Manufacturing Process Affects Coagulation Kinetics of Ortho-R, an Injectable Chitosan–Platelet-Rich Plasma Biomaterial for Tissue Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Manufacturing of the Chitosan Freeze-Dried Product
2.2. Isolation of Platelet-Rich Plasma
2.3. Preparation of Ortho-R (Chitosan–PRP)
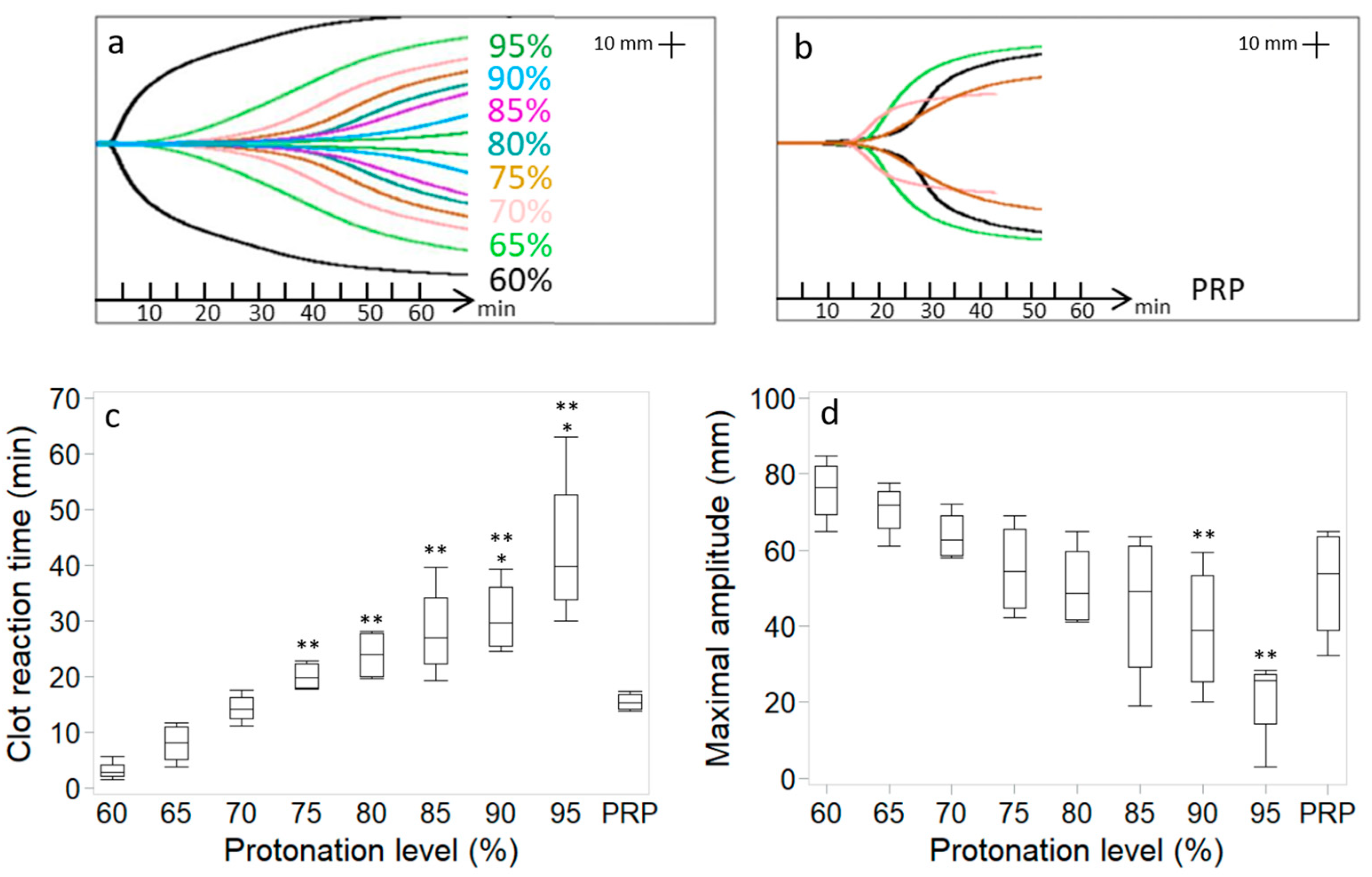
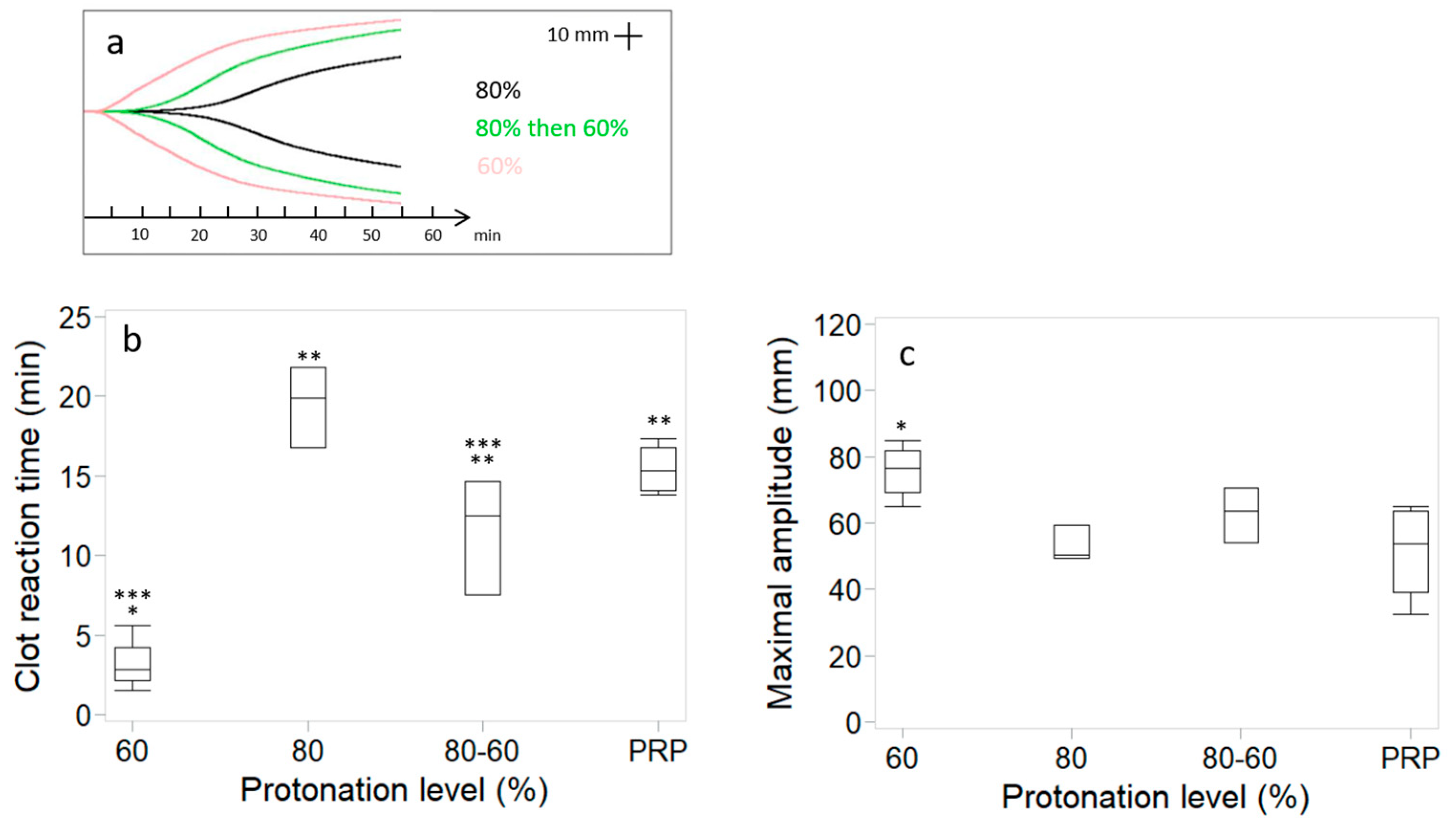
2.4. Assessment of Coagulation Kinetics with Thromboelastography
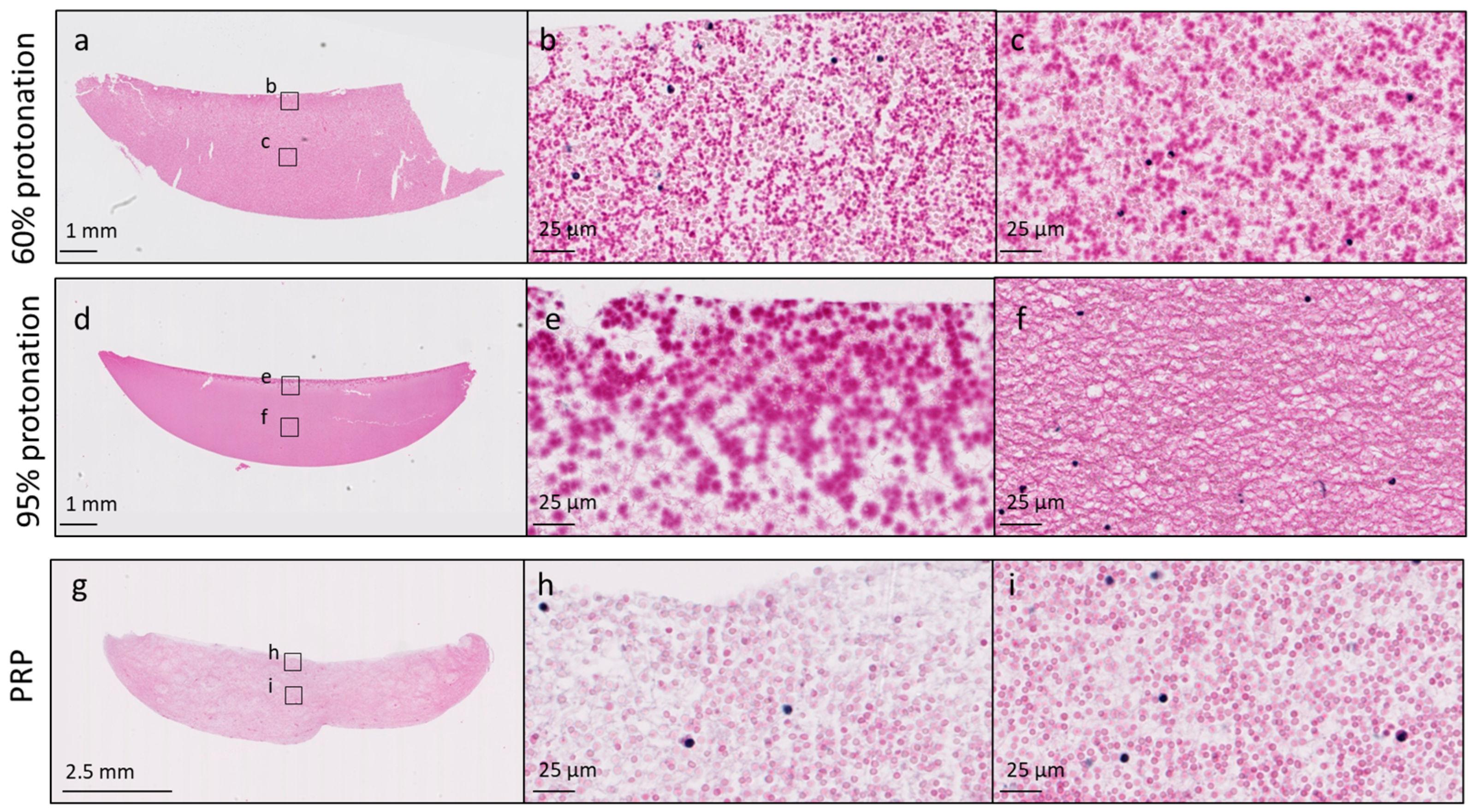
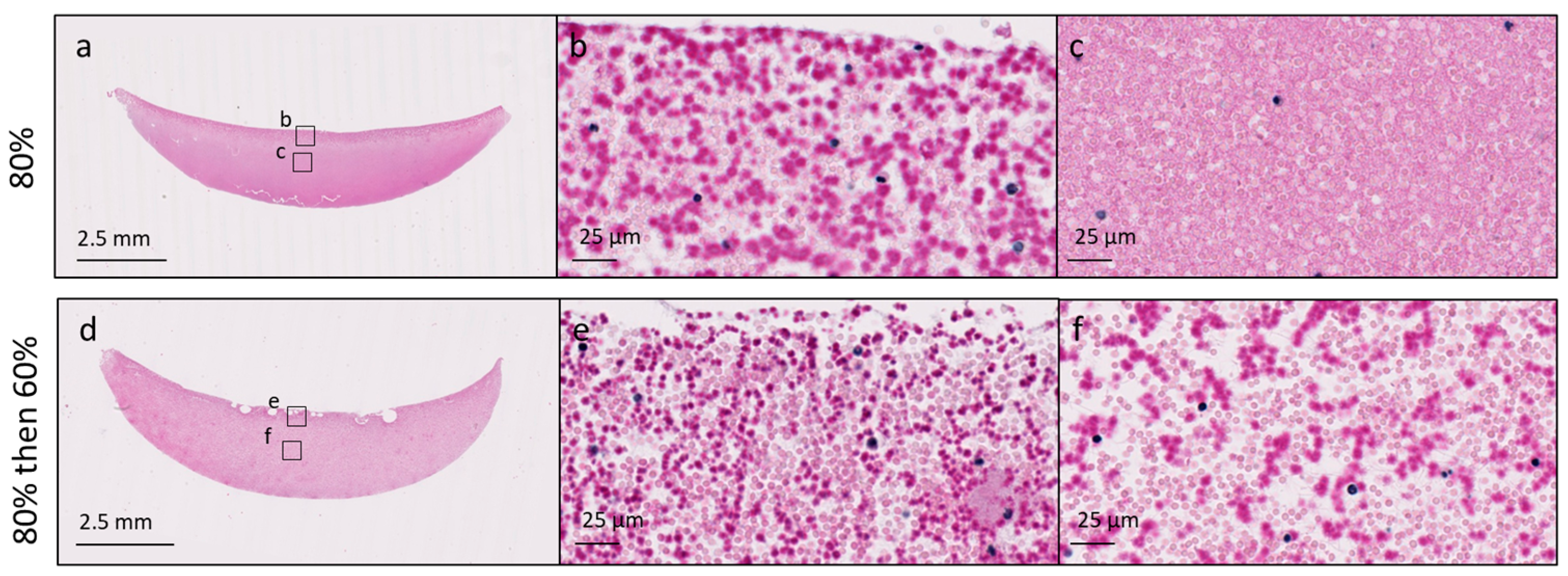
2.5. Assessment of Clot Homogeneity with Histology
2.6. Statistical Analysis
3. Results
3.1. Protonation Level of the Chitosan Amino Groups in the Freeze-Dried Product Affects Chitosan–PRP Coagulation Kinetics and Distribution of Chitosan in the Chitosan–PRP Clots
3.2. Changes in Coagulation Kinetics, Clot Strength and Chitosan Distribution Induced by High Protonation of the Chitosan Amino Groups Are Partially Reversible
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chevrier, A.; Darras, V.; Picard, G.; Nelea, M.; Veilleux, D.; Lavertu, M.; Hoemann, C.D.; Buschman, M.D. Injectable chitosan-platelet-rich plasma (PRP) implants to promote tissue regeneration: In vitro properties, in vivo residence, degradation, cell recruitment and vascularization. J. Tissue Eng. Regen. Med. 2018, 12, 217–228. [Google Scholar] [PubMed]
- Deprés-Tremblay, G.; Chevrier, A.; Tran-Khanh, N.; Nelea, M.; Buschmann, M.D. Chitosan inhibits platelet-mediated clot retraction, increases platelet-derived growth factor release, and increases residence time and bioactivity of platelet-rich plasma in vivo. Biomed. Mater. 2017, 13, 015005. [Google Scholar]
- Ghazi Zadeh, L.; Chevrier, A.; Lamontagne, M.; Buschmann, M.D.; Hoemann, C.D.; Lavertu, M. Multiple platelet-rich plasma preparations can solubilize freeze-dried chitosan formulations to form injectable implants for orthopedic indications. Biomed. Mater. Eng. 2019, 30, 349–364. [Google Scholar] [PubMed]
- Dwivedi, G.; Chevrier, A.; Hoemann, C.D.; Buschmann, M.D. Injectable freeze-dried chitosan-platelet-rich-plasma implants improve marrow-stimulated cartilage repair in a chronic-defect rabbit model. J. Tissue Eng. Regen. Med. 2019, 13, 599–611. [Google Scholar]
- Ghazi Zadeh, L.; Chevrier, A.; Hurtig, M.B.; Farr, J.; Rodeo, S.; Hoemann, C.D.; Buschmann, M.D. Freeze-dried chitosan-PRP injectable surgical implants for meniscus repair: Pilot feasibility studies in ovine models. Reg. Med. Ther. 2017, 1, 16–29. [Google Scholar]
- Deprés-Tremblay, G.; Chevrier, A.; Snow, M.; Rodeo, S.; Buschmann, M.D. Freeze-dried chitosan-platelet-rich plasma implants improve supraspinatus tendon attachment in a transosseous rotator cuff repair model in the rabbit. J. Biomed. App 2019, 33, 792–807. [Google Scholar]
- Deprés-Tremblay, G.; Chevrier, A.; Chevrier, A.; Hurtig, M.B.; Snow, M.; Rodeo, S.; Buschmann, M.D. Freeze-dried chitosan-platelet-rich plasma implants for rotator cuff tear repair: Pilot ovine studies. ACS Biomat Sci. Eng. 2018, 4, 3737–3746. [Google Scholar]
- Chevrier, A.; Hurtig, M.B.; Lavertu, M. Chitosan-platelet-rich plasma implants improve rotator cuff repair in a large animal model: Pilot study. J. Biomed. App 2022, 37, 183–194. [Google Scholar]
- Chevrier, A.; Hurtig, M.B.; Lavertu, M. Chitosan–Platelet-Rich Plasma Implants Improve Rotator Cuff Repair in a Large Animal Model: Pivotal Study. Pharmaceutics 2021, 13, 1955. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Ana 2003, 32, 1149–1158. [Google Scholar]
- Nguyen, S.; Winnik, F.M.; Buschmann, M.D. Improved reproducibility in the determination of the molecular weight of chitosan by analytical size exclusion chromatography. Carbohydr. Polym. 2009, 75, 528–533. [Google Scholar]
- Rossomacha, E.; Hoemann, C.D.; Shive, M.S. Simple methods for staining chitosan in biotechnological applications. J. Histotechnol. 2004, 27, 31–36. [Google Scholar]
- Sidonio, R.F., Jr.; Hofmman, M.; Kenet, G.; Dargaud, Y. Thrombin generation and implications for hemophilia therapies: A narrative review. Res. Pract. Thromb. Haemost. 2023, 7, 100018. [Google Scholar] [PubMed]
- Cap, A.; Hunt, B.J. The pathogenesis of traumatic coagulopathy. Anaesthesia 2015, 70 (Suppl. S1), 96–101. [Google Scholar]
- Davenport, R. Pathogenesis of acute traumatic coagulopathy. Transfusion 2013, 53 (Suppl. S1), 23S–27S. [Google Scholar]
- Maegele, M.; Schöchl, H.; Cohen, M.J. An update on the coagulopathy of trauma. Shock. 2014, 41 (Suppl. S1), 21–25. [Google Scholar]
- De Robertis, E.; Kozek-Langenecker, S.A.; Tufano, R.; Romano, G.M.; Piazza, O.; Zito Marinosci, G. Coagulopathy induced by acidosis, hypothermia and hypocalcaemia in severe bleeding. Minerva Anestesiol. 2015, 81, 65–75. [Google Scholar]
- Thorsen, K.; Ringdal, K.G.; Strand, K.; Søreide, K.; Hagemo, J.; Søreide, K. Clinical and cellular effects of hypothermia, acidosis and coagulopathy in major injury. Br. J. Surg. 2011, 98, 894–907. [Google Scholar]
- Darlington, D.N.; Kheirabadi, B.S.; Delgado, A.V.; Scherer, M.R.; Martini, W.Z.; Dubick, M.A. Coagulation Changes to Systemic Acidosis and Bicarbonate Correction in Swine. J. Trauma Inj. Infect. Crit. Care 2011, 71, 1271–1277. [Google Scholar]
- Darlington, D.N.; Kheirabadi, B.S.; Scherer, M.R.; Martini, W.Z.; Cap, A.P.; Dubick, M.A. Acidosis and correction of acidosis does not affect rFVIIa function in swine. Int. J. Burn. Trauma. 2012, 2, 145–157. [Google Scholar]
- Martini, W.Z. Coagulopathy by hypothermia and acidosis: Mechanisms of thrombin generation and fibrinogen availability. J. Trauma. 2009, 67, 202–209. [Google Scholar] [PubMed]
- Martini, W.Z.; Holcomb, J.B. Acidosis and coagulopathy: The differential effects on fibrinogen synthesis and breakdown in pigs. Ann. Surg. 2007, 246, 831–835. [Google Scholar] [PubMed]
- Martini, W.Z.; Dubick, M.A.; Pusateri, A.E.; Park, M.S.; Ryan, K.L.; Holcomb, J.B. Does bicarbonate correct coagulation function impaired by acidosis in swine? J. Trauma. 2006, 61, 99–106. [Google Scholar] [PubMed]
- Martini, W.Z.; Pusateri, A.E.; Uscilowicz, J.M.; Delgado, A.V.; Holcomb, J.B. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J. Trauma. 2005, 58, 1002–1010. [Google Scholar] [PubMed]
- Chaimoff, C.; Creter, D.; Djaldetti, M. The effect of pH on platelet and coagulation factor activities. Am. J. Surg. 1978, 136, 257–259. [Google Scholar]
- Green, F.W., Jr.; Kaplan, M.M.; Curtis, L.E.; Levine, P.H. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology 1978, 74, 38–43. [Google Scholar]
- Engström, M.; Schött, U.; Romner, B.; Reinstrup, B. Acidosis Impairs the Coagulation: A Thromboelastographic Study. J. Trauma. Acute Care Surg. 2006, 61, 624–628. [Google Scholar]
- Ramaker, A.J.; Meyer, P.; van der Meer, J.; Struys, M.M.; Lisman, T.; van Oeveren, W.; Hendriks, H.G. Effects of acidosis, alkalosis, hyperthermia and hypothermia on haemostasis: Results of point-of-care testing with the thromboelastography analyser. Blood Coagul. Fibrinolysis 2009, 20, 436–439. [Google Scholar]
- Campbell, J.E.; Aden, J.K.; Cap, A.P. Acute traumatic coagulopathy: Whole blood thrombelastography measures the tip of the iceberg. J. Trauma. Acute Care Surg. 2015, 78, 955–961. [Google Scholar]
- Scharbert, G.; Franta, G.; Wetzel, L.; Kozek-Langenecker, S. Effect of pH levels on platelet aggregation and coagulation: A whole blood in vitro study. Crit. Care 2011, 15, P446. [Google Scholar]
- Meng, Z.H.; Wolberg, A.S. The effect of temperature and pH on the activity of factor VIIa: Implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J. Trauma. 2003, 55, 886–891. [Google Scholar] [PubMed]
- Marchand, C.; Rivard, G.E.; Sun, J.; Hoemann, C.D. Solidification mechanisms of chitosan-glycerol phosphate/blood implant for articular cartilage repair. Osteoarthr. Cartil. 2009, 17, 953–960. [Google Scholar]
- Marumo, M.; Suehiro, A.; Kakishita, E.; Groschner, K.; Wakabayashi, I. Extracellular pH Affects Platelet Aggregation Associated with Modulation of Store-Operated Ca2+ Entry. Thromb. Res. 2001, 104, 353–360. [Google Scholar]
- Etulain, J.; Negrotto, S.; Carestia, A.; Pozner, A.G.; Romaniuk, M.A.; D’Atri, L.P.; Klement, G.L.; Schattner, M. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb. Haemost. 2012, 107, 99–110. [Google Scholar] [PubMed]

| Chitosan Properties | Chitosan 1 DDA 84.8% Mn 32 kDa | Chitosan 2 DDA 81.7% Mn 37 kDa | Chitosan 3 DDA 82.7% Mn 39 kDa |
|---|---|---|---|
| 60% protonation | 30 mM HCl | 29 mM HCl | 29 mM HCl |
| 65% protonation | 33 mM HCl | 31 mM HCl | 32 mM HCl |
| 70% protonation | 35 mM HCl | 34 mM HCl | 34 mM HCl |
| 75% protonation | 38 mM HCl | 36 mM HCl | 37 mM HCl |
| 80% protonation | 41 mM HCl | 39 mM HCl | 39 mM HCl |
| 85% protonation | 43 mM HCl | 41 mM HCl | 42 mM HCl |
| 90% protonation | 46 mM HCl | 44 mM HCl | 44 mM HCl |
| 95% protonation | 48 mM HCl | 46 mM HCl | 47 mM HCl |
| 80% protonation then 60% | 41 mM HCl then 11 mM NaOH | 39 mM HCl then 10 mM NaOH | 39 mM HCl then 10 mM NaOH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevrier, A.; Lavertu, M. Manufacturing Process Affects Coagulation Kinetics of Ortho-R, an Injectable Chitosan–Platelet-Rich Plasma Biomaterial for Tissue Repair. Bioengineering 2024, 11, 929. https://doi.org/10.3390/bioengineering11090929
Chevrier A, Lavertu M. Manufacturing Process Affects Coagulation Kinetics of Ortho-R, an Injectable Chitosan–Platelet-Rich Plasma Biomaterial for Tissue Repair. Bioengineering. 2024; 11(9):929. https://doi.org/10.3390/bioengineering11090929
Chicago/Turabian StyleChevrier, Anik, and Marc Lavertu. 2024. "Manufacturing Process Affects Coagulation Kinetics of Ortho-R, an Injectable Chitosan–Platelet-Rich Plasma Biomaterial for Tissue Repair" Bioengineering 11, no. 9: 929. https://doi.org/10.3390/bioengineering11090929
APA StyleChevrier, A., & Lavertu, M. (2024). Manufacturing Process Affects Coagulation Kinetics of Ortho-R, an Injectable Chitosan–Platelet-Rich Plasma Biomaterial for Tissue Repair. Bioengineering, 11(9), 929. https://doi.org/10.3390/bioengineering11090929






