Thermal Evaluation of Bone Drilling: Assessing Drill Bits and Sequential Drilling
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Sequential drilling using the manufacturer’s recommended drill bits and corresponding spindle speeds;
- (2)
- Sequential peck drilling using the manufacturer’s recommended drill bits and corresponding spindle speeds;
- (3)
- Sequential drilling using the manufacturer’s recommended drill bits with a constant spindle speed of 1500 rpm.
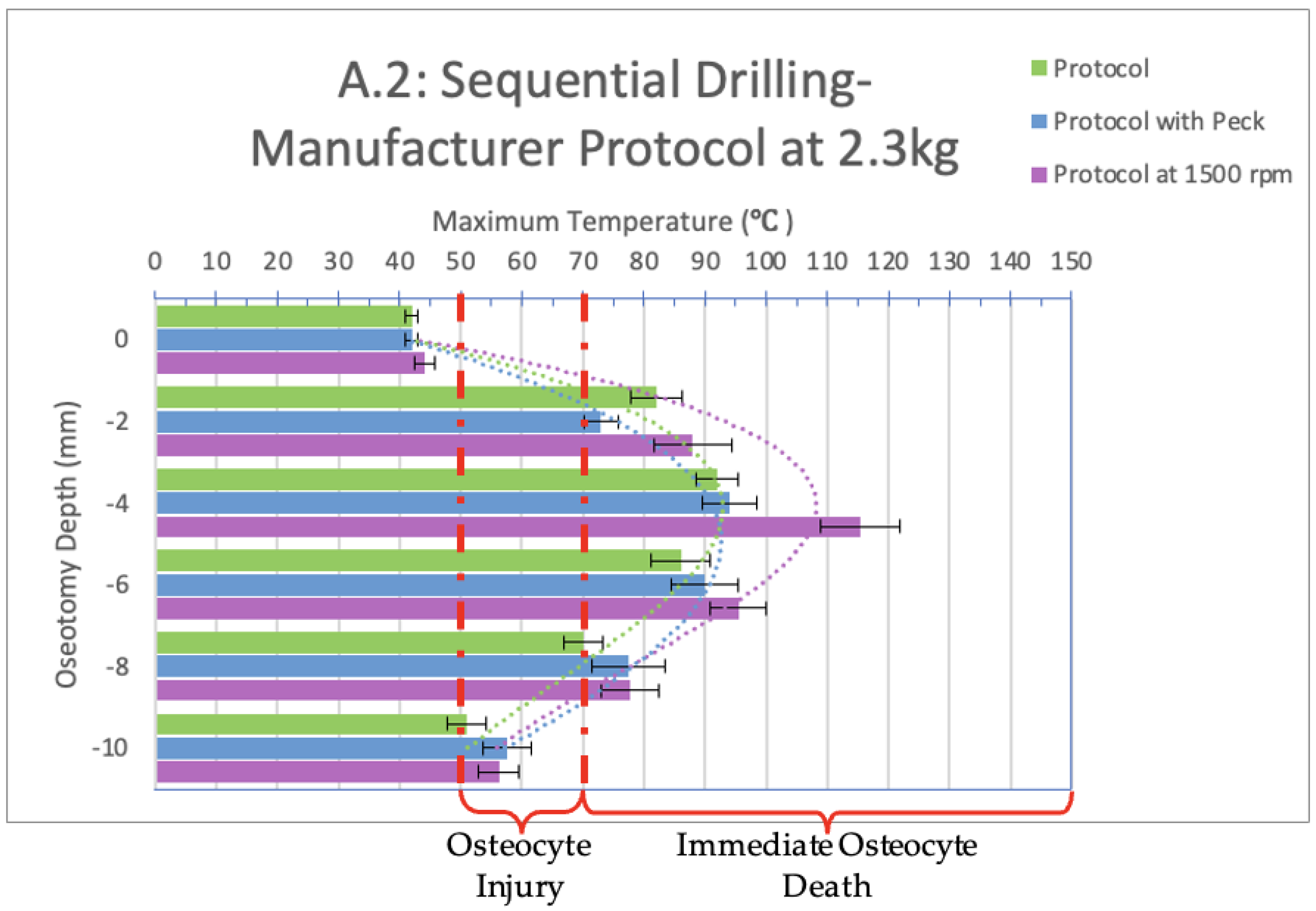
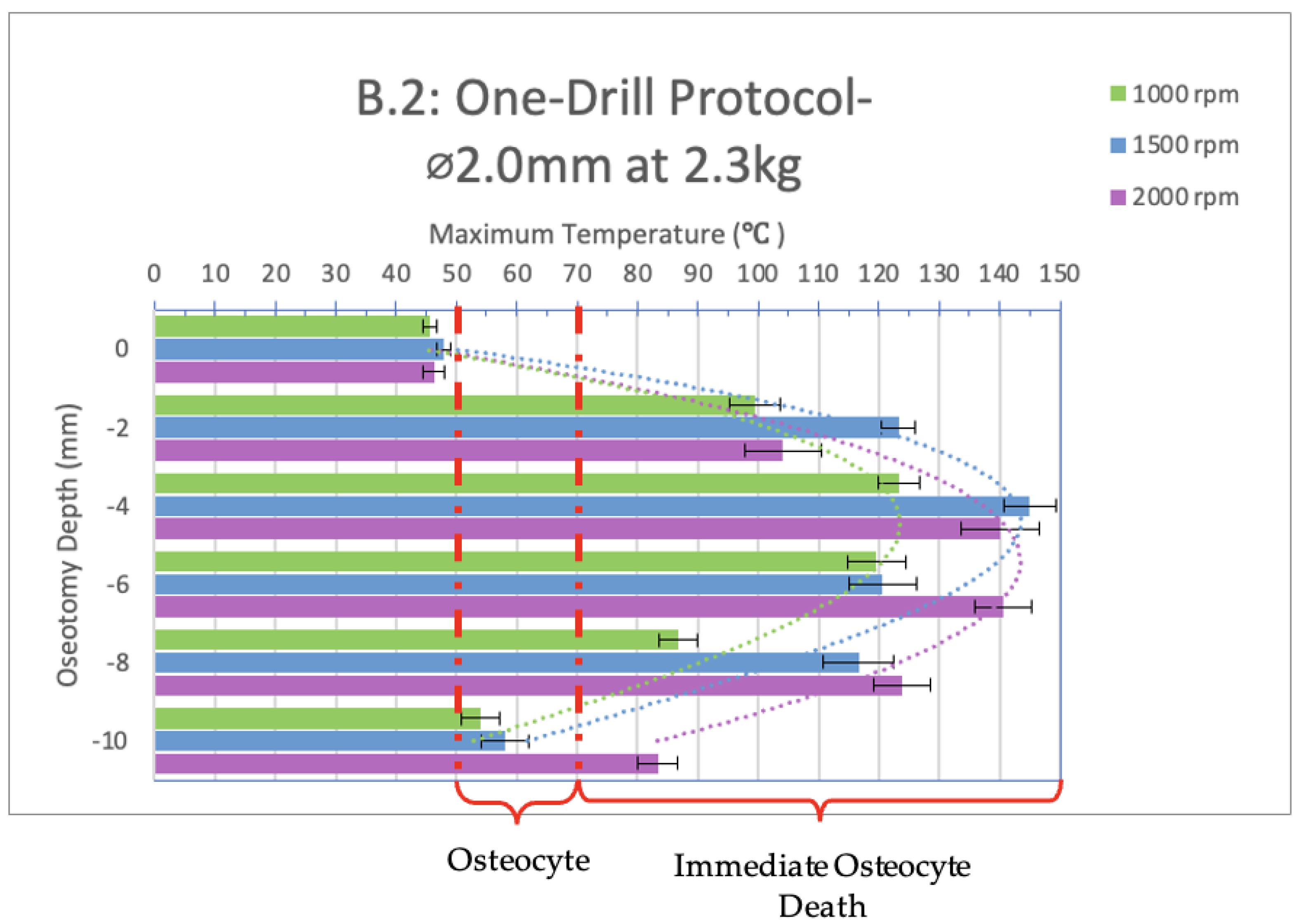
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Eriksson, A.; Albrektsson, T.; Grane, B.; McQueen, D. Thermal injury to bone. A vital-microscopic description of heat effects. Int. J. Oral Surg. 1982, 11, 115–121. [Google Scholar] [CrossRef]
- Lundskog, J. Heat and bone tissue. An experimental investigation of the thermal properties of bone and threshold levels for thermal injury. Scand. J. Plast. Reconstr. Surg. 1972, 9, 1–80. [Google Scholar]
- Eriksson, R.A.; Albrektsson, T.; Magnusson, B. Assessment of bone viability after heat trauma. A histological, histochemical and vital microscopic study in the rabbit. Scand. J. Plast. Reconstr. Surg. 1984, 18, 261–268. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Adell, R. Temperatures during drilling for the placement of implants using the osseointegration technique. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1986, 44, 4–7. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T.; Albrektsson, B. Heat caused by drilling cortical bone. Temperature measured in vivo in patients and animals. Acta Orthop. Scand. 1984, 55, 629–631. [Google Scholar] [CrossRef]
- Lavelle, C.; Wedgwood, D. Effect of internal irrigation on frictional heat generated from bone drilling. J. Oral Surg. 1980, 38, 499–503. [Google Scholar]
- Karmani, S.; Lam, F. The design and function of surgical drills and K-wires. Curr. Orthop. 2004, 18, 484–490. [Google Scholar] [CrossRef]
- Karmani, S. The thermal properties of bone and the effects of surgical intervention. Curr. Orthop. 2006, 20, 52–58. [Google Scholar] [CrossRef]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical bone drilling and thermal osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef]
- Heuzeroth, R.; Pippenger, B.E.; Sandgren, R.; Bellón, B.; Kühl, S. Thermal exposure of implant osteotomies and its impact on osseointegration-A preclinical in vivo study. Clin. Oral Implant. Res. 2021, 32, 672–683. [Google Scholar] [CrossRef]
- Tabassum, A.; Wismeijer, D.; Hogervorst, J.; Tahmaseb, A. Comparison of Proliferation and Differentiation of Human Osteoblast-like Cells Harvested During Implant Osteotomy Preparation Using Two Different Drilling Protocols. Int. J. Oral Maxillofac. Implant. 2020, 35, 141–149. [Google Scholar] [CrossRef]
- Rugova, S.; Abboud, M. Standardized Testing for Thermal Evaluation of Bone Drilling: Towards Predictive Assessment of Thermal Trauma. Bioengineering 2024, 11, 642. [Google Scholar] [CrossRef]
- Markovic, A.; Mišić, T.; Mančić, D.; Jovanović, I.; Šćepanović, M.; Jezdić, Z. Real-time thermographic analysis of low-density bone during implant placement: A randomized parallel-group clinical study comparing lateral condensation with bone drilling surgical technique. Clin. Oral. Implant. Res. 2014, 25, 910–918. [Google Scholar] [CrossRef]
- Berman, A.T.; Reid, J.S.; Yanicko, D.R., Jr.; Sih, G.C.; Zimmerman, M.R. Thermally induced bone necrosis in rabbits. Relation to implant failure in humans. Clin. Orthop. Relat. Res. 1984, 186, 284–292. [Google Scholar] [CrossRef]
- Akhbar, M.F.A.; Sulong, A.W. Surgical Drill Bit Design and Thermomechanical Damage in Bone Drilling: A Review. Ann. Biomed. Eng. 2021, 49, 29–56. [Google Scholar] [CrossRef]
- Islam, M.A.; Kamarrudin, N.S.; Daud, R.; Mohd Noor, S.N.F.; Azmi, A.I.; Razlan, Z.M. A Review of Surgical Bone Drilling and Drill Bit Heat Generation for Implantation. Metals 2022, 12, 1900. [Google Scholar] [CrossRef]
- Rugova, S. Implant Bed Preparation Testing. Master’s Thesis, Stony Brook University, Stony Brook, NY, USA, 2015. [Google Scholar]
- Kalidindi, V. Optimization of Drill Design and Coolant Systems during Dental Implant Surgery. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2004. [Google Scholar]
- Cordioli, G.; Majzoub, Z. Heat generation during implant site preparation: An in vitro study. Int. J. Oral Maxillofac. Implant. 1997, 12, 186–193. [Google Scholar]
- Timon, C.; Keady, C. Thermal Osteonecrosis Caused by Bone Drilling in Orthopedic Surgery: A Literature Review. Cureus 2019, 11, e5226. [Google Scholar] [CrossRef]
- Isler, S.C.; Cansiz, E.; Tanyel, C.; Soluk, M.; Selvi, F.; Cebi, Z. The effect of irrigation temperature on bone healing. Int. J. Med. Sci. 2011, 8, 704–708. [Google Scholar] [CrossRef]
- Pandey, R.K.; Panda, S.S. Drilling of bone: A comprehensive review. J. Clin. Orthop. Trauma 2013, 4, 15–30. [Google Scholar] [CrossRef]
- Javed, F.; Kellesarian, S.V.; Abduljabbar, T.; Abduljabbar, A.T.; Akram, Z.; Vohra, F.; Rahman, I.; Romanos, G.E. Influence of involuntary cigarette smoke inhalation on osseointegration: A systematic review and meta-analysis of preclinical studies. Int. J. Oral Maxillofac. Surg. 2018, 47, 764–772. [Google Scholar] [CrossRef]
- César-Neto, J.B.; Benatti, B.B.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H., Jr. The influence of cigarette smoke inhalation and its cessation on the tooth-supporting alveolar bone: A histometric study in rats. J. Periodontal Res. 2006, 41, 118–123. [Google Scholar] [CrossRef]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontol. 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- César-Neto, J.B.; Benatti, B.B.; Sallum, E.A.; Nociti, F.H., Jr. Bone density around titanium implants may benefit from smoking cessation: A histologic study in rats. Int. J. Oral Maxillofac. Implant. 2005, 20, 713–719. [Google Scholar]
- Morris, H.E.; Ochi, S.; Crum, P.; Orenstein, I.; Plezia, R. Bone density: Its influence on implant stability after uncovering. J. Oral Implantol. 2003, 29, 263–269. [Google Scholar] [CrossRef]
- Dalago, H.R.; Schuldt Filho, G.; Rodrigues, M.A.; Renvert, S.; Bianchini, M.A. Risk indicators for Peri-implantitis. A cross-sectional study with 916 implants. Clin. Oral Implant. Res. 2017, 28, 144–150. [Google Scholar] [CrossRef]



| Drilling Groups | Drill Bit Diameter | RPM | |
|---|---|---|---|
| A.1: Sequential drilling at 1.2 kg A.2: Sequential drilling at 2.3 kg | Sequential drilling— manufacturer recommendation | Ø2.0 | 2000 |
| Ø2.5 | 1500 | ||
| Ø3.2 | 1000 | ||
| Ø3.7 | 1000 | ||
| Ø4.1 | 1000 | ||
| A.1: Sequential drilling at 1.2 kg A.2: Sequential drilling at 2.3 kg | Sequential drilling— manufacturer recommendation, including peck drilling | Ø2.0 | 2000 |
| Ø2.5 | 1500 | ||
| Ø3.2 | 1000 | ||
| Ø3.7 | 1000 | ||
| Ø4.1 | 1000 | ||
| A.1: Sequential drilling at 1.2 kg A.2: Sequential drilling at 2.3 kg | Sequential drilling— manufacturer recommendation with constant spindle speed | Ø2.0 | 1500 |
| Ø2.5 | 1500 | ||
| Ø3.2 | 1500 | ||
| Ø3.7 | 1500 | ||
| Ø4.1 | 1500 | ||
| B.1 One-drill protocol—Ø2.0 mm drill bit at 1.2 kg B.2 One-drill protocol—Ø2.0 mm drill bit at 2.3 kg | 1000 | ||
| 1500 | |||
| 2000 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugova, S.; Abboud, M. Thermal Evaluation of Bone Drilling: Assessing Drill Bits and Sequential Drilling. Bioengineering 2024, 11, 928. https://doi.org/10.3390/bioengineering11090928
Rugova S, Abboud M. Thermal Evaluation of Bone Drilling: Assessing Drill Bits and Sequential Drilling. Bioengineering. 2024; 11(9):928. https://doi.org/10.3390/bioengineering11090928
Chicago/Turabian StyleRugova, Sihana, and Marcus Abboud. 2024. "Thermal Evaluation of Bone Drilling: Assessing Drill Bits and Sequential Drilling" Bioengineering 11, no. 9: 928. https://doi.org/10.3390/bioengineering11090928
APA StyleRugova, S., & Abboud, M. (2024). Thermal Evaluation of Bone Drilling: Assessing Drill Bits and Sequential Drilling. Bioengineering, 11(9), 928. https://doi.org/10.3390/bioengineering11090928






