Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast
Abstract
1. Introduction
2. Types of Yeasts Used in Beta-Glucan Fermentation
3. Fermentation Process for Beta-Glucan Production
3.1. Substrate Selection
3.2. Inoculum Development
3.3. Fermentation Conditions and Parameters
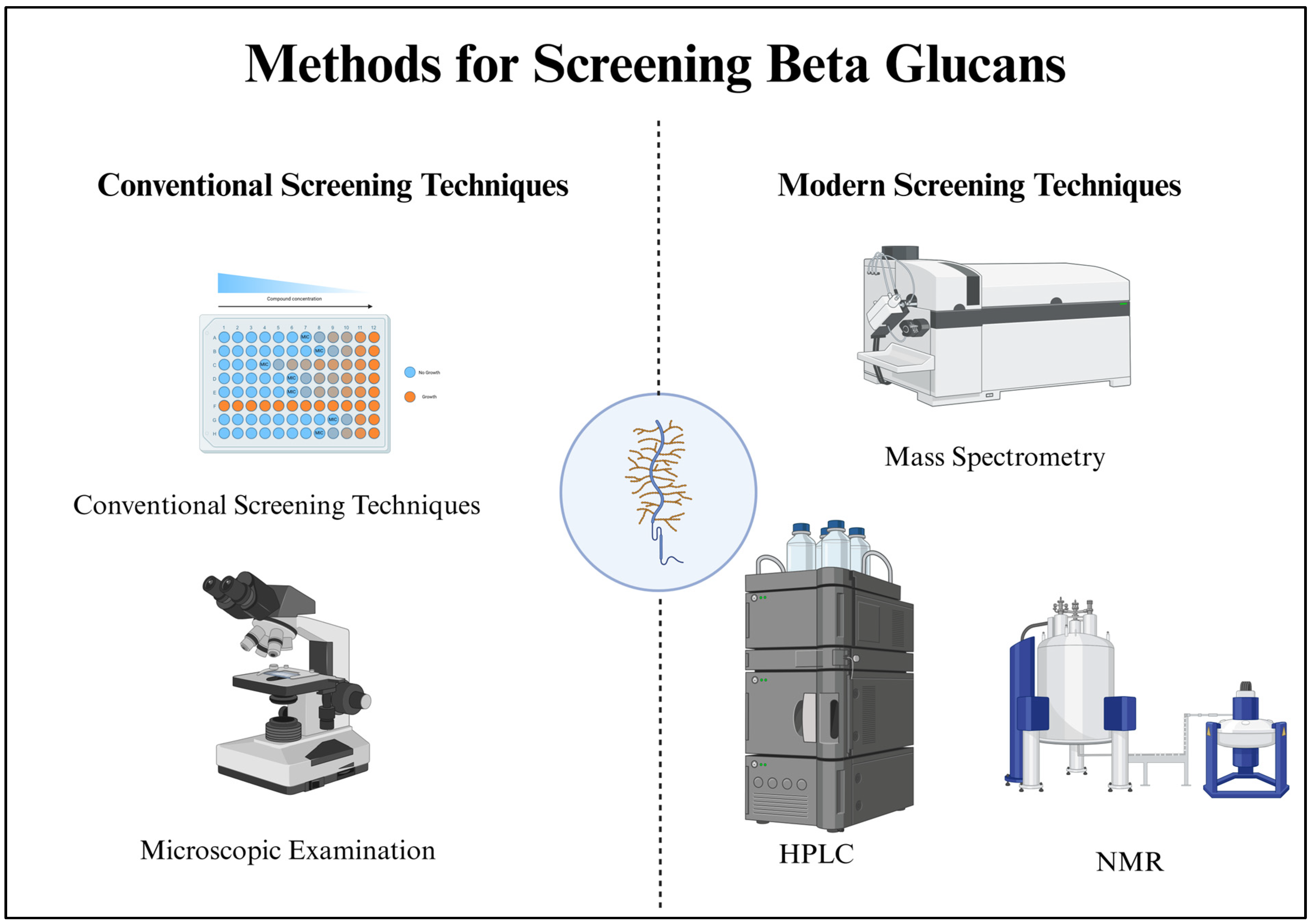
4. Methods for Screening Beta-Glucans
4.1. Chemical Assays for Beta-Glucan Detection
4.2. Microscopic Examination
4.3. Modern Screening Techniques
5. Extraction and Recovery of Beta-Glucan
5.1. Pretreatment of Yeast Biomass to Enhance Extraction of Beta-Glucan
5.2. Mechanical Methods
5.2.1. Bead Milling/Bead-Assisted Extraction
5.2.2. High-Pressure Homogenization-Based Extraction
5.2.3. Ultrasonication-Based Extraction
5.3. Non-Mechanical Methods
5.3.1. Pulsed Electric Field-Based Extraction
5.3.2. Enzyme-Assisted Extraction
5.3.3. Alkaline Extraction
5.3.4. Acidic Extraction
5.4. Purification and Characterization
6. Health Benefits of Beta-Glucans
6.1. Immunomodulatory Effects
6.2. Anti-Tumor Properties
6.3. Antioxidant Activity
6.4. Cholesterol-Lowering Effect
6.5. Diabetes Management
6.6. Anti-Inflammatory Effects
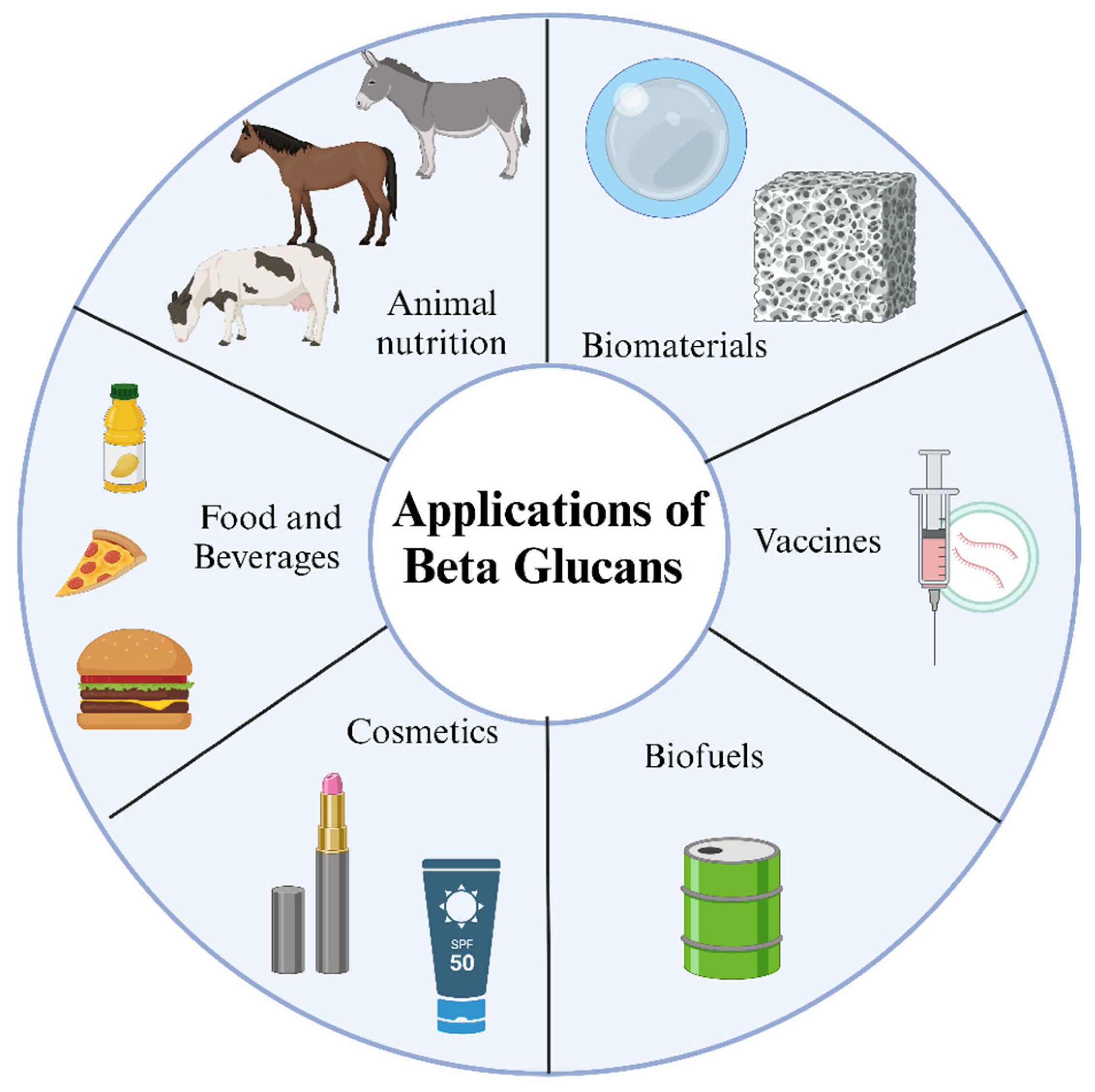
7. Applications for Beta-Glucans
7.1. Functional Foods and Beverages
7.2. Nutraceuticals and Dietary Supplements
7.3. Pharmaceutical Formulations
7.4. Cosmetic and Skincare Products
7.5. Animal Feed and Veterinary Applications
7.6. Industrial and Biotechnological Uses
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Rieder, A.; Samuelsen, A.B. Do cereal mixed-linked β-glucans possess immune-modulating activities? Mol. Nutr. Food Res. 2012, 56, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Xu, B. Oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) of β-glucans from different sources with various molecular weight. Bioact. Carbohydr. Diet. Fibre 2014, 3, 11–16. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.; Malfatti, C.R.M. Biological activities of derivatized d-glucans: A review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; da Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.; Dekker, R.F.; Barbosa, A.M.; Teixeira, S.D.; Salomé, K. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): Preparation, characterization and antioxidant evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Onofre, S.B.; Bertoldo, I.C.; Abatti, D.; Refosco, D. Chemical Composition of the Biomass of Saccharomyces cerevisiae—(Meyen ex E. C. Hansen, 1883) Yeast obtained from the Beer Manufacturing Process. Int. J. Environ. Agric. Biotechnol. 2017, 2, 558–562. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Iqbal, S.; Anjum, A.A. Nutraceutical, Anti-Inflammatory, and Immune Modulatory Effects of β-Glucan Isolated from Yeast. BioMed Res. Int. 2017, 2017, 8972678. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Oliveira, P.G.; Gaspar, V.M.; Mano, J.F.; Coimbra, M.A.; Coelho, E. Brewer’s yeast polysaccharides—A review of their exquisite structural features and biomedical applications. Carbohydr. Polym. 2022, 277, 118826. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.H.; Mustafa, S.; Man, Y.B.C. Microbial Polysaccharides and Their Modification Approaches: A Review. Int. J. Food Prop. 2014, 18, 332–347. [Google Scholar] [CrossRef]
- Carpenter, K.C.; Breslin, W.L.; Davidson, T.; Adams, A.; McFarlin, B.K. Baker’s yeast β-glucan supplementation increases monocytes and cytokines post-exercise: Implications for infection risk? Br. J. Nutr. 2013, 109, 478–486. [Google Scholar] [CrossRef]
- Kim, H.; Yun, J. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J. Appl. Microbiol. 2005, 99, 728–738. [Google Scholar] [CrossRef] [PubMed]
- York, W.S. A conformational model for cyclic β-(1→2)-linked glucans based on NMR analysis of the β-glucans produced by Xanthomonas campestris. Carbohydr. Res. 1995, 278, 205–225. [Google Scholar] [CrossRef]
- Kambhampati, N.S.V.; Kar, S.; Pinnepalli, S.S.K.; Chelli, J.; Doble, M. Microbial cyclic β-(1→3),(1→6)-glucans as potential drug carriers: Interaction studies between cyclic β-glucans isolated from Bradyrhizobium japonicum and betulinic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Fontaine, T.; Aimanianda, V.; Latgé, J.-P. Aspergillus Cell Wall and Biofilm. Mycopathologia 2014, 178, 371–377. [Google Scholar] [CrossRef]
- Coen, M.L.; Lerner, C.G.; Capobianco, J.O.; Goldman, R.C. Synthesis of yeast cell wall glucan and evidence for glucan metabolism in a Saccharomyces cerevisiae whole cell system. Microbiology 1994, 140, 2229–2237. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Sivamaruthi, B.S.; Sirilun, S.; Peerajan, S.; Kesika, P.; Chaiyasut, K.; Chaiyasut, C. Extraction of β-glucan from Saccharomyces cerevisiae: Comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Sci. Technol. 2016, 37, 124–130. [Google Scholar] [CrossRef]
- Kim, S.-R.; Kang, H.-W.; Ro, H.-S. Generation and Evaluation of High β-Glucan Producing Mutant Strains of Sparassis crispa. Mycobiology 2013, 41, 159–163. [Google Scholar] [CrossRef]
- Moriya, N.; Moriya, Y.; Nomura, H.; Kusano, K.; Asada, Y.; Uchiyama, H.; Park, E.Y.; Okabe, M. Improved β-glucan yield using an Aureobasidium pullulans M-2 mutant strain in a 200-L pilot scale fermentor targeting industrial mass production. Biotechnol. Bioprocess Eng. 2013, 18, 1083–1089. [Google Scholar] [CrossRef]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1→3)-β-d-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Shan, K.; Liu, W.; Xi, C.; Zhang, Y.; Wang, W.; Wang, C.; Cao, R.; Zhu, W.; Wang, H.; et al. Effect of Different Initial Fermentation pH on Exopolysaccharides Produced by Pseudoalteromonas agarivorans Hao 2018 and Identification of Key Genes Involved in Exopolysaccharide Synthesis via Transcriptome Analysis. Mar. Drugs 2022, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L.plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.-L.; Yu, J.-Y.; Hsieh, C.; Wu, J.-Y. Production and characterization of curdlan by Agrobacterium sp. Biochem. Eng. J. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Dey, B.; Maity, K.K.; Patra, S.; Mandal, S.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Isolation and characterization of an immunoenhancing glucan from alkaline extract of an edible mushroom, Lentinus squarrosulus (Mont.) Singer. Carbohydr. Res. 2011, 346, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.A.; Seviour, R.J. The production of exopolysaccharides by Aureobasidium pullulans in fermenters with low-shear configurations. Appl. Microbiol. Biotechnol. 1998, 49, 168–174. [Google Scholar] [CrossRef]
- Alanio, A.; Gits-Muselli, M.; Guigue, N.; Denis, B.; Bergeron, A.; Touratier, S.; Hamane, S.; Bretagne, S. Prospective comparison of (1,3)-beta-D-glucan detection using colorimetric and turbidimetric assays for diagnosing invasive fungal disease. Med. Mycol. 2021, 59, 882–889. [Google Scholar] [CrossRef]
- Şener, G.; Sert, G.; Şehirli, A.Ö.; Arbak, S.; Uslu, B.; Gedik, N.; Ayanoglu-Dulger, G. Pressure ulcer-induced oxidative organ injury is ameliorated by β-glucan treatment in rats. Int. Immunopharmacol. 2006, 6, 724–732. [Google Scholar] [CrossRef]
- van Avezathe, A.V.; Brandhoff, P.; van Bourgondiën, M.; Krijger, G. Rapid screening methods for beta-emitters in food samples. J. Environ. Radioact. 2015, 141, 130–137. [Google Scholar] [CrossRef]
- Bobade, H.; Gupta, A.; Sharma, S. Beta-glucan. In Nutraceuticals and Health Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–358. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; Francois, J. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rawling, M.; Schiavone, M.; Apper, E.; Merrifield, D.L.; Castex, M.; Leclercq, E.; Foey, A. Yeast cell wall extracts from Saccharomyces cerevisiae varying in structure and composition differentially shape the innate immunity and mucosal tissue responses of the intestine of zebrafish (Danio rerio). Front. Immunol. 2023, 14, 1158390. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of Winery Spent Yeast Waste Biomass as a New Source for the Production of β-Glucan. Waste Biomass Valorization 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Thielen, U.; Glenney, P.; Moran, C. A Study of Saccharomyces cerevisiae Cell Wall Glucans. J. Inst. Brew. 2009, 115, 151–158. [Google Scholar] [CrossRef]
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An evaluation study of different methods for the production of β-D-glucan from yeast biomass. Drug Test. Anal. 2015, 8, 46–55. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M. β-Glucan as a Food Ingredient. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–381. [Google Scholar] [CrossRef]
- Joseph, R.; Bachhawat, A.K. Yeasts. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 823–830. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Guluarte, C.; Angulo, C. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus peru. Microb. Pathog. 2020, 143, 104141. [Google Scholar] [CrossRef] [PubMed]
- Bzducha-Wróbel, A.; Błażejak, S.; Molenda, M.; Reczek, L. Biosynthesis of β(1,3)/(1,6)-glucans of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. Eur. Food Res. Technol. 2014, 240, 1023–1034. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Boonlum, N.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Phalinphattharakit, K.; Nimitkeatkai, H.; Jarerat, A. Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers 2022, 14, 1582. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell disruption and permeabilization methods for obtaining yeast bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell wall polysaccharides: Before and after autolysis of brewer’s yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G.; Lv, M. Extraction, characterization and antioxidant activities of mannan from yeast cell wall. Int. J. Biol. Macromol. 2018, 118, 952–956. [Google Scholar] [CrossRef]
- Eom, S.J.; Lim, T.-G.; Jhun, H.; Lee, N.H.; Kang, M.-C.; Song, K.-M. Inhibitory effect of Saccharomyces cerevisiae extract obtained through ultrasound-assisted extraction on melanoma cells. Ultrason. Sonochem. 2021, 76, 105620. [Google Scholar] [CrossRef] [PubMed]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the Efficiency of Different Disruption Methods on Yeast Cell Wall Preparation for β-Glucan Isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef] [PubMed]
- Dallies, N.; François, J.; Paquet, V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Avramia, I.; Amariei, S. A Simple and Efficient Mechanical Cell Disruption Method Using Glass Beads to Extract β-Glucans from Spent Brewer’s Yeast. Appl. Sci. 2022, 12, 648. [Google Scholar] [CrossRef]
- Imatoukene, N.; Koubaa, M.; Perdrix, E.; Benali, M.; Vorobiev, E. Combination of cell disruption technologies for lipid recovery from dry and wet biomass of Yarrowia lipolytica and using green solvents. Process Biochem. 2019, 90, 139–147. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Tsantes, M.; Taoukis, P. Effect of high pressure homogenization on the production of yeast extract via autolysis and beta-glucan recovery. Innov. Food Sci. Emerg. Technol. 2020, 62, 102340. [Google Scholar] [CrossRef]
- Tam, Y.J.; Allaudin, Z.N.; Lila, M.A.M.; Bahaman, A.R.; Tan, J.S.; Rezaei, M.A. Enhanced cell disruption strategy in the release of recombinant hepatitis B surface antigen from Pichia pastoris using response surface methodology. BMC Biotechnol. 2012, 12, 70. [Google Scholar] [CrossRef]
- Follows, M.; Hetherington, P.J.; Dunnill, P.; Lilly, M.D. Release of enzymes from bakers’ yeast by disruption in an industrial homogenizer. Biotechnol. Bioeng. 1971, 13, 549–560. [Google Scholar] [CrossRef]
- Moore, E.; Hoare, M.; Dunnill, P. Disruption of baker’s yeast in a high-pressure homogenizer: New evidence on mechanism. Enzym. Microb. Technol. 1990, 12, 764–770. [Google Scholar] [CrossRef]
- Spiden, E.M.; Scales, P.J.; Kentish, S.E.; Martin, G.J. Critical analysis of quantitative indicators of cell disruption applied to Saccharomyces cerevisiae processed with an industrial high pressure homogenizer. Biochem. Eng. J. 2012, 70, 120–126. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Cui, S.; Liu, H. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Lorincz, A. Ultrasonic Cellular Disruption of Yeast in Water-based Suspensions. Biosyst. Eng. 2004, 89, 297–308. [Google Scholar] [CrossRef]
- Doulah, M.S. Mechanism of disintegration of biological cells in ultrasonic cavitation. Biotechnol. Bioeng. 1977, 19, 649–660. [Google Scholar] [CrossRef]
- Guerrero, S.; López-Malo, A.; Alzamora, S. Effect of ultrasound on the survival of Saccharomyces cerevisiae: Influence of temperature, pH and amplitude. Innov. Food Sci. Emerg. Technol. 2001, 2, 31–39. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Xie, Y.; Wu, X.; Wu, T. Releasing polysaccharide and protein from yeast cells by ultrasound: Selectivity and effects of processing parameters. Ultrason. Sonochem. 2014, 21, 576–581. [Google Scholar] [CrossRef]
- Yuan, H.; He, Y.; Zhang, H.; Ma, X. Ultrasound-assisted enzymatic hydrolysis of yeast β-glucan catalyzed by β-glucanase: Chemical and microstructural analysis. Ultrason. Sonochem. 2022, 86, 106012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, Q.; Luo, X.; Xiao, Y.; Cai, W.; Ma, H. Effects and mechanisms of ultrasound- and alkali-assisted enzymolysis on production of water-soluble yeast β-glucan. Bioresour. Technol. 2019, 273, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Naglak, T.J.; Wang, H.Y. Protein Release from the Yeast Pichia Pastoris by Chemical Permeabilization: Comparison to Mechanical Disruption and Enzymatic Lysis. In Separations for Biotechnology 2; Springer: Dordrecht, The Netherlands, 1990; pp. 55–64. [Google Scholar] [CrossRef]
- Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules 2019, 25, 57. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Pankiewicz, U.; Sujka, M.; Jamroz, J. Bioaccumulation of the Selected Metal Ions in Saccharomyces cerevisiae Cells Under Treatment of the Culture with Pulsed Electric Field (PEF). J. Membr. Biol. 2015, 248, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. Pulsed-Electric-Fields-Induced Effects in Plant Tissues: Fundamental Aspects and Perspectives of Applications. In Electrotechnologies for Extraction from Food Plants and Biomaterials. Food Engineering Series; Springer: New York, NY, USA, 2009; pp. 39–81. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Stefanou, N.; Andreou, V.; Taoukis, P. Effect of pulsed electric fields on the production of yeast extract by autolysis. Innov. Food Sci. Emerg. Technol. 2018, 48, 287–295. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.A.; Zanette, C.M.; Spier, M.R. An overview of cell disruption methods for intracellular biomolecules recovery. Prep. Biochem. Biotechnol. 2020, 50, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ovalle, R. Cell Wall Architecture in Yeast: New Structure and New Challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Qiao, Y.; Ye, X.; Zhong, L.; Xia, C.; Zhang, L.; Yang, F.; Li, Y.; Fang, X.; Fu, L.; Huang, Y.; et al. Yeast β-1,3-glucan production by an outer membrane β-1,6-glucanase: Process optimization, structural characterization and immunomodulatory activity. Food Funct. 2022, 13, 3917–3930. [Google Scholar] [CrossRef] [PubMed]
- Salazar, O.; Asenjo, J.A. Enzymatic lysis of microbial cells. Biotechnol. Lett. 2007, 29, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.S.; Cleveland, N.S.; Yeap, R.Y.; Dong, T.; Ramirez, K.J.; Nagle, N.J.; Lowell, A.C.; Beckham, G.T.; McMillan, J.D.; Biddy, M.J. Recovery of Fuel-Precursor Lipids from Oleaginous Yeast. ACS Sustain. Chem. Eng. 2018, 6, 2921–2931. [Google Scholar] [CrossRef]
- Javmen, A.; Grigiškis, S.; Gliebutė, R. β-glucan extraction from Saccharomyces cerevisiae yeast using Actinomyces rutgersensis 88 yeast lyzing enzymatic complex. Biologija 2012, 58, 2486. [Google Scholar] [CrossRef]
- Borchani, C.; Fonteyn, F.; Jamin, G.; Paquot, M.; Blecker, C.; Thonart, P. Enzymatic process for the fractionation of baker’s yeast cell wall (Saccharomyces cerevisiae). Food Chem. 2014, 163, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Marson, G.V.; Machado, M.T.d.C.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process. Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the Production of β-Glucan from Saccharomyces carlsbergensis RU01 by Using Tannic Acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Yun, H.S. Production of soluble β-glucan from the cell wall of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2006, 39, 496–500. [Google Scholar] [CrossRef]
- Lee, J.-N.; Lee, D.-Y.; Ji, I.-H.; Kim, G.-E.; Kim, H.N.; Sohn, J.; Kim, S.; Kim, C.-W. Purification of Soluble β-Glucan with Immune-enhancing Activity from the Cell Wall of Yeast. Biosci. Biotechnol. Biochem. 2001, 65, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, P.; Jiang, W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass-Valorization 2017, 10, 1131–1140. [Google Scholar] [CrossRef]
- Ashraf, Z.U.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.; Noor, N. Nanoreduction as a technology to exploit β-Glucan from cereal and fungal sources for enhancing its nutraceutical potential. Carbohydr. Polym. 2021, 258, 117664. [Google Scholar] [CrossRef]
- Byrtusová, D.; Shapaval, V.; Holub, J.; Šimanský, S.; Rapta, M.; Szotkowski, M.; Kohler, A.; Márová, I. Revealing the Potential of Lipid and β-Glucans Coproduction in Basidiomycetes Yeast. Microorganisms 2020, 8, 1034. [Google Scholar] [CrossRef]
- Xin, Y.; Ji, H.; Cho, E.; Roh, K.-B.; You, J.; Park, D.; Jung, E. Immune-enhancing effect of water-soluble beta-glucan derived from enzymatic hydrolysis of yeast glucan. Biochem. Biophys. Rep. 2022, 30, 101256. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics 2023, 15, 1615. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, Q. New insight into the structure-dependent two-way immunomodulatory effects of water-soluble yeast β-glucan in macrophages. Carbohydr. Polym. 2022, 291, 119569. [Google Scholar] [CrossRef] [PubMed]
- van Steenwijk, H.P.; Bast, A.; de Boer, A. Immunomodulating Effects of Fungal Beta-Glucans: From Traditional Use to Medicine. Nutrients 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, P.; Jiang, Z.; Sun, X.; He, H.; Yan, P.; Xu, Y.; Liu, Y. Bioinspired yeast-based β-glucan system for oral drug delivery. Carbohydr. Polym. 2023, 319, 121163. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, H.; Liu, G.; Li, M.; He, X.; Yin, C.; Tu, Q.; Shen, X.; Bai, W.; Wang, Q.; et al. Yeast β-D-glucan exerts antitumour activity in liver cancer through impairing autophagy and lysosomal function, promoting reactive oxygen species production and apoptosis. Redox Biol. 2020, 32, 101495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Rezoagli, E.; Abidin, I.Z.; Major, I.; Murray, P.; Murphy, E.J. β-Glucans from Yeast—Immunomodulators from Novel Waste Resources. Appl. Sci. 2022, 12, 5208. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, W.; Wang, W.; Zhao, S.; Lin, C.; Ru, X.; Guan, J.; Cong, H.; Yang, Q. Area Gene Regulates the Synthesis of β-Glucan with Antioxidant Activity in the Aureobasidium pullulans. Foods 2023, 12, 660. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Ma, X.; Dong, L.; He, Y.; Chen, S. Effects of ultrasound-assisted H2O2 on the solubilization and antioxidant activity of yeast β-glucan. Ultrason. Sonochem. 2022, 90, 106210. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Diet-Type (Fats Constant) and Blood Lipids in Man. J. Nutr. 1960, 70, 257–266. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kusmiati. Cholesterol-Lowering Effect of Beta Glucan Extracted from Saccharomyces cerevisiae in Rats. Sci. Pharm. 2016, 84, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Rahar, S.; Swami, G.; Nagpal, N.; Nagpal, M.; Singh, G.; Ma, N.; Gs, S. Preparation, characterization, and biological properties of β-glucans. J. Adv. Pharm. Technol. Res. 2011, 2, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Mitchelson, K.A.J.; Tran, T.T.T.; Dillon, E.T.; Vlckova, K.; Harrison, S.M.; Ntemiri, A.; Cunningham, K.; Gibson, I.; Finucane, F.M.; O’Connor, E.M.; et al. Yeast β-Glucan Improves Insulin Sensitivity and Hepatic Lipid Metabolism in Mice Humanized with Obese Type 2 Diabetic Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2100819. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Państwowego Zakładu Hig. 2019, 70, 315–324. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.; Zheng, X.; Nie, S.; Xu, X. Inhibition of dextran sodium sulfate-induced colitis in mice by baker’s yeast polysaccharides. Carbohydr. Polym. 2019, 207, 371–381. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, L.; Yi, Y.; Meng, Y.; Peng, K.; Jiang, X.; Wang, H. Synthesis, characterization and anti-inflammatory activity of selenium nanoparticles stabilized by aminated yeast glucan. Int. J. Biol. Macromol. 2023, 245, 125187. [Google Scholar] [CrossRef]
- Zhou, J.; Fu, Y.; Qi, G.; Dai, J.; Zhang, H.; Wang, J.; Wu, S. Yeast cell-wall polysaccharides improve immunity and attenuate inflammatory response via modulating gut microbiota in LPS-challenged laying hens. Int. J. Biol. Macromol. 2022, 224, 407–421. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Mykhalevych, A.; Polishchuk, G.; Nassar, K.; Osmak, T.; Buniowska-Olejnik, M. β-Glucan as a Techno-Functional Ingredient in Dairy and Milk-Based Products—A Review. Molecules 2022, 27, 6313. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of new ingredients obtained from brewer’s spent yeast on bread characteristics. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef]
- Suwannarong, S.; Wongsagonsup, R.; Suphantharika, M. Effect of spent brewer’s yeast β-D-glucan on properties of wheat flour dough and bread during chilled storage. Int. J. Biol. Macromol. 2020, 156, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Andrzej, K.M.; Małgorzata, M.; Sabina, K.; Horbańczuk, O.K.; Rodak, E. Application of rich in β-glucan flours and preparations in bread baked from frozen dough. Food Sci. Technol. Int. 2019, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Barbut, S. Effect of inulin, β-Glucan and their mixtures on emulsion stability, color and textural parameters of cooked meat batters. Meat Sci. 2013, 94, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. β-Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2005, 20, 68–78. [Google Scholar] [CrossRef]
- Guedes, J.d.S.; Pimentel, T.C.; Diniz-Silva, H.T.; Almeida, E.T.d.C.; Tavares, J.F.; de Souza, E.L.; Garcia, E.F.; Magnani, M. Protective effects of β-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. LWT 2019, 116, 108496. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2016, 83, 8–19. [Google Scholar] [CrossRef]
- Pillemer, L.; Schoenberg, M.D.; Blum, L.; Wurz, L. Properdin System and Immunity: II. Interaction of the Properdin System with Polysaccharides. Science 1955, 122, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. β(1-3)-D-glucan affects adipogenesis, wound healing and inflammation. Orient. Pharm. Exp. Med. 2011, 11, 169–175. [Google Scholar] [CrossRef]
- Dhurat, R.; Sharma, A.; Surve, R.; McCoy, J.; Kovacevic, M.; Goren, A.; Tan, Y.; Zou, Y.; Goldust, M.; Situm, M.; et al. Novel yeast extract is superior to colloidal oatmeal in providing rapid itch relief. J. Cosmet. Dermatol. 2021, 20, 207–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Zou, Y.; Bulat, V.; Mihic, L.L.; Kovacevic, M.; Lotti, T.; Verner, I.; Stanimirovic, A.; Situm, M.; et al. Yeast extract demonstrates rapid itch relief in chronic pruritus. J. Cosmet. Dermatol. 2020, 19, 2131–2134. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Meurens, F.; Berri, M.; Chevaleyre, C.; Melo, S.; Auclair, E.; Salmon, H. Saccharomyces cerevisiae decreases inflammatory responses induced by F4+ enterotoxigenic Escherichia coli in porcine intestinal epithelial cells. Veter. Immunol. Immunopathol. 2011, 141, 133–138. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Candelaria, J.I.; Villaseñor, A.; Woodruff, S.I.; Sallis, J.F. Concordance Between Parental and Children’s Reports of Parental Smoking Prompts. Chest 2004, 125, 429–434. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kang, M.-K.; Lee, E.-J.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Kim, K.-H.; Park, S.-J.; Choi, Y.-J.; et al. Dried Yeast Extracts Curtails Pulmonary Oxidative Stress, Inflammation and Tissue Destruction in a Model of Experimental Emphysema. Antioxidants 2019, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef]
- Kong, C.-S.; Kim, J.-A.; Kil Eom, T.; Kim, S.-K. Phosphorylated glucosamine inhibits adipogenesis in 3T3-L1 adipocytes. J. Nutr. Biochem. 2010, 21, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Yang, J.-Y.; Della-Fera, M.A.; Park, H.J.; Ambati, S.; Baile, C.A. Anti-Obesity Effects of Xanthohumol Plus Guggulsterone in 3T3-L1 Adipocytes. J. Med. Food 2009, 12, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.P.; Hunt, T.K.; Weiss, J.B.; Taylor, C.M.; Hanson, A.N.; Davies, G.H.; Halliday, B.J. Peptides From Live Yeast Cell Derivative Stimulate Wound Healing. Arch. Surg. 1990, 125, 641–646. [Google Scholar] [CrossRef]
- Draelos, Z.; Dahl, A.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Dyspigmentation, skin physiology, and a novel approach to skin lightening. J. Cosmet. Dermatol. 2013, 12, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Césarini, J.P.; Michel, L.; Maurette, J.M.; Adhoute, H.; Béjot, M. Immediate effects of UV radiation on the skin: Modification by an antioxidant complex containing carotenoids. Photodermatol. Photoimmunol. Photomed. 2003, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Camargo, F.B., Jr.; Gianeti, M.D.; Campos, P.M. Evaluation of dermatological effects of cosmetic formulations containing Saccharomyces cerevisiae extract and vitamins. Food Chem. Toxicol. 2008, 46, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Kaelle, G.C.B.; Souza, C.M.M.; Bastos, T.S.; Vasconcellos, R.S.; de Oliveira, S.G.; Félix, A.P. Diet digestibility and palatability and intestinal fermentative products in dogs fed yeast extract. Ital. J. Anim. Sci. 2022, 21, 802–810. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.-S. Clinical and Physiological Perspectives of β-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Cuesta, A.; Ortuño, J.; Meseguer, J. Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol. 2001, 11, 303–315. [Google Scholar] [CrossRef]
- Pongpet, J.; Ponchunchoovong, S.; Payooha, K. Partial replacement of fishmeal by brewer’s yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti). Aquac. Nutr. 2015, 22, 575–585. [Google Scholar] [CrossRef]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from spent brewer’s yeast: Preparation, analysis and use as a potential immunostimulant in shrimp feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Andrews, S.R.; Sahu, N.; Pal, A.; Mukherjee, S.; Kumar, S. Yeast extract, brewer’s yeast and spirulina in diets for Labeo rohita fingerlings affect haemato-immunological responses and survival following Aeromonas hydrophila challenge. Res. Veter. Sci. 2010, 91, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Huff, G.R.; Huff, W.E.; Farnell, M.B.; Rath, N.C.; Santos, F.S.d.L.; Donoghue, A.M. Bacterial clearance, heterophil function, and hematological parameters of transport-stressed turkey poults supplemented with dietary yeast extract. Poult. Sci. 2010, 89, 447–456. [Google Scholar] [CrossRef]
- Huff, G.; Dutta, V.; Huff, W.; Rath, N. Effects of dietary yeast extract on turkey stress response and heterophil oxidative burst activity. Br. Poult. Sci. 2011, 52, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.; Choct, M. Dietary modulation of gut microflora in broiler chickens: A review of the role of six kinds of alternatives to in-feed antibiotics. World’s Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Tan, C.; Li, J.; Ji, Y.; Yang, Y.; Zhao, X.; Chen, M.; Xin, Z.; Wen, L.; Cui, Z.; Shu, G.; et al. Effects of dietary supplementation of different amounts of yeast extract on oxidative stress, milk components, and productive performance of sows. Anim. Feed. Sci. Technol. 2020, 274, 114648. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Huang, X.; Guo, T.; Wen, W.; Feng, L.; Wei, L. The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp Litopenaeus vannamei. Aquac. Res. 2015, 48, 311–320. [Google Scholar] [CrossRef]
- Huynh, D.; Kaschabek, S.R.; Schlömann, M. Effect of inoculum history, growth substrates and yeast extract addition on inhibition of Sulfobacillus thermosulfidooxidans by NaCl. Res. Microbiol. 2020, 171, 252–259. [Google Scholar] [CrossRef]
- Proust, L.; Sourabié, A.; Pedersen, M.; Besançon, I.; Haudebourg, E.; Monnet, V.; Juillard, V. Insights Into the Complexity of Yeast Extract Peptides and Their Utilization by Streptococcus thermophilus. Front. Microbiol. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Proust, L.; Haudebourg, E.; Sourabié, A.; Pedersen, M.; Besançon, I.; Monnet, V.; Juillard, V. Multi-omics Approach Reveals How Yeast Extract Peptides Shape Streptococcus thermophilus Metabolism. Appl. Environ. Microbiol. 2020, 86, e01446-20. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Hillier, A.J.; Lees, G.J.; Jago, G.R. The nature of the stimulation of the growth of Streptococcus lactis by yeast extract. J. Dairy Res. 1975, 42, 123–138. [Google Scholar] [CrossRef]
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2014, 12, 88–91. [Google Scholar] [CrossRef]
- Hernández-Cortés, G.; Valle-Rodríguez, J.O.; Herrera-López, E.J.; Díaz-Montaño, D.M.; González-García, Y.; Escalona-Buendía, H.B.; Córdova, J. Improvement on the productivity of continuous tequila fermentation by Saccharomyces cerevisiae of Agave tequilana juice with supplementation of yeast extract and aeration. AMB Express 2016, 6, 47. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, L.; Tian, Q.; Piao, X. Comparative digestibility of energy and ileal amino acids in yeast extract and spray-dried porcine plasma fed to pigs. Arch. Anim. Nutr. 2017, 72, 76–84. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Wang, X.; Liao, Q.; Liu, H.; Wang, Q. Yeast Extract Affecting the Transformation of Biogenic Tooeleite and Its Stability. Appl. Sci. 2022, 12, 3290. [Google Scholar] [CrossRef]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett. 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed]


| Method | Advantage | Disadvantage |
|---|---|---|
| Chemical Methods | ||
| Alkaline Extraction | Efficient extraction of β-glucans; alkaline conditions enhance solubility. | It may cause degradation of some polysaccharides, requires careful pH control, and can be harsh on labile structures. |
| Acidic Extraction | Efficient extraction of β-glucans from fungal cell walls; cost-effective and straightforward process. | May lead to partial degradation of β-glucans due to acid hydrolysis; requires careful optimization to avoid undesired side effects. |
| Enzymatic Extraction | Enzymatic extraction of β-glucans is efficient, specific, and yields high purity, avoiding harsh chemical treatments. | It may be costlier than chemicals, and enzyme availability and specificity can vary, influencing extraction efficiency. |
| Physical Extraction Methods | ||
| Hot Water Extraction | Hot water extraction of β-glucans is a simple, cost-effective method that retains bioactivity and is environmentally friendly. | High temperatures may cause degradation of β-glucans, and the extraction process may not yield high purity compared to other methods. |
| Ultrasonic Extraction | Ultrasonic extraction of β-glucans is rapid and efficient, providing high yields quickly. | The potential for sample heating, degradation, and equipment costs may be higher than traditional methods. |
| Microwave-Assisted Extraction | Microwave-assisted extraction of β-glucans offers rapid extraction, reduced solvent usage, and enhanced yields compared to conventional methods. | Potential sample degradation due to high temperatures and equipment costs may limit accessibility for some laboratories. |
| Solid–Liquid Separation | ||
| Centrifugation | Efficient separation of β-glucans from other components, providing high purity in a relatively short processing time. | May require specialized equipment, and the high force involved could affect the structural integrity of β-glucans. |
| Filtration | Filtration extraction of β-glucans allows for a selective isolation process, yielding a purified product with reduced impurities. | It may require specialized equipment, and the process could be time-consuming, potentially limiting its scalability for large-scale production. |
| Sedimentation | Efficient method for isolating β-glucans from natural sources, providing a relatively simple and cost-effective process. | This may result in variable purity, which might be time-consuming compared to some modern extraction techniques. |
| Concentration Methods | ||
| Evaporation | An efficient extraction method for β-glucans, concentrating the target compound by evaporation. | May be time-consuming, and some heat-sensitive compounds could degrade during evaporation. |
| Spray Drying | Efficient method for extracting β-glucans from various sources, producing a dry and easily transportable product. | This may lead to the degradation of heat-sensitive compounds, and the process can be energy-intensive. |
| Freeze Drying | Preserves the structural integrity and bioactivity of β-glucans, providing a high-quality product with extended shelf life. | It requires specialized equipment, is time-consuming, and may result in higher production costs than alternative extraction methods. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, N.; Mahajan, A.A.; Pathak, S.; Seth, P.; Chowdhury, A.; Ghose, I.; Das, S.; Chowdhury, R.; Bera, A.; Dey, A.; et al. Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast. Bioengineering 2025, 12, 365. https://doi.org/10.3390/bioengineering12040365
Sarkar N, Mahajan AA, Pathak S, Seth P, Chowdhury A, Ghose I, Das S, Chowdhury R, Bera A, Dey A, et al. Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast. Bioengineering. 2025; 12(4):365. https://doi.org/10.3390/bioengineering12040365
Chicago/Turabian StyleSarkar, Nirmal, Atharva Anand Mahajan, Sagarjyoti Pathak, Prakriti Seth, Ankita Chowdhury, Indrilla Ghose, Shrimanti Das, Rajanyaa Chowdhury, Aishi Bera, Anuvab Dey, and et al. 2025. "Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast" Bioengineering 12, no. 4: 365. https://doi.org/10.3390/bioengineering12040365
APA StyleSarkar, N., Mahajan, A. A., Pathak, S., Seth, P., Chowdhury, A., Ghose, I., Das, S., Chowdhury, R., Bera, A., Dey, A., Dutta, A., Majumder, I., Ghosh, S., Rajendran, R. L., & Gangadaran, P. (2025). Beta-Glucans in Biotechnology: A Holistic Review with a Special Focus on Yeast. Bioengineering, 12(4), 365. https://doi.org/10.3390/bioengineering12040365











