Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sample Size Estimation
2.2. Experimental Protocol and Equipment
2.2.1. Camera Setup and Calibration
2.2.2. Video Collection and Pose Estimation
2.2.3. Physics-Based Modeling and Simulation
2.3. Experimental Procedure
2.4. Data Collection and Processing
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
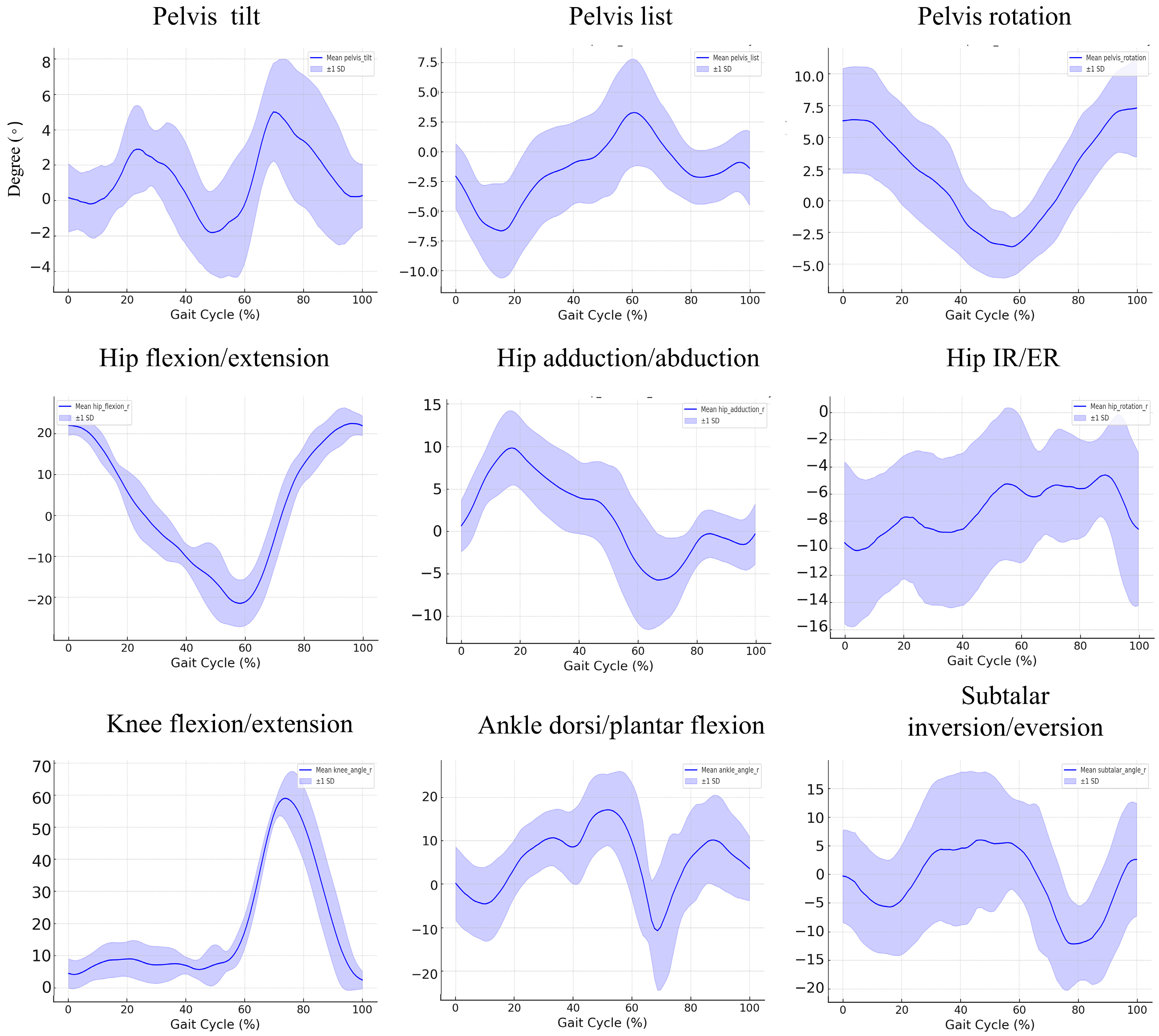
3.2. Kinematic Findings during the Gait Cycle in the Control Group
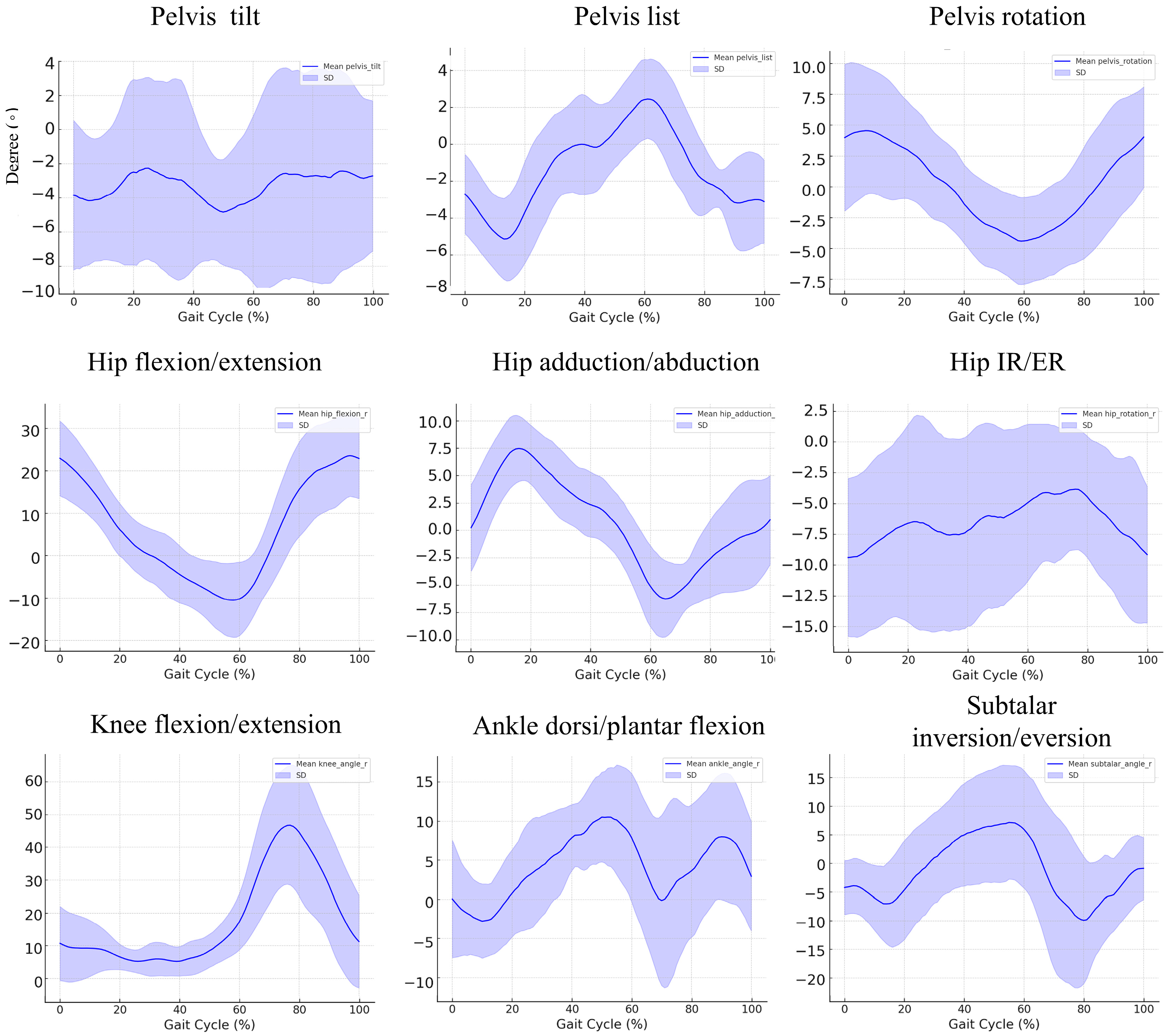
3.3. Kinematic Findings during the Gait Cycle in the Patient Group
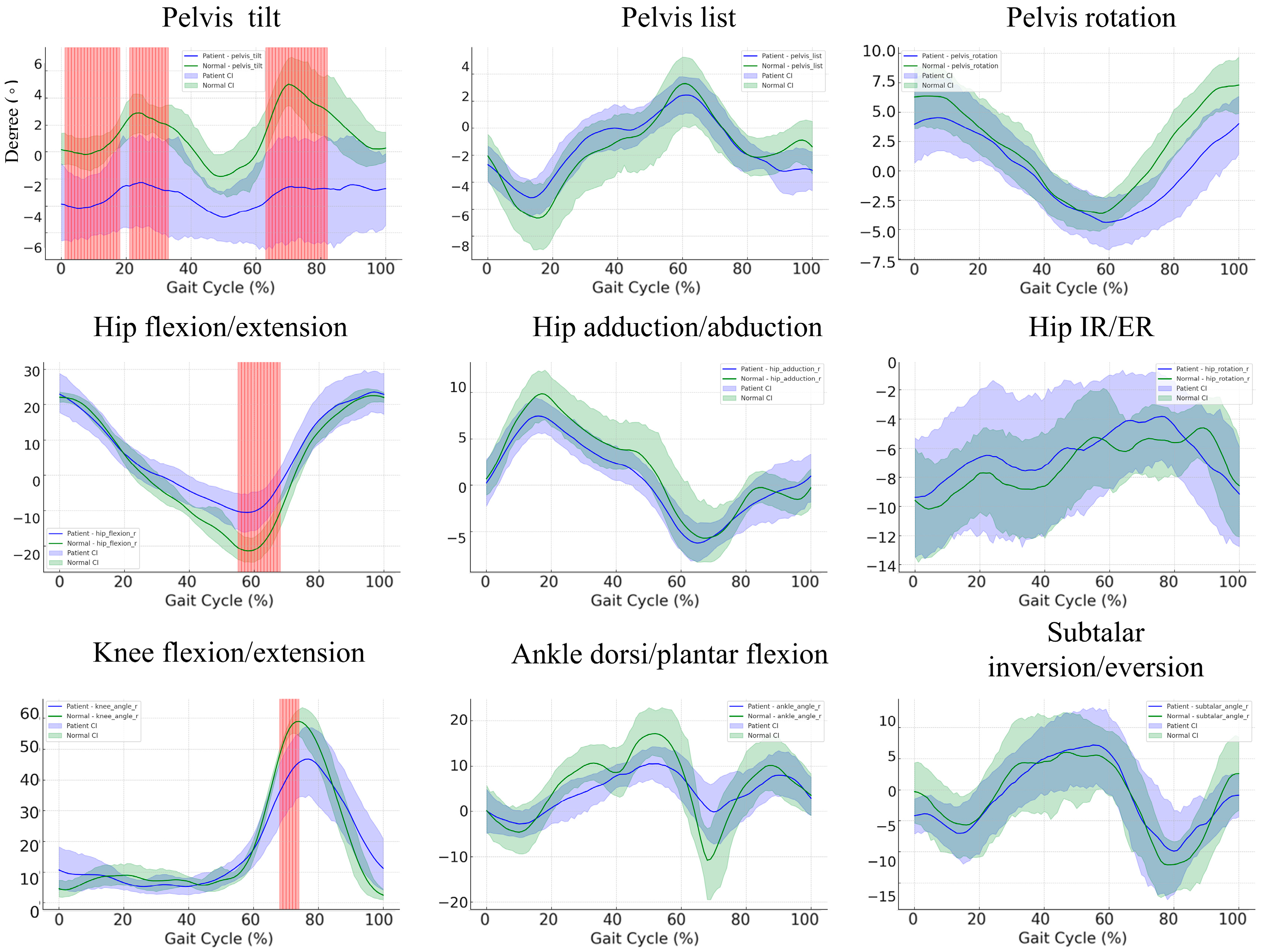
3.4. Kinematic Differences during the Gait Cycle: A Comparison between Control and Patient Groups
3.5. Gait Cycle Kinematics in Stroke Patients versus Controls: A Subgroup Comparison
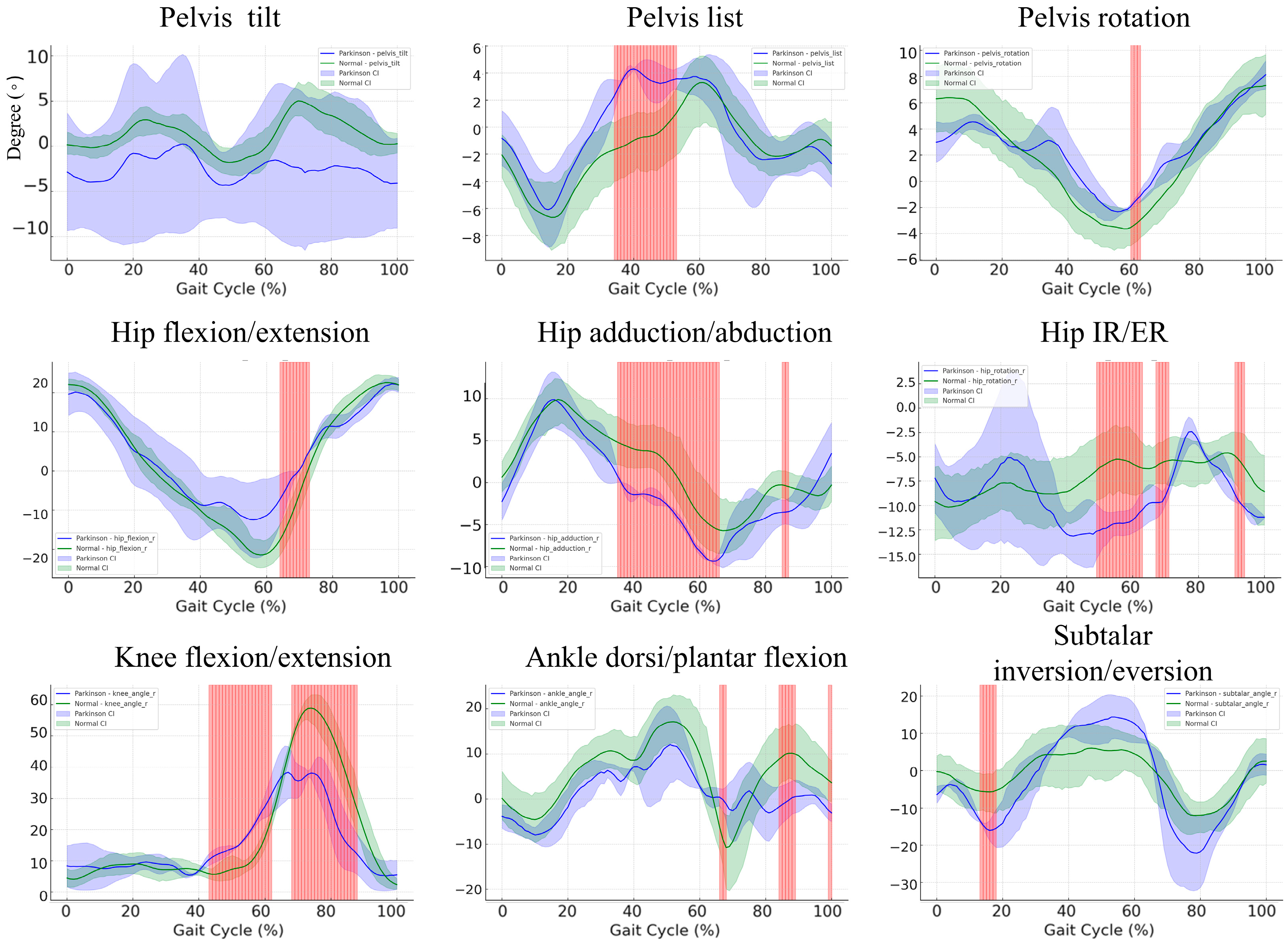
3.6. Gait Cycle Kinematics in Parkinson’s Disease Patients versus Healthy Controls: A Subgroup Comparison
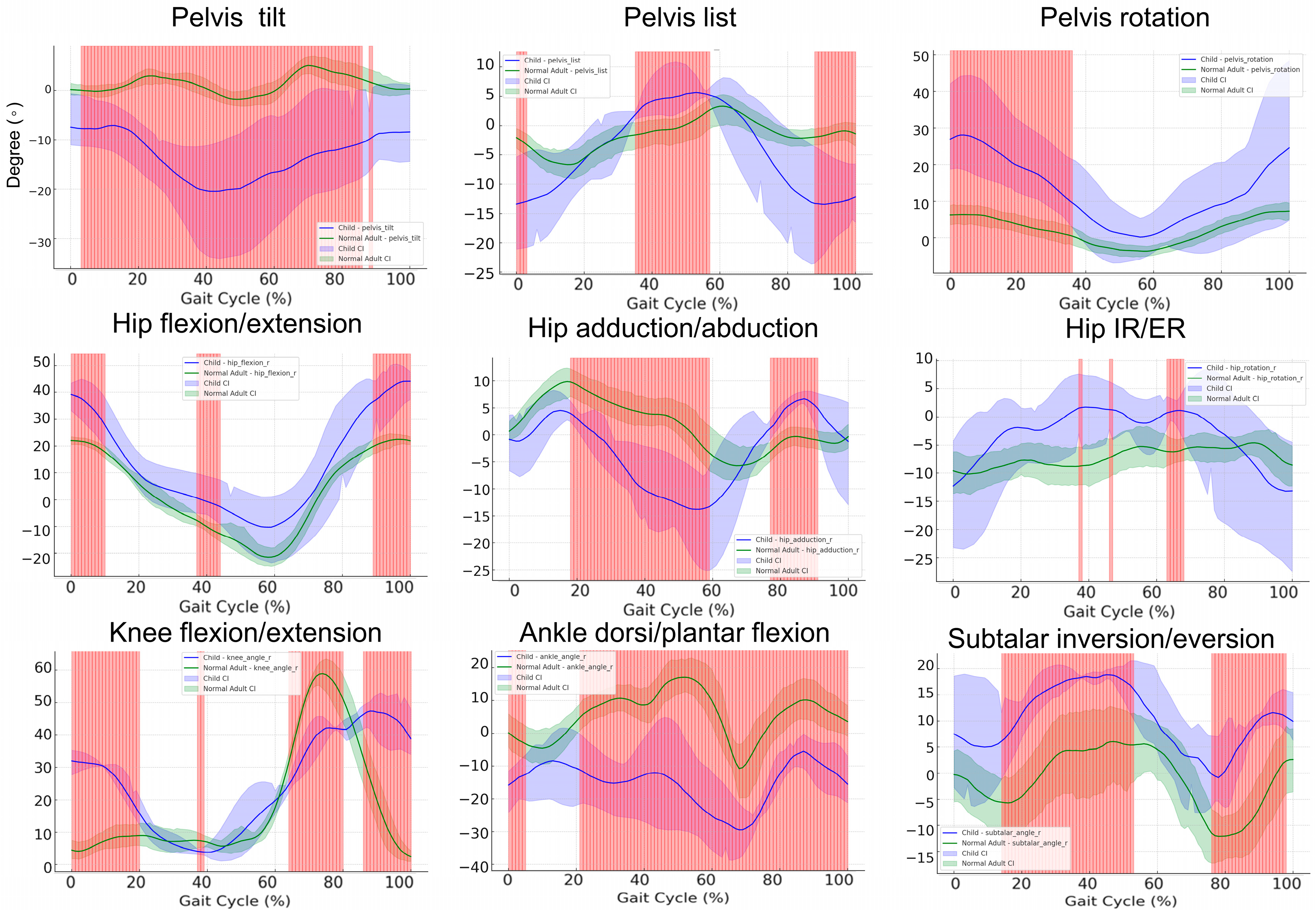
3.7. Gait Cycle Kinematics in Pediatric Patients versus Healthy Controls: A Subgroup Comparison
3.8. Kinetic Differences during the Gait Cycle: A Comparison between Control and Patient Groups
4. Discussion
4.1. Comparison of Temporospatial Gait Parameters and Efficiency of Data Acquisition in Neurological Conditions
4.2. Comparative Analysis of Kinematic Gait Patterns in Controls and Patients with Neurological Disorders
4.3. Comparative Analysis of Kinematic Gait Patterns between Healthy Controls and Pediatric Patients with Neurological Disorders
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gage, J.R. Gait Analysis in Cerebral Palsy; Cambridge University Press: Cambridge, UK, 1991; ISBN 978-0-521-41277-3. [Google Scholar]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The Updated European Consensus 2009 on the Use of Botulinum Toxin for Children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Shrader, W.; Shih, C.; McDonald, T. Instrumented Gait Analysis in the Care of Children with Cerebral Palsy: Current Concept Revew. J. Pediatr. Orthop. Soc. N. Am. 2021, 3, 237. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Pedersen, N.W.; Overgaard, S.; Hansen, L.K.; Dunkhase-Heinl, U.; Petkov, Y.; Engell, V.; Baker, R.; Holsgaard-Larsen, A. The Use of Instrumented Gait Analysis for Individually Tailored Interdisciplinary Interventions in Children with Cerebral Palsy: A Randomised Controlled Trial Protocol. BMC Pediatr. 2015, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.B.; Salgado, D.P.; Catháin, C.Ó.; O’Connor, N.; Murray, N. Human Gait Assessment Using a 3D Marker-Less Multimodal Motion Capture System. Multimed. Tools Appl. 2020, 79, 2629–2651. [Google Scholar] [CrossRef]
- Uhlrich, S.D.; Falisse, A.; Kidziński, Ł.; Muccini, J.; Ko, M.; Chaudhari, A.S.; Hicks, J.L.; Delp, S.L. OpenCap: Human Movement Dynamics from Smartphone Videos. PLoS Comput. Biol. 2023, 19, e1011462. [Google Scholar] [CrossRef]
- Cimolin, V.; Vismara, L.; Ferraris, C.; Amprimo, G.; Pettiti, G.; Lopez, R.; Galli, M.; Cremascoli, R.; Sinagra, S.; Mauro, A.; et al. Computation of Gait Parameters in Post Stroke and Parkinson’s Disease: A Comparative Study Using RGB-D Sensors and Optoelectronic Systems. Sensors 2022, 22, 824. [Google Scholar] [CrossRef]
- Steffensen, E.A.; Magalhães, F.; Knarr, B.A.; Kingston, D.C. Comparison of Markerless and Marker-Based Motion Capture of Gait Kinematics in Individuals with Cerebral Palsy and Chronic Stroke: A Case Study Series. Res. Sq. 2023, rs.3.rs-2557403. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Ismail Ibrahim Ismail Alali, S.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-Stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Alberto, S.; Cabral, S.; Proença, J.; Pona-Ferreira, F.; Leitão, M.; Bouça-Machado, R.; Kauppila, L.A.; Veloso, A.P.; Costa, R.M.; Ferreira, J.J.; et al. Validation of Quantitative Gait Analysis Systems for Parkinson’s Disease for Use in Supervised and Unsupervised Environments. BMC Neurol. 2021, 21, 331. [Google Scholar] [CrossRef]
- di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef]
- Cao, Z.; Hidalgo, G.; Simon, T.; Wei, S.-E.; Sheikh, Y. OpenPose: Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 43, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Kanko, R.M.; Laende, E.K.; Davis, E.M.; Selbie, W.S.; Deluzio, K.J. Concurrent Assessment of Gait Kinematics Using Marker-Based and Markerless Motion Capture. J. Biomech. 2021, 127, 110665. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.O.; Silverman, B.W. Functional Data Analysis; Springer Series in Statistics; Springer: New York, NY, USA, 2005; ISBN 978-0-387-40080-8. [Google Scholar]
- Carpenter, J.; Bithell, J. Bootstrap Confidence Intervals: When, Which, What? A Practical Guide for Medical Statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics Notes: Bootstrap Resampling Methods. Br. Med. J. 2015, 350, h2622. [Google Scholar] [CrossRef]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 39, 1137–1149. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, B.; Liu, D.; Wang, J. Deep High-Resolution Representation Learning for Human Pose Estimation. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 20 June 2019; pp. 5686–5696. [Google Scholar]
- Jin, S.; Xu, L.; Xu, J.; Wang, C.; Liu, W.; Qian, C.; Ouyang, W.; Luo, P. Whole-Body Human Pose Estimation in the Wild. arXiv 2020, arXiv:2007.11858. [Google Scholar]
- Zhang, F.; Zhu, X.; Dai, H.; Ye, M.; Zhu, C. Distribution-Aware Coordinate Representation for Human Pose Estimation. arXiv 2019, arXiv:1910.06278. [Google Scholar]
- Rajagopal, A.; Dembia, C.L.; DeMers, M.S.; Delp, D.D.; Hicks, J.L.; Delp, S.L. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans. Biomed. Eng. 2016, 63, 2068–2079. [Google Scholar] [CrossRef]
- Lai, A.K.M.; Arnold, A.S.; Wakeling, J.M. Why Are Antagonist Muscles Co-Activated in My Simulation? A Musculoskeletal Model for Analysing Human Locomotor Tasks. Ann. Biomed. Eng. 2017, 45, 2762–2774. [Google Scholar] [CrossRef]
- Uhlrich, S.D.; Jackson, R.W.; Seth, A.; Kolesar, J.A.; Delp, S.L. Muscle Coordination Retraining Inspired by Musculoskeletal Simulations Reduces Knee Contact Force. Sci. Rep. 2022, 12, 9842. [Google Scholar] [CrossRef]
- Seth, A.; Hicks, J.L.; Uchida, T.K.; Habib, A.; Dembia, C.L.; Dunne, J.J.; Ong, C.F.; DeMers, M.S.; Rajagopal, A.; Millard, M.; et al. OpenSim: Simulating Musculoskeletal Dynamics and Neuromuscular Control to Study Human and Animal Movement. PLoS Comput. Biol. 2018, 14, e1006223. [Google Scholar] [CrossRef] [PubMed]
- Ferraty, F.; Vieu, P. Nonparametric Functional Data Analysis; Springer Series in Statistics; Springer: New York, NY, USA, 2006; ISBN 978-0-387-30369-7. [Google Scholar]
- Edwards, W.B.; Derrick, T.R.; Hamill, J. Time Series Analysis in Biomechanics. In Handbook of Human Motion; Müller, B., Wolf, S.I., Brueggemann, G.-P., Deng, Z., McIntosh, A., Miller, F., Selbie, W.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–24. ISBN 978-3-319-30808-1. [Google Scholar]
- Ullah, S.; Finch, C.F. Applications of Functional Data Analysis: A Systematic Review. BMC Med. Res. Methodol. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Tok, F. Gait Disturbances in Patients with Stroke. Phys. Med. Rehabil. 2014, 6, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Burpee, J.L.; Lewek, M.D. Biomechanical Gait Characteristics of Naturally Occurring Unsuccessful Foot Clearance during Swing in Individuals with Chronic Stroke. Clin. Biomech. 2015, 30, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Nanhoe-Mahabier, W.; Snijders, A.H.; Delval, A.; Weerdesteyn, V.; Duysens, J.; Overeem, S.; Bloem, B.R. Walking Patterns in Parkinson’s Disease with and without Freezing of Gait. Neuroscience 2011, 182, 217–224. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Giladi, N.; Peretz, C.; Herman, T.; Gruendlinger, L.; Hausdorff, J.M. Treadmill Walking as an External Pacemaker to Improve Gait Rhythm and Stability in Parkinson’s Disease. Mov. Disord. 2005, 20, 1109–1114. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Kwakkel, G.; Rochester, L.; Jones, D.; van Wegen, E.; Willems, A.M.; Chavret, F.; Hetherington, V.; Baker, K.; Lim, I. Cueing Training in the Home Improves Gait-Related Mobility in Parkinson’s Disease: The RESCUE Trial. J. Neurol. Neurosurg. Psychiatry 2007, 78, 134–140. [Google Scholar] [CrossRef]
- Õunpuu, S.; Davis, R.B.; DeLuca, P.A. Joint Kinetics: Methods, Interpretation and Treatment Decision-Making in Children with Cerebral Palsy and Myelomeningocele. Gait Posture 1996, 4, 62–78. [Google Scholar] [CrossRef]
- Adde, L.; Helbostad, J.L.; Jensenius, A.R.; Taraldsen, G.; Grunewaldt, K.H.; Støen, R. Early Prediction of Cerebral Palsy by Computer-Based Video Analysis of General Movements: A Feasibility Study. Dev. Med. Child. Neurol. 2010, 52, 773–778. [Google Scholar] [CrossRef]
- Mizuta, N.; Hasui, N.; Kai, T.; Inui, Y.; Sato, M.; Ohnishi, S.; Taguchi, J.; Nakatani, T. Characteristics of Limb Kinematics in the Gait Disorders of Post-Stroke Patients. Sci. Rep. 2024, 14, 3082. [Google Scholar] [CrossRef]
- Morris, M.E. Movement Disorders in People With Parkinson Disease: A Model for Physical Therapy. Phys. Ther. 2000, 80, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.S.; Liu, M.Q.; Schwartz, M.H.; Ounpuu, S.; Delp, S.L. The Role of Estimating Muscle-Tendon Lengths and Velocities of the Hamstrings in the Evaluation and Treatment of Crouch Gait. Gait Posture 2006, 23, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Ounpuu, S.; Arnold, A.S.; Gage, J.R.; Delp, S.L. Kinematic and Kinetic Factors That Correlate with Improved Knee Flexion Following Treatment for Stiff-Knee Gait. J. Biomech. 2006, 39, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Ziziene, J.; Daunoraviciene, K.; Juskeniene, G.; Raistenskis, J. Comparison of Kinematic Parameters of Children Gait Obtained by Inverse and Direct Models. PLoS ONE 2022, 17, e0270423. [Google Scholar] [CrossRef]
- Mundt, M.; Born, Z.; Goldacre, M.; Alderson, J. Estimating Ground Reaction Forces from Two-Dimensional Pose Data: A Biomechanics-Based Comparison of AlphaPose, BlazePose, and OpenPose. Sensors 2023, 23, 78. [Google Scholar] [CrossRef]
- Colyer, S.; Needham, L.; Evans, M.; Wade, L.; Cosker, D.; Cazzola, D.; McGuigan, P.; Bilzon, J. Estimation of Ground Reaction Forces from Markerless Kinematics and Comparison Against Measured Force Plate Data. ISBS Proc. Arch. 2023, 41, 23. [Google Scholar]
- Solanki, D.; Lahiri, U. Design of Instrumented Shoes for Gait Characterization: A Usability Study With Healthy and Post-Stroke Hemiplegic Individuals. Front. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef]
- Falisse, A.; Serrancolí, G.; Dembia, C.L.; Gillis, J.; De Groote, F. Algorithmic Differentiation Improves the Computational Efficiency of OpenSim-Based Trajectory Optimization of Human Movement. PLoS ONE 2019, 14, e0217730. [Google Scholar] [CrossRef]
- Andersson, J.; Åkesson, J.; Diehl, M. CasADi: A Symbolic Package for Automatic Differentiation and Optimal Control. In Recent Advances in Algorithmic Differentiation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 297–307. [Google Scholar]
- Stenum, J.; Hsu, M.M.; Pantelyat, A.Y.; Roemmich, R.T. Clinical Gait Analysis Using Video-Based Pose Estimation: Multiple Perspectives, Clinical Populations, and Measuring Change. PLoS Digit. Health 2024, 3, e0000467. [Google Scholar] [CrossRef]
- Werling, K.; Bianco, N.A.; Raitor, M.; Stingel, J.; Hicks, J.L.; Collins, S.H.; Delp, S.L.; Liu, C.K. AddBiomechanics: Automating Model Scaling, Inverse Kinematics, and Inverse Dynamics from Human Motion Data through Sequential Optimization. PLoS ONE 2023, 18, e0295152. [Google Scholar] [CrossRef]
- Needham, L.; Evans, M.; Wade, L.; Cosker, D.P.; McGuigan, M.P.; Bilzon, J.L.; Colyer, S.L. The Development and Evaluation of a Fully Automated Markerless Motion Capture Workflow. J. Biomech. 2022, 144, 111338. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 10) Mean (SD) | Patient (n = 10) Mean (SD) | p-Value | |
|---|---|---|---|
| Age (years) | 31.30 (11.55) | 51.60 (24.45) | 0.034 * |
| Sex (male/female) | 3M/7F | 4M/6F | 1.000 |
| Height (m) | 1.68 (0.09) | 1.59 (0.21) | 0.230 |
| Weight (kg) | 60.50 (16.13) | 62.50 (18.91) | 0.802 |
| BMI (kg/m2) | 21.18 (3.83) | 24.26 (3.95) | 0.093 |
| Gait speed (m/s) | 1.10 (0.13) | 0.67 (0.31) | 0.002 * |
| Stride length (m) | 1.29 (0.15) | 0.81 (0.31) | 0.001 * |
| Step width (cm) | 12.17 (3.10) | 15.58 (3.92) | 0.045 * |
| Cadence (step/min) | 104.60 (9.93) | 94.70 (28.92) | 0.328 |
| Double support (%cycle) | 29.35 (2.72) | 36.69 (12.50) | 0.100 |
| Step length asymmetry (%) | 91.23 (11.70) | 107.43 (16.76) | 0.023 * |
| Joint | Peak Value (Degree) | Control Mean (SD) | Patients Mean (SD) | p-Value |
|---|---|---|---|---|
| Hip | Flexion | 24.225 (2.348) | 26.146 (10.074) | 0.57 |
| Extension | −21.736 (5.938) | −11.52 (8.642) | 0.007 * | |
| Adduction | 10.599 (5.291) | 8.431 (3.068) | 0.281 | |
| Abduction | −7.937 (2.402) | −7.544 (2.465) | 0.722 | |
| Internal Rotation | −1.208 (3.307) | 1.121 (6.209) | 0.313 | |
| External Rotation | −13.663 (5.101) | −13.629 (6.114) | 0.989 | |
| Knee | Flexion | 61.492 (5.702) | 53.952 (12.767) | 0.113 |
| Extension | 1.152 (1.013) | 2.168 (2.458) | 0.25 | |
| Ankle | Dorsiflexion | 20.416 (10.514) | 16.253 (6.256) | 0.299 |
| Plantarflexion | −14.189 (13.056) | −6.667 (8.03) | 0.142 | |
| Subtalar | Inversion | 17.496 (7.34) | 14.758 (8.779) | 0.459 |
| Eversion | −15.709 (6.618) | −11.021 (12.62) | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Y.-S.; Jung, T.-D.; Lee, Y.-S.; Kwon, Y.; Kim, H.J.; Kim, H.C.; Lee, J.C.; Park, E. Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders. Bioengineering 2024, 11, 911. https://doi.org/10.3390/bioengineering11090911
Min Y-S, Jung T-D, Lee Y-S, Kwon Y, Kim HJ, Kim HC, Lee JC, Park E. Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders. Bioengineering. 2024; 11(9):911. https://doi.org/10.3390/bioengineering11090911
Chicago/Turabian StyleMin, Yu-Sun, Tae-Du Jung, Yang-Soo Lee, Yonghan Kwon, Hyung Joon Kim, Hee Chan Kim, Jung Chan Lee, and Eunhee Park. 2024. "Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders" Bioengineering 11, no. 9: 911. https://doi.org/10.3390/bioengineering11090911
APA StyleMin, Y.-S., Jung, T.-D., Lee, Y.-S., Kwon, Y., Kim, H. J., Kim, H. C., Lee, J. C., & Park, E. (2024). Biomechanical Gait Analysis Using a Smartphone-Based Motion Capture System (OpenCap) in Patients with Neurological Disorders. Bioengineering, 11(9), 911. https://doi.org/10.3390/bioengineering11090911







