Environmental Impacts and Strategies for Bioremediation of Dye-Containing Wastewater
Abstract
1. Introduction
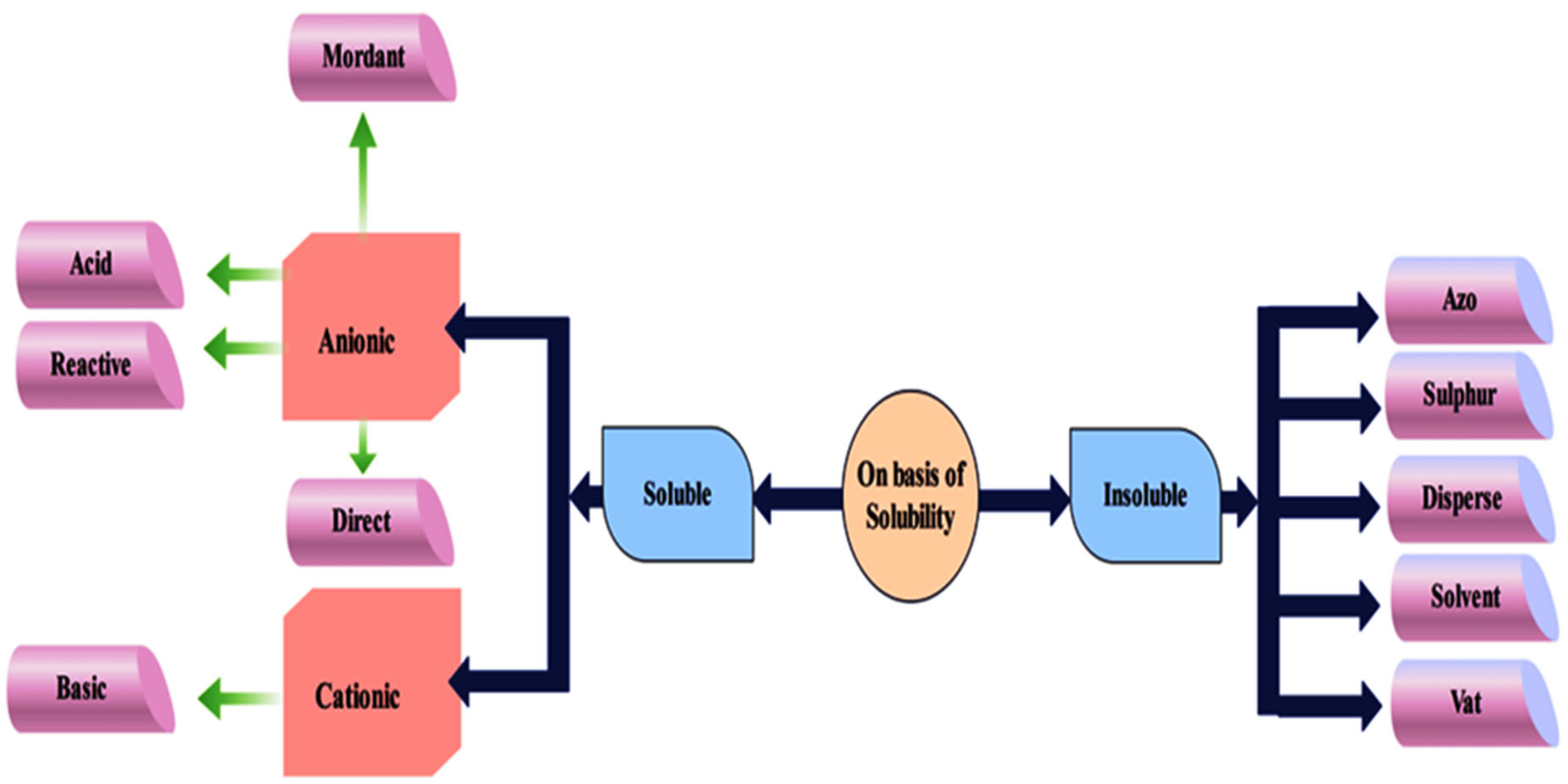
1.1. Classification of Dyes
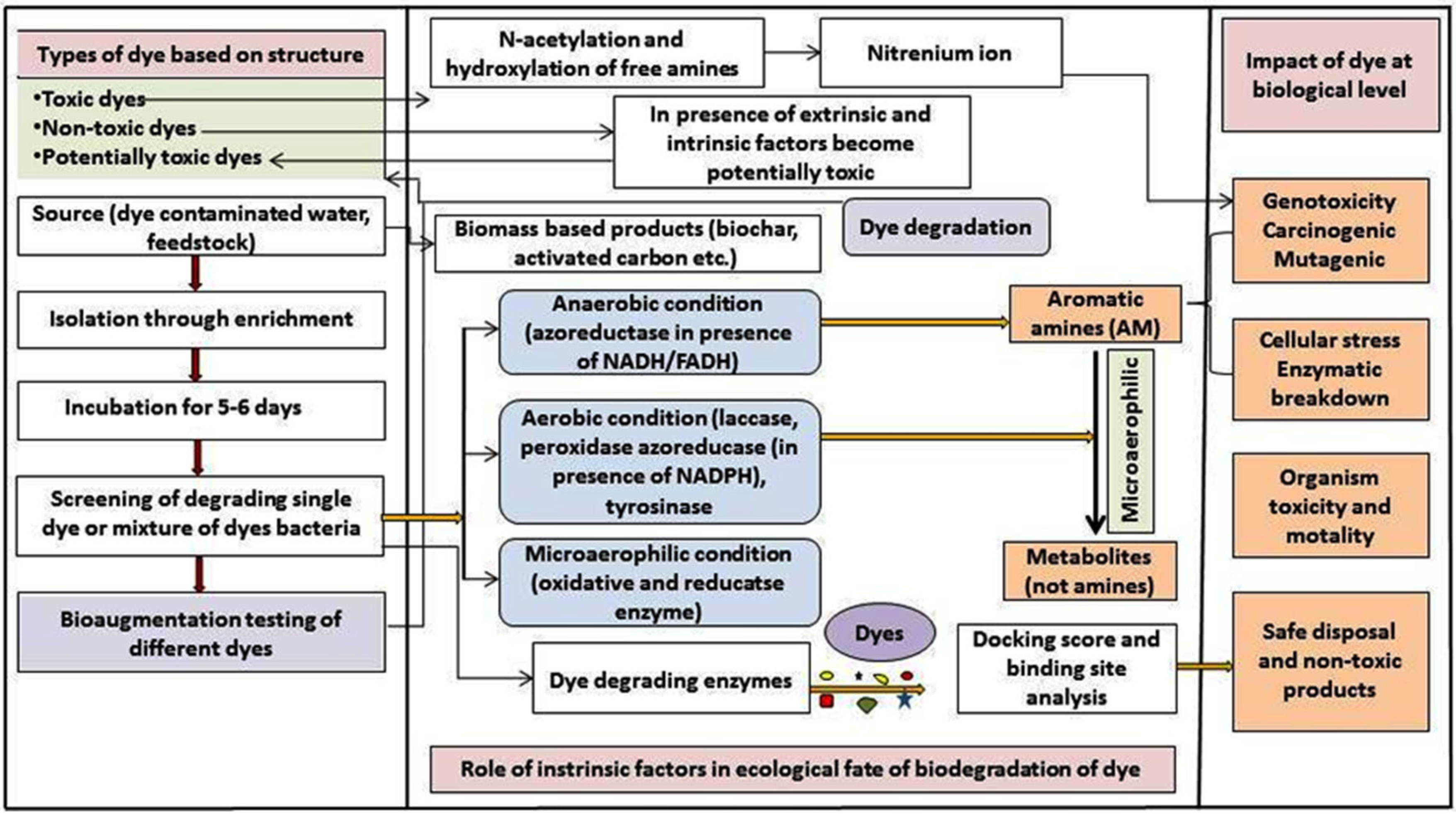
1.2. Harmful Effects of Textile Wastewater
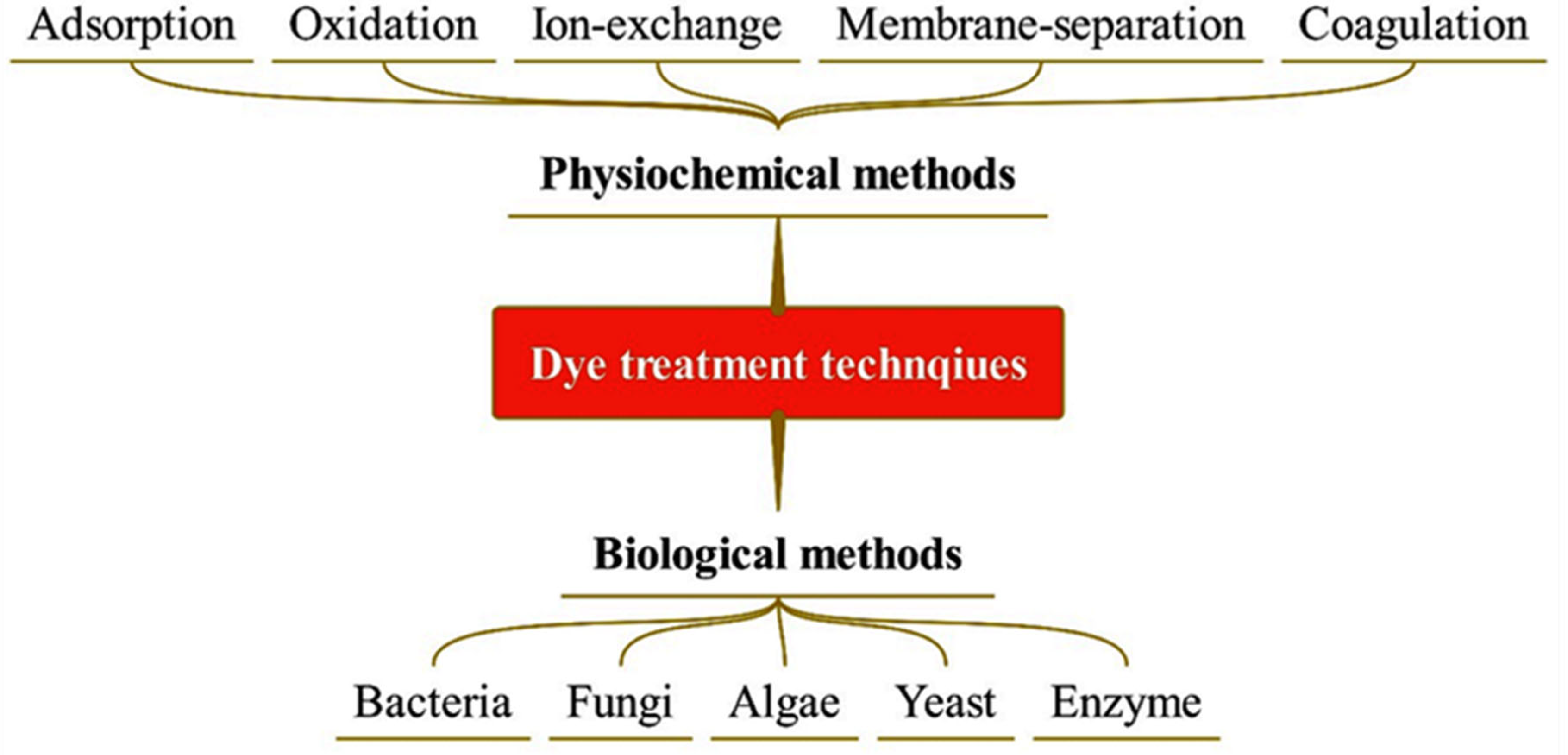
1.3. Treatment of Dyes
1.3.1. Physicochemical Methods
Adsorption
Remediation by Oxidation of Dyes
Ion Exchange
Membrane Separation
Coagulation
1.3.2. Biological Methods
2. Bioremediation of Dyes
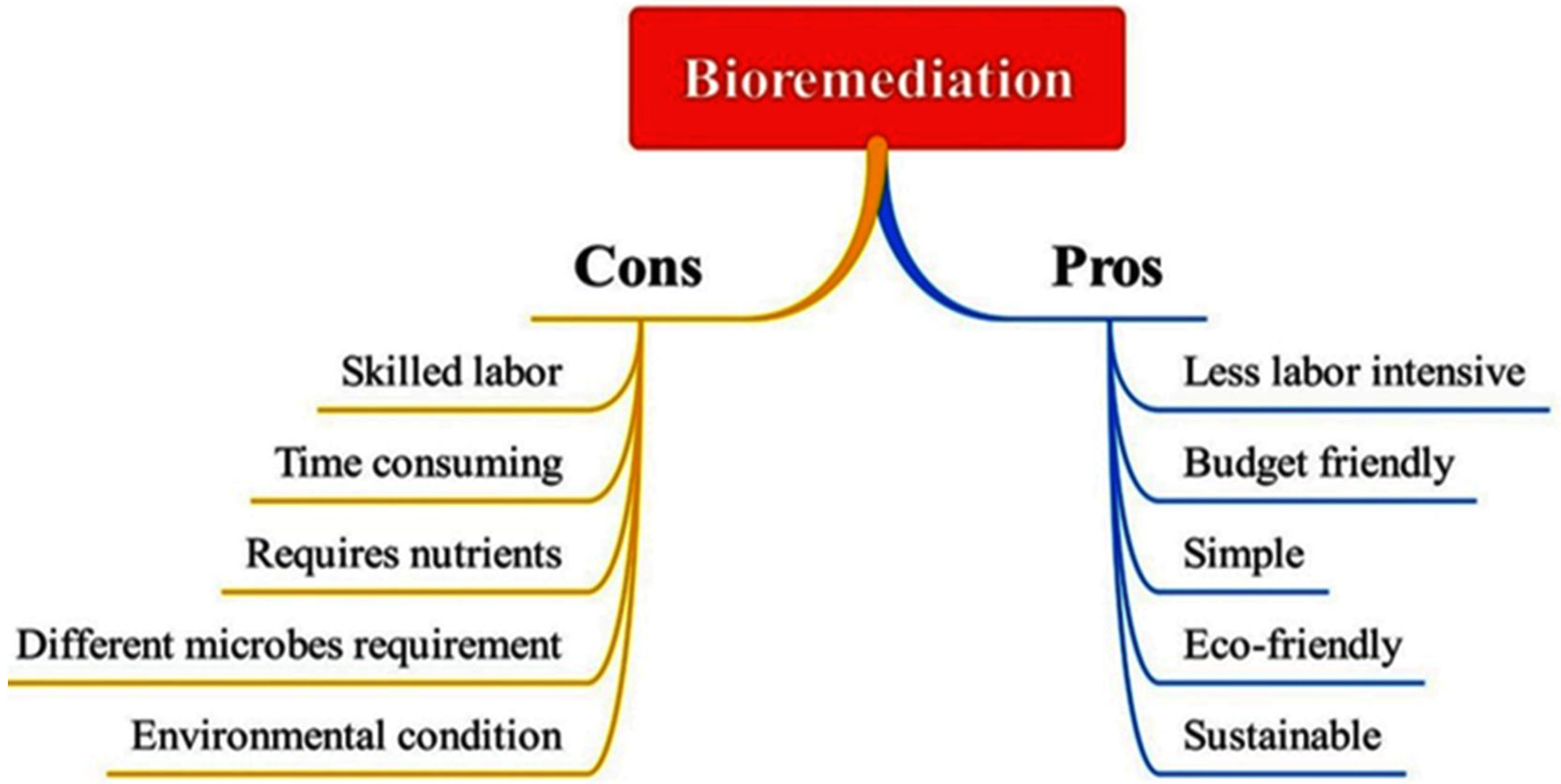
2.1. Advantages and Limitations of Bioremediation
2.2. Bioremediation of Dye Using Bacterial Strains
2.3. Bioremediation of Dye Using Fungal Strains
2.4. Bioremediation of Dye Using Algae Strains
2.5. Bioremediation of Dye Using Yeast Strains
2.6. Bioremediation of Dye Using Enzymes Strains
3. Bioreactor Advancement for Bioremediation of Dyes
3.1. Conventional Bioreactors
3.1.1. Membrane Bioreactor
3.1.2. Stirred Tank Bioreactors(STRs)
3.1.3. Wave Bioreactors(WBRs)
3.1.4. Airlift Bioreactor
3.1.5. Fixed-Bed Bioreactor
3.1.6. Fluidized Bed Bioreactor
3.1.7. Modern Bioreactors
3.1.8. Combined or Sequential Bioreactors
3.1.9. Hybrid Bioreactors
4. Microbial Fuel Cell for Removal of Dyes
4.1. Types of MFC
4.1.1. Single-Chamber MFC
4.1.2. Dual-Chamber MFCs
4.2. MFC Microorganisms
4.3. MFC-Based Bioremediation Mechanism
4.4. Future Scope and Challenges Associated with MFCs
5. Genetically Engineered Microorganisms (GEMs)
6. Nanoparticle-Based Bioremediation
7. Comparisons of Different Bioremediation Techniques
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| ASP | Activated sludge process |
| AO7 | Acid orange 7 |
| BOD | Biochemical oxygen demand |
| CNT | Carbon nanotube |
| COD | Chemical oxygen demand |
| CR | Congo red |
| FADH | Flavin adenine dinucleotide hydrogen |
| MFC | Microbial fuel cells |
| MOF | Metal-organic framework |
| NF | Nano filtration |
| NADH | Nicotinamide adenine dinucleotide hydrogen |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| RB5 | Reactive black 5 |
| RO | Reverse osmosis |
| TDS | Total dissolved solid |
| TSS | Total suspended solid |
| UF | Ultra filtration |
References
- Mishra, A.; Singh, R.S.; Mishra, V.; Giri, B.S.; Singh, D. Kinetics studies and effect of the process parameters on the biodegradation of methyl orange dye. J. Indian Chem. Soc. 2024, 101, 101334. [Google Scholar] [CrossRef]
- Cui, M.H.; Cui, D.; Gao, L.; Wang, A.J.; Cheng, H.Y. Azo dye decolorization in an up-flow bioelectrochemical reactor with domestic wastewater as a cost-effective yet highly efficient electron donor source. Water Res. 2016, 105, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Mishra, V.; Kushwaha, J.; Sengar, M.; Sinha, S.; Singh, S.; Giri, B.S. Strategies for biological treatment of waste water: A critical review. J. Clean. Prod. 2024, 454, 142266. [Google Scholar] [CrossRef]
- Cai, S.; Huang, C.; Wang, C.; Zhang, L.; Huang, K.; Dong, H.; Luo, H.; Chen, K.; Yao, S.; Zhu, H.; et al. New breakthrough in dye removal: Ultrafast removal of high concentration MB with biochar-based organic photocatalysts under indoor light (30W/m2) drive. J. Clean. Prod. 2024, 449, 141539. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye-containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Su, C.X.H.; Low, L.W.; Teng, T.T.; Wong, Y.S. Combination and hybridisation of treatments in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2016, 4, 3618–3631. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, C.; Ospina-Betancourth, C.; Sanabria, J. High Resistance of a Sludge Enriched with Nitrogen-Fixing Bacteria to Ammonium Salts and Its Potential as a Biofertilizer. Bioengineering 2021, 8, 55. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Periyasamy, A.P. A review of bioremediation of textile dye containing wastewater. Clean. Water 2025, 4, 100092. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Jamal, A.; Ilyas, M.; Zubair, M.; Khan, G.; Atieh, M.A. Bioremediation of dyes: Current status and prospects. J. Water Process Eng. 2020, 38, 101680. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Sun, J.; Fareed, M.F.; Kenawy, E.R.; Ali, S.S. Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmatomyces halophilus SSA1575, valued for textile azo dye wastewater processing and detoxification. Sci. Rep. 2020, 10, 12370. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, S.; Pathak, S.; Kasaudhan, J.; Mishra, A.; Bala, S.; Garg, D.; Singh, R.; Singh, P.; Singh, P.K.; et al. Recent Strategies for the Remediation of Textile Dyes from Wastewater: A Systematic Review. Toxics 2023, 11, 940. [Google Scholar] [CrossRef]
- Zahuri, A.A.; Abdul Patah, M.F.; Kamarulzaman, Y.; Hashim, N.H.; Thirumoorthi, T.; Wan Mohtar, W.H.M.; Mohd Hanafiah, Z.; Amir, Z.; Wan-Mohtar, W.A.A.Q.I. Decolourisation of Real Industrial and Synthetic Textile Dye Wastewater Using Activated Dolomite. Water 2023, 15, 1172. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M. Process parameters for decolorization and biodegradation of orange II (Acid Orange 7) in dye-simulated minimal salt medium and subsequent textile effluent treatment by Bacillus cereus (MTCC 9777) RMLAU1. Environ. Monit. Assess. 2013, 185, 8909–8923. [Google Scholar] [CrossRef]
- Goswami, M.; Chaturvedi, P.; Kumar Sonwani, R.; Dutta Gupta, A.; Rani Singhania, R.; Shekher Giri, B.; Nath Rai, B.; Singh, H.; Yadav, S.; Sharan Singh, R. Application of Arjuna (Terminalia arjuna) seed biochar in hybrid treatment system for the bioremediation of Congo red dye. Bioresour. Technol. 2020, 307, 123203. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.; Moody, S.C. Filamentous Fungi Are Potential Bioremediation Agents of Semi-Synthetic Textile Waste. J. Fungi. 2023, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Shahrak, M.N.; Tanhaei, B.; Sillanpaa, M. Emerging adsorptive removal of azo dye by metal–organic frameworks. Chemosphere 2016, 160, 30–44. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Khandare, R.V.; Govindwar, S.P. Phytoremediation of textile dyes and effluents: Current scenario and future prospects. Biotechno Advan. 2015, 33, 1697–1714. [Google Scholar] [CrossRef]
- Xie, R.; Danso, B.; Sun, J.; Al-Zahrani, M.; Dar, M.A.; Al-Tohamy, R.; Ali, S.S. Biorefinery and Bioremediation Strategies for Efficient Management of Recalcitrant Pollutants Using Termites as an Obscure yet Promising Source of Bacterial Gut Symbionts: A Review. Insects 2024, 15, 908. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Huang, X.; Bo, X.; Zhao, Y.; Gao, B.; Wang, Y.; Sun, S.; Yue, Q.; Li, Q. Effects of compound bioflocculant on coagulation performance and floc properties for dye removal. Bioresour. Technol. 2014, 165, 116–121. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Duan, D.; Zhang, Z.; Liu, C.; Cai, W.; Zhao, Z. Environmental Impacts and Biological Technologies Toward Sustainable Treatment of Textile Dyeing Wastewater: A Review. Sustainability 2024, 16, 10867. [Google Scholar] [CrossRef]
- Sravan, J.S.; Matsakas, L.; Sarkar, O.P. Advances in Biological Wastewater Treatment Processes: Focus on Low-Carbon Energy and Resource Recovery in Biorefinery Context. Bioengineering 2024, 11, 281. [Google Scholar] [CrossRef]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioremediation of chromium complex dyes and treatment of sludge generated during the process. Int. Biodeterior. Biodegrad. 2017, 119, 448–460. [Google Scholar] [CrossRef]
- Ito, T.; Adachi, Y.; Yamanashi, Y.; Shimada, Y. Long–term natural remediation process in textile dye–polluted river sediment driven by bacterial community changes. Water Res. 2016, 100, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, W.; Zhang, Z.; Yang, T.; Xu, Z.; Zhang, C.; Guo, B.; Lu, W. Efficient Bioremediation of Petroleum-Contaminated Soil by Immobilized Bacterial Agent of Gordonia alkanivorans W33. Bioengineering 2023, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Chen, X.; Dai, R.; Luo, Y.; Ma, P.; Ni, S.; Ma, C. Anaerobic digestion of recalcitrant textile dyeing sludge with alternative pretreatment strategies. Bioresour. Technol. 2016, 222, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Brüschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef]
- Gheorghe, V.; Gheorghe, C.G.; Popovici, D.R.; Mihai, S.; Dragomir, R.E.; Somoghi, R. Reduction of Oxigen Production by Algal Cells in the Presence of O-ChlorobenzylideneMalononitrile. Bioengineering 2024, 11, 623. [Google Scholar] [CrossRef]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015, 14, 158–174. [Google Scholar] [CrossRef]
- Xie, X.H.; Zheng, X.L.; Yu, C.Z.; Zhang, Q.Y.; Wang, Y.Q.; Cong, J.H.; Liu, N.; He, Z.J.; Yang, B.; Liu, J.S. High-efficient biodegradation of refractory dye by a new bacterial flora DDMY1 under different conditions. Int. J. Environ. Sci. Technol. 2020, 17, 1491–1502. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial fuel cell as new technol ogy for bioelectricity generation: A review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Saroj, S.; Kumar, K.; Pareek, N.; Prasad, R.; Singh, R.P. Biodegradation of azo dyes Acid Red 183, Direct Blue 15 and Direct Red 75 by the isolate Penicillium oxalicum SAR-3. Chemosphere 2014, 107, 240–248. [Google Scholar] [CrossRef]
- Anastasi, A.; Parato, B.; Spina, F.; Tigini, V.; Prigione, V.; Varese, G.C. Decolourisation and detoxification in the fungal treatment of textile wastewaters from dyeing processes. N. Biotechnol. 2011, 29, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jonstrup, M.; Kumar, N.; Guieysse, B.; Murto, M.; Mattiasson, B. Decolorization of textile dyes by Bjerkandera sp. BOL 13 using waste biomass as carbon source. J. Chem. Technol. Biotechnol. 2013, 88, 388–394. [Google Scholar] [CrossRef]
- Neoh, C.H.; Lam, C.Y.; Lim, C.K.; Yahya, A.; Bay, H.H.; Ibrahim, Z.; Noor, Z.Z. Biodecolorization of recalcitrant dye as the sole sourceof nutrition using Curvularia clavata NZ2 and decolorization ability of its crude enzymes. Environ. Sci. Pollut. Res. 2015, 22, 11669–11678. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Demir, T.A.; Kiran, I.; Ozcan, A.; Ozcan, A.S.; Tunali, S. Biosorption potential of Neurospora crassa cells for decolorization of Acid Red 57 (AR57) dye. J. Chem. Technol. Biotechnol. 2006, 81, 1100–1106. [Google Scholar] [CrossRef]
- Arunprasath, T.; Sudalai, S.; Meenatchi, R.; Jeyavishnu, K.; Arumugam, A. Biodegradation of triphenylmethane dye malachite green by a newly isolated fungus strain. Biocatal. Agric. Biotechnol. 2019, 17, 672–679. [Google Scholar] [CrossRef]
- Martins, R.A.; Salgado, E.M.; Gonçalves, A.L.; Esteves, A.F.; Pires, J.C.M. Microalgae-Based Remediation of Real Textile Wastewater: Assessing Pollutant Removal and Biomass Valorisation. Bioengineering 2024, 11, 44. [Google Scholar] [CrossRef]
- Popovici, D.R.; Gheorghe, C.G.; escu-Vasile, C.M.D. Assessment of the active sludge microorganism’s population during wastewater treatment in a micro-pilot plant. Bioengineering 2024, 11, 1306. [Google Scholar] [CrossRef]
- Ozer, A.; Akkaya, G.; Turabik, M. The removal of Acid Red 274 from wastewater: Combined biosorption and biocoagulation with Spirogyra rhizopus. Dye. Pigment. 2006, 71, 83–89. [Google Scholar] [CrossRef]
- Yu, Z.; Wen, X. Screening and identification of yeasts for decolorizing synthetic dyes in industrial wastewater. Int. Biodeterior. Biodegrad. 2005, 56, 109–114. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Fu, C.C.; Juang, R.S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads. Chem. Eng. J. 2016, 304, 313–324. [Google Scholar] [CrossRef]
- Choi, Y.S.; Long, Y.; Kim, M.J.; Kim, J.J.; Kim, G.H. Decolorization and degradation of synthetic dyes by Irpex lacteus KUC8958. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2013, 48, 501–508. [Google Scholar] [CrossRef]
- Kushch, O.V.; Iryna, O.; Hordieieva; Zosenko, O.O.; Shendrik, A.N. Comparison of N-Hydroxy Compounds as Mediators in Laccase-Catalysed Decolorization of Indigo Carmine. Chem. Select. 2019, 4, 3905–3913. [Google Scholar] [CrossRef]
- Teerapatsakul, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int. Biodeterior. Biodegrad. 2017, 120, 52–57. [Google Scholar] [CrossRef]
- Buscio, V.; García-Jiménez, M.; Vilaseca, M.; López-Grimau, V.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of textile dyeing effluents treated with coupled nanofiltration and electrochemical processes. Materials 2016, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonashydrophila strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Telke, A.A.; Kalyani, D.C.; Govindwar, S.P. Decolorization and detoxification of sulfonatedazo dye methyl orange by Kocuriarosea MTCC 1532. J. Hazard. Mater. 2010, 176, 503–509. [Google Scholar] [CrossRef]
- Kalyani, D.C.; Telke, A.A.; Dhanve, R.S.; Jadhav, J.P. Ecofriendly biodegradation and detoxification of Reactive Red 2 textile dye by newly isolated Pseudomonas sp. SUK1. J. Hazard. Mater. 2009, 163, 735–742. [Google Scholar] [CrossRef]
- Bhatt, N.; Patel, K.C.; Keharia, H.; Madamwar, D. Decolorization of diazo-dye Reactive Blue 172 by Pseudomonas aeruginosa NBAR12. J. Basic Microbiol. 2005, 45, 407–418. [Google Scholar] [CrossRef]
- Saratale, R.G.; Gandhi, S.S.; Purankar, M.V.; Kurade, M.B.; Govindwar, S.P.; Oh, S.E.; Saratale, G.D. Decolorization and detoxification of sulfonatedazo dye C.I. Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS. J. Biosci. Bioeng. 2012, 115, 658–667. [Google Scholar] [CrossRef]
- Dawkar, V.V.; Jadhav, U.U.; Jadhav, M.U.; Kagalkar, A.N.; Govindwar, S.P. Decolorization and detoxification of sulphonatedazo dye Red HE7B by Bacillus sp.VUS. World J. Microbiol. Biotechnol. 2010, 26, 909–916. [Google Scholar] [CrossRef]
- Kurade, M.B.; Waghmode, T.R.; Kagalkar, A.N.; Govindwar, S.P. Decolorization of textile industry effluent containing disperse dye Scarlet RR by a newly developed bacterial-yeast consortium BL-GG. Chem. Eng. J. 2012, 184, 33–41. [Google Scholar] [CrossRef]
- Pandi, A.; Kuppuswami, G.M.; Ramudu, K.N.; Palanivel, S. A sustainable approach for degradation of leather dyes by a new fungal laccase. J. Clean. Prod. 2019, 211, 590–597. [Google Scholar] [CrossRef]
- Zhang, S.J.; Yang, M.; Yang, Q.X.; Zhang, Y.; Xin, B.P.; Pan, F. Biosorption of reactive dyes by the mycelium pellets of a new isolate of Penicilliumoxalicum. Biotechnol. Lett. 2003, 25, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Bhowal, J.; Das, A.R.; Guha, A.K. Adsorption behavior of rhodamine B on Rhizopusoryzae biomass. Langmuir 2006, 22, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ghada, W.A.S.; Mostafa, M.E.L. Biodegradation of basic fuchsin and methyl red by the blue green algae Hydrocoleumoligotrichum and Oscillatoria limnetica. Environ. Eng. Manag. J. 2016, 15, 279–286. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.W.; Jang, T.; Yoon, S.; Choi, J.H.; Hong, E.; Park, J.A. Adsorption of cationic dyes using Sargassumhorneri and Ulvaaustralis biosorbent. Desalination Water Treat. 2025, 323, 101351. [Google Scholar] [CrossRef]
- Labena, A.; Abdelhamid, A.E.; Amin, A.S.; Husien, S.; Hamid, L.; Safwat, G.; Diab, A.; Gobouri, A.A.; Azab, E. Removal of Methylene Blue and Congo Red Using Adsorptive Membrane Impregnated with Dried Ulvafasciata and Sargassumdentifolium. Plants 2021, 10, 384. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Quan, X.; Zhang, J.; Zhao, H.; Chen, S. Effects of an electric field and zero valent iron on anaerobic treatment of azo dye wastewater and microbial community structures. Bioresour. Technol. 2011, 102, 2578–2584. [Google Scholar] [CrossRef]
- Yang, H.Y.; He, C.S.; Li, L.; Zhang, J.; Shen, J.Y.; Mu, Y.; Yu, H.Q. Process and kinetics of azo dye decolourization in bioelectrochemical systems: Effect of several key factors. Sci. Rep. 2016, 6, 27243. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Motamedi, E.; Salekdeh, G.H. Highly efficient removal of dyes from wastewater using nanocellulose from quinoa husk as a carrier for immobilization of laccase. Bioresour. Technol. 2022, 349, 126833. [Google Scholar] [CrossRef]
- Riaz, A.; Kalsoom, U.; Bhatti, H.N.; Jesionowski, T.; Bilal, M. Citrus limon peroxidase-assisted biocatalytic approach for biodegradation of reactive 1847 colfax blue P3R and 621 colfax blue R dyes. Bioprocess Biosyst. Eng. 2023, 46, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J. Recent advances in bioreactor engineering. Korean J. Chem. Eng. 2010, 27, 1035–1041. [Google Scholar] [CrossRef]
- Agrawal, S.; Tipre, D.; Dave, S.; Jamil, N.; Kumar, P.; Batool, R. (Eds.) Soil Microenvironment for Bioremediation and Polymer Production; Wiley: New York, NY, USA, 2019; ISBN 1119592054. [Google Scholar]
- Narayanan, C.M.; Narayan, V. Biological wastewater treatment and bioreactor design: A review. Sustain. Environ. Res. 2019, 1, 33. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Yan, B.; Li, T.; Jegatheesan, V.; Zhang, Y. Treatment of anthraquinone dye textile wastewater using anaerobic dynamic membrane bioreactor: Performance and microbial dynamics. Chemosphere 2020, 238, 124539. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Teng, J.Y. Anaerobic decolorization bacteria for the treatment of azo dye in a sequential anaerobic and aerobic membrane bioreactor. J. Taiwan Inst. Chem. Eng. 2009, 40, 500–504. [Google Scholar] [CrossRef]
- Bai, Y.N.; Wang, X.N.; Zhang, F.; Wu, J.; Zhang, W.; Lu, Y.Z.; Fu, L.; Lau, T.C.; Zeng, R.J. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard. Mater. 2020, 388, 121753. [Google Scholar] [CrossRef]
- Schirmer, C.; Nussbaumer, T.; Schöb, R.; Pörtner, R.; Eibl, R.; Eibl, D. Development, Engineering and Biological Characterization of Stirred Tank Bioreactors. Biopharmaceuticals 2018, 5, 87–107. [Google Scholar] [CrossRef]
- Powell, E.E.; Hill, G.A. Optimization of continuously stirred tank bioreactor design for cost minimization: Effect of microbial species and operating conditions. Int. J. Chem. React. Eng. 2008, 6, 1–26. [Google Scholar] [CrossRef]
- Khelifi, E.; Gannoun, H.; Touhami, Y.; Bouallagui, H.; Hamdi, M. Aerobic decolourization of the indigo dye-containing textile wastewater using continuous combined bioreactors. J. Hazard. Mater. 2008, 152, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology 1999, 30, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Courtois, D.; Hénault, N.; Cuvier, A.; Bastin, M.; Aknin, A.; Dubreuil, J.; Pétiard, V. Two new disposable bioreactors for plant cell culture: The wave and undertow bioreactor and the slug bubble bioreactor. Biotechnol. Bioeng. 2007, 96, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Sodaneath, H.; Lee, J.I.; Yang, S.O.; Jung, H.; Ryu, H.W.; Cho, K.S. Decolorization of textile dyes in an air-lift bioreactor inoculated with Bjerkandera adusta OBR105. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 1099–1111. [Google Scholar] [CrossRef]
- Kurade, M.B.; Waghmode, T.R.; Patil, S.M.; Jeon, B.H.; Govindwar, S.P. Monitoring the gradual biodegradation of dyes in a simulated textile effluent and development of a novel triple layered fixed bed reactor using a bacterium-yeast consortium. Chem. Eng. J. 2017, 307, 1026–1036. [Google Scholar] [CrossRef]
- Khataee, A.; Vahid, B.; Aghdasinia, H.; Bagheri, R. Semi-pilot scale fluidized bed reactor for removal of a textile dye through heterogeneous Fenton process using natural pyrite. Int. J. Environ. Sci. Technol. 2018, 15, 289–300. [Google Scholar] [CrossRef]
- Setty, S. Multistage fluidized bed bioreactor for dye decolorization using immobilized polyurethane foam: A novel approach. Biochem. Eng. J. 2019, 152, 107368. [Google Scholar] [CrossRef]
- Rene, E.R.; Kim, S.J.; Park, H.S. Effect of COD/N ratio and salinity on the performance of sequencing batch reactors. Bioresour. Technol. 2008, 99, 839–846. [Google Scholar] [CrossRef]
- Zou, H.; Wang, Y. Azo dyes wastewater treatment and simultaneous electricity generation in a novel process of electrolysis cell combined with microbial fuel cell. Bioresour. Technol. 2017, 235, 167–175. [Google Scholar] [CrossRef]
- Ulson, S.M.D.A.G.; Bonilla, K.A.S.; de Souza, A.A.U. Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J. Hazard. Mater. 2010, 179, 35–42. [Google Scholar] [CrossRef]
- Santos, A.B.D.; Madrid, M.P.D.; Stams, A.J.M.; Lier, J.B.V.; Cervantes, F.J. Azo Dye Reduction by Mesophilic and Thermophilic Anaerobic. Biotechnol. Prog. 2005, 21, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Coates, J.D. Electrotrophy. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Y.; Quan, X.; Liu, Y.; Onu, P. A built-in zero valent iron anaerobic reactor to enhance treatment of azo dye wastewater. Water Sci. Technol. 2011, 63, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Quan, X.; Li, Y.; Chen, S.; Zhao, H.; Wang, D. An anaerobic reactor packed with a pair of Fe-graphite plate electrodes for bioaugmentation of azo dye wastewater treatment. Biochem. Eng. J. 2012, 63, 31–37. [Google Scholar] [CrossRef]
- Wang, H.C.; Cheng, H.Y.; Wang, S.S.; Cui, D.; Han, J.L.; Hu, Y.P.; Su, S.G.; Wang, A.J. Efficient treatment of azo dye containing wastewater in a hybrid acidogenic bioreactor stimulated by biocatalyzed electrolysis. J. Environ. Sci. 2016, 39, 198–207. [Google Scholar] [CrossRef]
- Moussa, H.; Hamid, S.; Mameri, A.; Lekmine, S.; Tahraoui, H.; Kebir, M.; Touzout, N.; Dahmoune, F.; Ola, M.S.; Zhang, J.; et al. From Green Chemistry to Healthy Environments: Silver Nanoparticles as a Dual Antioxidant and Antibacterial Agents for Advancing Biomedicine and Sustainable Wastewater Treatment. Bioengineering 2024, 11, 1205. [Google Scholar] [CrossRef]
- Long, X.; Pan, Q.; Wang, C.; Wang, H.; Li, H.; Li, X. Microbial fuel cell-photoelectrocatalytic cell combined system for the removal of azo dye wastewater. Bioresour. Technol. 2017, 244, 182–191. [Google Scholar] [CrossRef]
- Logroño, W.; Pérez, M.; Urquizo, G.; Kadier, A.; Echeverría, M.; Recalde, C.; Rákhely, G. Single chamber microbial fuel cell (SCMFC) with a cathodic microalgal biofilm: A preliminary assessment of the generation of bioelectricity and biodegradation of real dye textile wastewater. Chemosphere 2017, 176, 378–388. [Google Scholar] [CrossRef]
- Khalili, H.B.; Mohebbi-Kalhori, D.; Afarani, M.S. Microbial fuel cell (MFC) using commercially available unglazed ceramic wares: Low-cost ceramic separators suitable for scale-up. Int. J. Hydrogen Energy 2017, 42, 8233–8241. [Google Scholar] [CrossRef]
- Solanki, K.; Subramanian, S.; Basu, S. Microbial fuel cells for azo dye treatment with electricity generation: A review. Bioresour. Technol. 2013, 131, 564–571. [Google Scholar] [CrossRef]
- Liu, L.; Tsyganova, O.; Lee, D.J.; Chang, J.S.; Wang, A.; Ren, N. Double-chamber microbial fuel cells started up under room and low temperatures. Int. J. Hydrogen Energy 2013, 38, 15574–15579. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J. Microbial fuel cells as pollutant treatment units: Research updates. Bioresour. Technol. 2016, 217, 121–128. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.C.; Chen, H.R.; Chen, C.Y.; Tseng, M.J.; Cheng, K.C.; Shih, R.C.; Chang, Y.F. A single-chamber microbial fuel cell without an air cathode. Int. J. Mol. Sci. 2012, 13, 3933–3948. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Logan, B.E. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 2010, 105, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Malik, A. Degradation of Reactive Black 5 dye by a newly isolated bacterium Pseudomonas entomophila BS1. Can. J. Microbiol. 2016, 62, 220–232. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, J.; Lu, Y.M.; Jia, R. Degradation and detoxification of the triphenylmethane dye malachite green catalyzed by crude manganese peroxidase from Irpexlacteus F17. Environ. Sci. Pollut. Res. 2016, 23, 9585–9597. [Google Scholar] [CrossRef]
- Miran, W.; Rasool, K.; Nawaz, M.; Kadam, A.; Shin, S.; Heo, J.; Jang, J.; Sung Lee, D. Simultaneous electricity production and Direct Red 80 degradation using a dual chamber microbial fuel cell. Desalination Water Treat. 2016, 57, 9051–9059. [Google Scholar] [CrossRef]
- Yuan, G.E.; Li, Y.; Lv, J.; Zhang, G.; Yang, F. Integration of microbial fuel cell and catalytic oxidation reactor with iron phthalocyanine catalyst for Congo red degradation. Biochem. Eng. J. 2017, 120, 118–124. [Google Scholar] [CrossRef]
- Qin, L.J.; Han, K.; Yueh, P.L.; Hsueh, C.C.; Chen, B.Y. Interactive influences of decolorized metabolites on electron-transfer characteristics of microbial fuel cells. Biochem. Eng. J. 2016, 109, 297–304. [Google Scholar] [CrossRef]
- Cetinkaya, A.Y.; Ozkaya, B.; Taskan, E.; Karadag, D.; Cakmakci, M. The production of electricity from dual-chambered microbial fuel cell fueled by old age leachate. Energy Sources, Part A Recover. Util. Environ. Eff. 2016, 38, 1544–1552. [Google Scholar] [CrossRef]
- Erable, B.; Byrne, N.; Etcheverry, L.; Achouak, W.; Bergel, A. Single medium microbial fuel cell: Stainless steel and graphite electrode materials select bacterial communities resulting in opposite electrocatalytic activities. Int. J. Hydrogen Energy 2017, 42, 26059–26067. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ellis, M.W.; Nain, A.S.; Behkam, B. Effect of electrode sub-micron surface feature size on current generation of Shewanella oneidensis in microbial fuel cells. J. Power Sources 2017, 347, 270–276. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Wei, S.; Yang, F.; Li, X. Bio-cathode materials evaluation and configuration optimization for power output of vertical subsurface flow constructed wetland—Microbial fuel cell systems. Bioresour. Technol. 2014, 166, 575–583. [Google Scholar] [CrossRef]
- Tursun, H.; Liu, R.; Li, J.; Abro, R.; Wang, X.; Gao, Y.; Li, Y. Carbon material optimized biocathode for improving microbial fuel cell performance. Front. Microbiol. 2016, 7, 6. [Google Scholar] [CrossRef]
- Khan, M.D.; Abdulateif, H.; Ismail, I.M.; Sabir, S.; Khan, M.Z. Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. PLoS ONE 2015, 10, e0138448. [Google Scholar] [CrossRef]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous cellulose degradation and electricity production by Enterobacter cloacae in a microbial fuel cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef]
- Liu, L.; Bilal, M.; Duan, X.; Iqbal, H.M. Mitigation of environmental pollution by genetically engineered bacteria- current challenges and future perspectives. Sci. Total Environ. 2019, 667, 444–454. [Google Scholar] [CrossRef]
- Jafari, M.; Danesh, Y.R.; Goltapeh, E.M.; Varma, A. Bioremediation and Genetically Modified Organisms. In Fungi as Bioremediators; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 433–451. [Google Scholar]
- Bu, T.; Yang, R.; Zheng, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site directed mutagenesis. Peer J. 2020, 8, e10267. [Google Scholar] [CrossRef]
- Dixit, S.; Garg, S. Biodegradation of environmentally hazardous azo-dyes and aromatic amines using Klebsiella pneumoniae. J. Environ. Eng. 2018, 144, 04018035. [Google Scholar] [CrossRef]
- Chang, J.S.; Kuo, T.S.; Chao, V.P.; Ho, J.Y.; Lin, P.J. Azo dye decolorization with a mutant E. coli strain. Biotechnol. Lett. 2000, 22, 807–812. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, P.; Singh, R.; Bala, S.; Pathak, N.; Singh, S.; Chauhan, R.S.; Singh, P.K. Microbial biosorbent for remediation of dyes and heavy metal pollution: A green strategy for sustainable environment. Front. Microbiol. 2023, 14, 1168954. [Google Scholar] [CrossRef]
- Balrabe, Y.B.; Oumarou, I.N.M.; Korony, S.A.; Adjama, I.; Baraze, I.R.A. Photooxidation of organic dye by Fe2O3 nanoparticles; catalyst, electron acceptor and polyurethane membrane effects. J. Nanotechnol. 2023, 2023, 1292762. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Li, H.; Matter, I.A. Nano-bioremediation of textile industry wastewater using immobilized CuO-NPs myco-synthesized by a novel Cu-resistant Fusarium oxysporum OSF18. Environ. Sci. Pollut. Res. 2023, 30, 16694–16706. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Removal of organic dyes by functionalized nanomaterials. In Handbook of Green and Sustainable Nanotechnology; Shanker, U., Hussain, C.M., Rani, M., Eds.; Springer: Cham, Switzerland, 2023; Volume 4, pp. 1267–1298. [Google Scholar] [CrossRef]
- Singh, G.; Chaudhary, S.; Giri, B.S.; Mishra, V.K. Assessment of geochemistry and irrigation suitability of the River Ganga, Varanasi, India: PCA reduction for water quality index and health risk evaluation. Environ. Sci. Pollut. Res. 2025, 32, 4199–4218. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Giri, B.S.; Thivaharan, V.; Srivastava, A.K.; Kumar, S.; Singh, R.P.; Kumar, R. Sequestration of simulated carbon dioxide (CO2) using churning cementations waste and fly-ash in a thermo-stable batch reactor (TSBR). Environ. Sci. Pollut. Res. 2020, 27, 27470–27479. [Google Scholar] [CrossRef] [PubMed]


| Type of Species | Name of Species | Dye | Optimum Operating Conditions (Static) | Peak Removal Efficiency | Ref. |
|---|---|---|---|---|---|
| Bacteria | Pseudomonas entomophilaBS1 | Reactive black 5 | 5–9 pH, 37 °C, 120 h, 500 mg/L | 93% | [19] |
| Consortium of pseudomonas species SUK1 and pseudomonas rettgeri strain HSL1 | Reactive orange 16 | 30 ± 02 °C 18–24 h, 100 mg/L | 100% | [37] | |
| Disperse red 78 | 30 ± 02 °C 36–42 h, 100 mg/L | 100% | [37] | ||
| Reactive black 5 | 30 ± 02 °C 48 h, 100 mg/L | 58% | [37] | ||
| Direct red 81 | 30 ± 02 °C 48 h, 100 mg/L | 92% | [37] | ||
| DDMY1 | Reactive black 5 | 5.0 pH, 100 mg/L 30–40 °C | 87.45 ± 1.09% | [38] | |
| Reactive black 5 | 9.0 pH, 100 mg/L 30–40 °C | 88.89 ± 2.56%. | [38] | ||
| Bacteria | Aeromonashydrophila | Reactive black 5 | 24 h, 100 mg/L | 76% | [56] |
| Bacillus cereus (MTCC 9777) | Acid orange 7 | pH 8.0, 96 h, 100 mg/L | 52.5% | [20] | |
| Kocuriarosea MTCC 1532 | Methyl orange | 6.8 pH, 30 °C, 50 mg/L | 100% | [57] | |
| Pseudomona ssp. SUK1 | Reactive red 2 | 6.2–7.5 pH, 30 °C, 24 h, 5 mg/L | 91% | [58] | |
| Pseudomonas aeruginosa NBAR12 | Reactive blue 172 | 7.0 pH, 40 °C 42 h, 500 mg/L | 83% | [59] | |
| Lysinibacillus sp. RGS. | C.I. Remazol red | 7.8 pH, 30 °C, 48 h, 50 mg/L | 87% | [60] | |
| Bacillus sp.VUS | Red HE7B | 18 h, 50 mg/L | 100% | [61] | |
| Bacteria- yeast consortium | Brevibacilluslaterosporus MTCC 2298—Galactomycesgeotrichum MTCC1360 | Scarlet RR | 9.0 pH, 40 °C, 18 h, 50 mg/L | 98% | [62] |
| Fungus | Penicilliumoxalicum(SAR-3) | Direct red 75, direct blue 15 and acid red 183 | 7.0 pH, 30 °C, 120 h, 100 mg/L | 96.6 ± 3.25% | [41] |
| Fungus | Laccase from Peroneutypascoparia | Acid red 97 | 6.0 pH, 40 °C, 84 h, 100 mg/L | 75% | [63] |
| Bacilluscereus (MTCC 9777) RMLAU1 | Acid orange 7 | 8.0 pH, 33 °C, 96 h, 100 mg/L | 68.5% | [20] | |
| mycelium pellets of Penicilliumoxalicum | Reactive blue 19 | 2.0 pH, 20 °C, 100 mg/L | 91% | [64] | |
| Rhizopusoryzae MTCC 262 | Rhodamine B | 7.0 pH,40 °C, 05 h, 100 mg/L | 90% | [65] | |
| Neurosporacrassa | Acid red 57 | 1.0 pH, 20 °C, 40 min, 100 mg/L | 98.7% | [45] | |
| Curvularia clavate NZ2 | Congo red (CR) | 5.0 pH, 05 h, 100 mg/L | 96.1 ± 1% | [44] | |
| Curvularia clavate NZ2 | RB5 | 5.0 pH, 05 h, 100 mg/L | 90.3 ± 1.86% | [44] | |
| Curvularia clavate NZ2 | Acid orange 7 (AO7) | 5.0 pH, 05 h, 100 mg/L | 46.3 ± 1.86% | [44] | |
| Lasiodiplodia so. | Malachite green | 7.0 pH,30 °C, 24 h, 50 mg/L | 96.9% | [46] | |
| Algae | Hydrocoleumoligotrichum | Basic fuchsin | 7 days, 5 mg/L | 92.44% | [66] |
| Algae | Oscillatorialimnetica | Basic fuchsin | 7 days, 5 mg/L | 90.23% | [66] |
| Hydrocoleumoligotrichum | Methyl red | 7 days, 20 mg/L | 53.23% | [66] | |
| Oscillatorialimnetica | Methyl red | 7 days, 20 mg/L | 50.18% | [66] | |
| Chlorella vulgaris | Remazol black B | 2.0 pH, 35 °C, 800 mg/L | 53.2% | [67] | |
| Sargassumhorneri | Methylene blue | 5.0–5.5 pH, 25 °C, 02 h, 200 mg/L | 92.5% | [68] | |
| Sargassumhorneri | Methylene blue | 5.0–5.5 pH, 25 °C, 02 h, 200 mg/L | 89.7% | [68] | |
| Ulvaaustralis | Toluidine blue | 5.0–5.5 pH, 25 °C, 02 h, 200 mg/L | 95.3% | [68] | |
| Ulvaaustralis | Toluidine blue | 5.0–5.5 pH, 25 °C, 02 h, 200 mg/L | 96.4% | [68] | |
| Ulvafasciata | Methylene blue | 04 h, 100 mg/L | 88.9% | [69] | |
| Ulvafasciata | Congo red | 04 h, 50 mg/L | 79.6% | [69] | |
| Sargassumdentifolium | Methylene blue | 30 min, 100 mg/L | 82.1% | [69] | |
| Algae | Sargassumdentifolium | Congo red | 04 h, 100 mg/L | 85% | [69] |
| Yeast | Sterigmatomyces halophilus SSA1575 | Reactive black 5 | 5.0 pH, 30 °C, 18 h, 50 mg/L | 100% | [13] |
| Candida rugopelliculosa HXL-2 | Reactive blue 13 | 5.0 pH, 28 °C, 28 h, 50 mg/L | 90% | [70] | |
| Enzyme | Irpexlacteus F17 | Malachite green | 3.1 pH, 40 °C, 24 h, 200 mg/L | 96% | [71] |
| Nanocellulose immobilized laccase enzyme (PersiLac1) | Malachite green | 5.0 pH, 50 °C, 24 h, 150 mg/L | 98% | [72] | |
| Nanocellulose immobilized laccase enzyme (PersiLac1) | Congo red | 5.0 pH, 50 °C, 24 h, 150 mg/L | 60% | [72] | |
| Citrus limon peroxidase | 1847 Colafx blue P3R | 4.0 pH, 35 °C, 1 h, 200 mg/L | 83% | [73] | |
| Citrus limon peroxidase | 621 Colafx blue | 4.0 pH, 35 °C, 1 h, 200 mg/L | 99% | [73] |
| Technique | Advantages | Scalability | Economic Feasibility |
|---|---|---|---|
| ASP | Well-studied, effective | High | Moderate |
| Algal | Eco-friendly, multi-nutrient removal | Moderate | Moderate |
| Fungal | Effective for complex dyes | Moderate | Moderate |
| Bacterial | Fast, adaptable | High | High |
| Bioreactors | Controlled, efficient, compact | High | Moderate |
| Wetlands/phyto | Low-maintenance, sustainable | Moderate | High |
| Enzyme-based | High specificity, no biomass | Low–Moderate | Low |
| Algal | Eco-friendly, multi-nutrient removal | Moderate | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Mishra, A.; Patel, S.K.; Kushwaha, J.; Singh, S.; Mishra, V.; Singh, D.; Singh, V.; Giri, B.S.; Singhania, R.R.; et al. Environmental Impacts and Strategies for Bioremediation of Dye-Containing Wastewater. Bioengineering 2025, 12, 1043. https://doi.org/10.3390/bioengineering12101043
Kumar M, Mishra A, Patel SK, Kushwaha J, Singh S, Mishra V, Singh D, Singh V, Giri BS, Singhania RR, et al. Environmental Impacts and Strategies for Bioremediation of Dye-Containing Wastewater. Bioengineering. 2025; 12(10):1043. https://doi.org/10.3390/bioengineering12101043
Chicago/Turabian StyleKumar, Mukesh, Anshuman Mishra, Suresh Kumar Patel, Jyoti Kushwaha, Sunita Singh, Vinay Mishra, Deepak Singh, Vijay Singh, Balendu Shekher Giri, Reeta Rani Singhania, and et al. 2025. "Environmental Impacts and Strategies for Bioremediation of Dye-Containing Wastewater" Bioengineering 12, no. 10: 1043. https://doi.org/10.3390/bioengineering12101043
APA StyleKumar, M., Mishra, A., Patel, S. K., Kushwaha, J., Singh, S., Mishra, V., Singh, D., Singh, V., Giri, B. S., Singhania, R. R., & Singh, D. (2025). Environmental Impacts and Strategies for Bioremediation of Dye-Containing Wastewater. Bioengineering, 12(10), 1043. https://doi.org/10.3390/bioengineering12101043









