2000 2017, 73, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fok, M.R.; Jin, L. Learn, unlearn, and relearn post-extraction alveolar socket healing: Evolving knowledge and practices. J. Dent. 2024, 145, 104986. [Google Scholar] [CrossRef]
- El-Sioufi, I.; Oikonomou, I.; Koletsi, D.; Bobetsis, Y.A.; Madianos, P.N.; Vassilopoulos, S. Clinical evaluation of different alveolar ridge preservation techniques after tooth extraction: A randomized clinical trial. Clin. Oral Investig. 2023, 27, 4471–4480. [Google Scholar] [CrossRef]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef]
- MacBeth, N.; Mardas, N.; Davis, G.; Donos, N. Healing patterns of alveolar bone following ridge preservation procedures. Clin. Oral Implant. Res. 2024, 35, 1452–1466. [Google Scholar] [CrossRef]
- Li, C.; Su, M.; Mai, M.; Guo, Z.; Li, Y.; Chen, S.; Liu, Q.; Chen, D.; Wu, X.; Chen, Z.; et al. Calcium enrichment activity initiates extracellular calcium influx-dependent inflammatory response of biologically-derived hydroxyapatite. Mater. Today Bio 2024, 28, 101231. [Google Scholar] [CrossRef]
- Avery, D.; Morandini, L.; Sheakley, L.; Alajmi, A.; Bergey, L.; Donahue, H.J.; Martin, R.K.; Olivares-Navarrete, R. Obesity prolongs the pro-inflammatory response and attenuates bone healing on titanium implants. Acta Biomater. 2025, 192, 473–486. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Rodríguez-Morales, P.; Franklin, R.A. Macrophage phenotypes and functions: Resolving inflammation and restoring homeostasis. Trends Immunol. 2023, 44, 986–998. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W. The roles of ions on bone regeneration. Drug. Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of fluoridation of porcine hydroxyapatite on osteoblastic activity of human MG63 cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef]
- Wu, S.; Xia, B.; Mai, S.; Feng, Z.; Wang, X.; Liu, Y.; Liu, R.; Li, Z.; Xiao, Y.; Chen, Z.; et al. Sodium Fluoride under Dose Range of 2.4-24 μM, a Promising Osteoimmunomodulatory Agent for Vascularized Bone Formation. ACS Biomater. Sci. Eng. 2019, 5, 817–830. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, W.; Huang, B.; Chen, Z.; Fang, J.; Li, Z.; Chen, Z. Fluorination Enhances the Osteogenic Capacity of Porcine Hydroxyapatite. Tissue Eng. Part A 2018, 24, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Liu, Q.; Li, Z.; Zhang, H.; Chen, Z. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 2017, 18, 110–121. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, R.; Li, Z.; Luo, X.; Huang, B.; Liu, Q.; Chen, Z.; Tsoi, J.K.H.; Su, Y.X.; Cheung, K.M.C.; et al. Contribution of the in situ release of endogenous cations from xenograft bone driven by fluoride incorporation toward enhanced bone regeneration. Biomater. Sci. 2018, 6, 2951–2964. [Google Scholar] [CrossRef]
- Han, S.; Chen, Z.; Han, P.; Hu, Q.; Xiao, Y. Activation of Macrophages by Lipopolysaccharide for Assessing the Immunomodulatory Property of Biomaterials. Tissue Eng. Part A 2017, 23, 1100–1109. [Google Scholar] [CrossRef]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Brauneck, F.; Fischer, B.; Witt, M.; Muschhammer, J.; Oelrich, J.; da Costa Avelar, P.H.; Tsoka, S.; Bullinger, L.; Seubert, E.; Smit, D.J.; et al. TIGIT blockade repolarizes AML-associated TIGIT+ M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis. J. Immunother. Cancer 2022, 10, e004794. [Google Scholar] [CrossRef]
- Fujii, J.; Osaki, T. Involvement of Nitric Oxide in Protecting against Radical Species and Autoregulation of M1-Polarized Macrophages through Metabolic Remodeling. Molecules 2023, 28, 814. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, S.; Lin, D. Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation. Int. J. Mol. Sci. 2024, 26, 245. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Wu, C.; Liu, J.; Yu, H.; Zhou, X.; Zhang, J.; Wang, X.; He, S.; Xu, X.; et al. miR-765 inhibits the osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting BMP6 via regulating the BMP6/Smad1/5/9 signaling pathway. Stem Cell Res. Ther. 2020, 11, 62. [Google Scholar] [CrossRef]
- Wei, E.; Hu, M.; Wu, L.; Pan, X.; Zhu, Q.; Liu, H.; Liu, Y. TGF-β signaling regulates differentiation of MSCs in bone metabolism: Disputes among viewpoints. Stem Cell Res. Ther. 2024, 15, 156. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.; Hong, H.S. Priming with a Combination of FGF2 and HGF Restores the Impaired Osteogenic Differentiation of Adipose-Derived Stem Cells. Cells 2022, 11, 2042. [Google Scholar] [CrossRef]
- Chen, L.; Zou, X.; Zhang, R.X.; Pi, C.J.; Wu, N.; Yin, L.J.; Deng, Z.L. IGF1 potentiates BMP9-induced osteogenic differentiation in mesenchymal stem cells through the enhancement of BMP/Smad signaling. BMB Rep. 2016, 49, 122–127. [Google Scholar] [CrossRef]
- Abundo, R.; Dellavia, C.P.B.; Canciani, E.; Daniele, M.; Dioguardi, M.; Zambelli, M.; Perelli, M.; Mastrangelo, F. Alveolar Ridge Preservation with a Novel Cross-Linked Collagen Sponge: Histological Findings from a Case Report. J. Clin. Med. 2023, 12, 7599. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Lin, Y.; Zou, Y.; Yang, J.; Han, Z.; Guo, X.; Gao, X.; Xu, J.; Gong, Z.; Li, R.; Li, Z.; et al. Systemic T-cell and local macrophage interactions mediate granule size-dependent biological hydroxyapatite foreign body reaction. BMEMat 2025, e12133. [Google Scholar] [CrossRef]
- Calder, D.; Oveissi, F.; Maleknia, S.; Huang, T.; Koong, B.; Abrams, T.; Oar, A.; Chrzanowski, W.; Dehghani, F.; Fathi, A. Universal Hydrogel Carrier Enhances Bone Graft Success: Preclinical and Clinical Evaluation. Adv. Healthc. Mater. 2025, e2403930. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.O.; Spadaro, J.A.; Berg, E.W. The trace elements of human bone. J. Bone Jt. Surg. Am. 1968, 50, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, P.; Jiang, G.; Zhang, M.; Yu, F.; Dong, X.; Wang, L.; Chen, Y.; Zhang, W.; Qi, Y.; et al. A novel magnesium ion-incorporating dual-crosslinked hydrogel to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111868. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2021, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, H.; Chen, Z.; Matinlinna, J.P. Insight into bone-derived biological apatite: Ultrastructure and effect of thermal treatment. Biomed. Res. Int. 2015, 2015, 601025. [Google Scholar] [CrossRef]
- Mladenović, Ž.; Sahlin-Platt, A.; Andersson, B.; Johansson, A.; Björn, E.; Ransjö, M. In vitro study of the biological interface of Bio-Oss: Implications of the experimental setup. Clin. Oral Implants Res. 2013, 24, 329–335. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018, 75, 463–471. [Google Scholar] [CrossRef]
- Liu, R.; Lin, Y.; Lin, J.; Zhang, L.; Mao, X.; Huang, B.; Xiao, Y.; Chen, Z.; Chen, Z. Blood Prefabrication Subcutaneous Small Animal Model for the Evaluation of Bone Substitute Materials. ACS Biomater. Sci. Eng. 2018, 4, 2516–2527. [Google Scholar] [CrossRef]
- Sparks, D.S.; Saifzadeh, S.; Savi, F.M.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A.; et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Huang, R.; Chen, K.; Huang, B.; Liu, Q.; Chen, Z.; Li, Z. Effects of fluorinated porcine hydroxyapatite on lateral ridge augmentation: An experimental study in the canine mandible. Am. J. Transl. Res. 2020, 12, 2473–2487. [Google Scholar]
- Li, Z.P.; Liu, Y.X.; Huang, R.X.; Liu, C.W.; Liu, R.H.; Liu, Q.; Huang, B.X.; Chen, Z.T.; Chen, Z.F. Pre-clinical study of the effects of fluorinated porcine hydroxyapatite in repairing pe-ri-implant bone defects in canine mandible. Zhonghua Kou Qiang Yi Xue Za Zhi 2020, 55, 908–914. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Naidu, N.S.S.; Kancharla, A.K.; Nandigam, A.R.; Tasneem, S.M.; Gummaluri, S.S.; Dey, S.; Prathipaty, R.J. Comparative study of demineralized freeze-dried bone allograft and its combination with platelet-rich fibrin in the treatment of intrabony defects: A randomized clinical trial. Dent. Med. Probl. 2024, 61, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Duś-Ilnicka, I. Current Concepts and Challenges in the Treatment of Cleft Lip and Palate Patients-A Comprehensive Review. J. Pers. Med. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Misztal-Kunecka, A.; Prządka, P.; Dzimira, S. The Use of Hydroxyapatite Polymer with Curdlan in the Treatment of Bone Defects Associated with Ectopic Tooth Extraction in Dogs-A Case Series. Life 2024, 14, 879. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Cevey, Á.C.; Penas, F.N.; Alba Soto, C.D.; Mirkin, G.A.; Goren, N.B. IL-10/STAT3/SOCS3 Axis Is Involved in the Anti-inflammatory Effect of Benznidazole. Front. Immunol. 2019, 10, 1267. [Google Scholar] [CrossRef]
- Mestres, G.; Espanol, M.; Xia, W.; Persson, C.; Ginebra, M.P.; Ott, M.K. Inflammatory response to nano- and microstructured hydroxyapatite. PLoS ONE 2015, 10, e0120381. [Google Scholar] [CrossRef]
- Chen, Z.; Pang, Q.; Zhan, J.; Liu, J.; Zhao, W.; Dong, L.; Huang, W. MSCs-derived ECM functionalized hydrogel regulates macrophage reprogramming for osteoarthritis treatment by improving mitochondrial function and energy metabolism. Mater. Today Bio 2024, 29, 101340. [Google Scholar] [CrossRef]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef]
- Nakai, K.; Urushihara, M.; Kubota, Y.; Kosaka, H. Ascorbate enhances iNOS activity by increasing tetrahydrobiopterin in RAW 264.7 cells. Free Radic. Biol. Med. 2003, 35, 929–937. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Hong, F.F.; Yang, S.L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Wang, J.; Bausch, K.; von Schmädel, K.; Bode, C.; Hehrlein, C. Transient hyperoxic reoxygenation reduces cytochrome C oxidase activity by increasing superoxide dismutase and nitric oxide. J. Biol. Chem. 2010, 285, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Atif, A.R.; Pujari-Palmer, M.; Tenje, M.; Mestres, G. A microfluidics-based method for culturing osteoblasts on biomimetic hydroxyapatite. Acta Biomater. 2021, 127, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.B.D.S.; Dionizio, A.S.; Araujo, T.T.; Fernandes, M.D.S.; Iano, F.G.; Buzalaf, M.A.R. Proposed mechanism for understanding the dose- and time-dependency of the effects of fluoride in the liver. Toxicol. Appl. Pharmacol. 2018, 358, 68–75. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Xu, L.; Feng, J.; Yang, B.; Chen, K.; Liu, Y.; Wu, X.; Wu, S.; Li, Z.; Chen, S.; et al. Fluorine-Incorporated Biogenic Hydroxyapatite Enhances Socket Bone Healing via Addressing Macrophage-Mediated Inflammatory Response. Bioengineering 2025, 12, 396. https://doi.org/10.3390/bioengineering12040396
Liu C, Xu L, Feng J, Yang B, Chen K, Liu Y, Wu X, Wu S, Li Z, Chen S, et al. Fluorine-Incorporated Biogenic Hydroxyapatite Enhances Socket Bone Healing via Addressing Macrophage-Mediated Inflammatory Response. Bioengineering. 2025; 12(4):396. https://doi.org/10.3390/bioengineering12040396
Chicago/Turabian StyleLiu, Chengwu, Leyao Xu, Junming Feng, Bo Yang, Kaidi Chen, Yuanxiang Liu, Xiayi Wu, Shiyu Wu, Zhipeng Li, Shoucheng Chen, and et al. 2025. "Fluorine-Incorporated Biogenic Hydroxyapatite Enhances Socket Bone Healing via Addressing Macrophage-Mediated Inflammatory Response" Bioengineering 12, no. 4: 396. https://doi.org/10.3390/bioengineering12040396
APA StyleLiu, C., Xu, L., Feng, J., Yang, B., Chen, K., Liu, Y., Wu, X., Wu, S., Li, Z., Chen, S., & Chen, Z. (2025). Fluorine-Incorporated Biogenic Hydroxyapatite Enhances Socket Bone Healing via Addressing Macrophage-Mediated Inflammatory Response. Bioengineering, 12(4), 396. https://doi.org/10.3390/bioengineering12040396








