Comparing the Immune Response to PEEK as an Implant Material with and without P-15 Peptide as Bone Graft Material in a Rabbit Long Bone Model
Abstract
1. Introduction
2. Materials and Methods
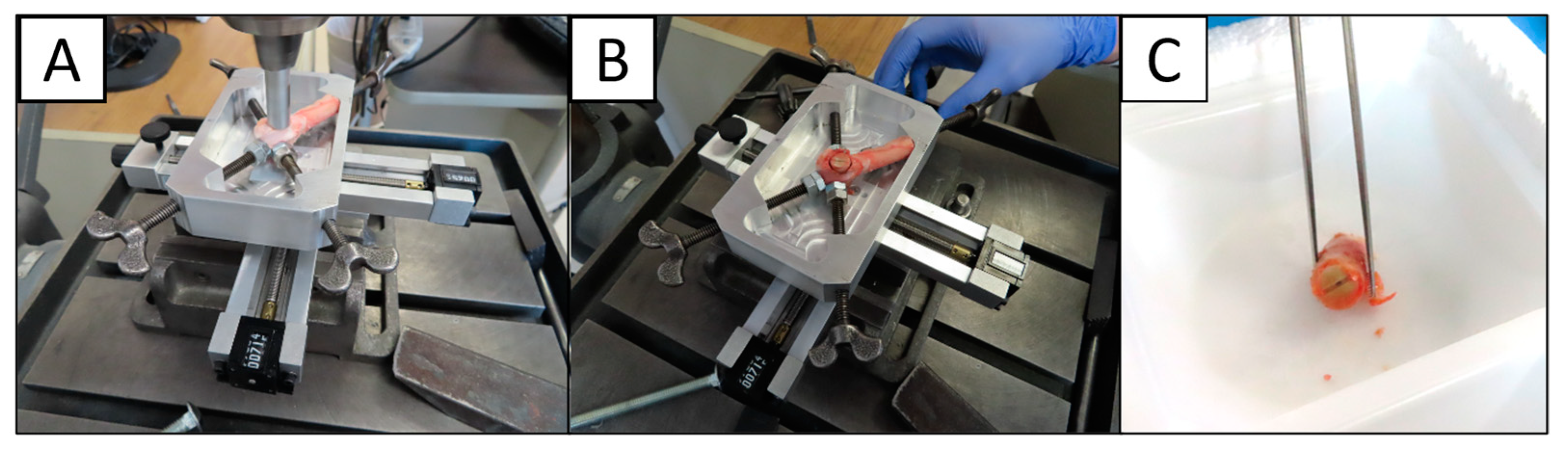
2.1. Preparation of Bone Dowels
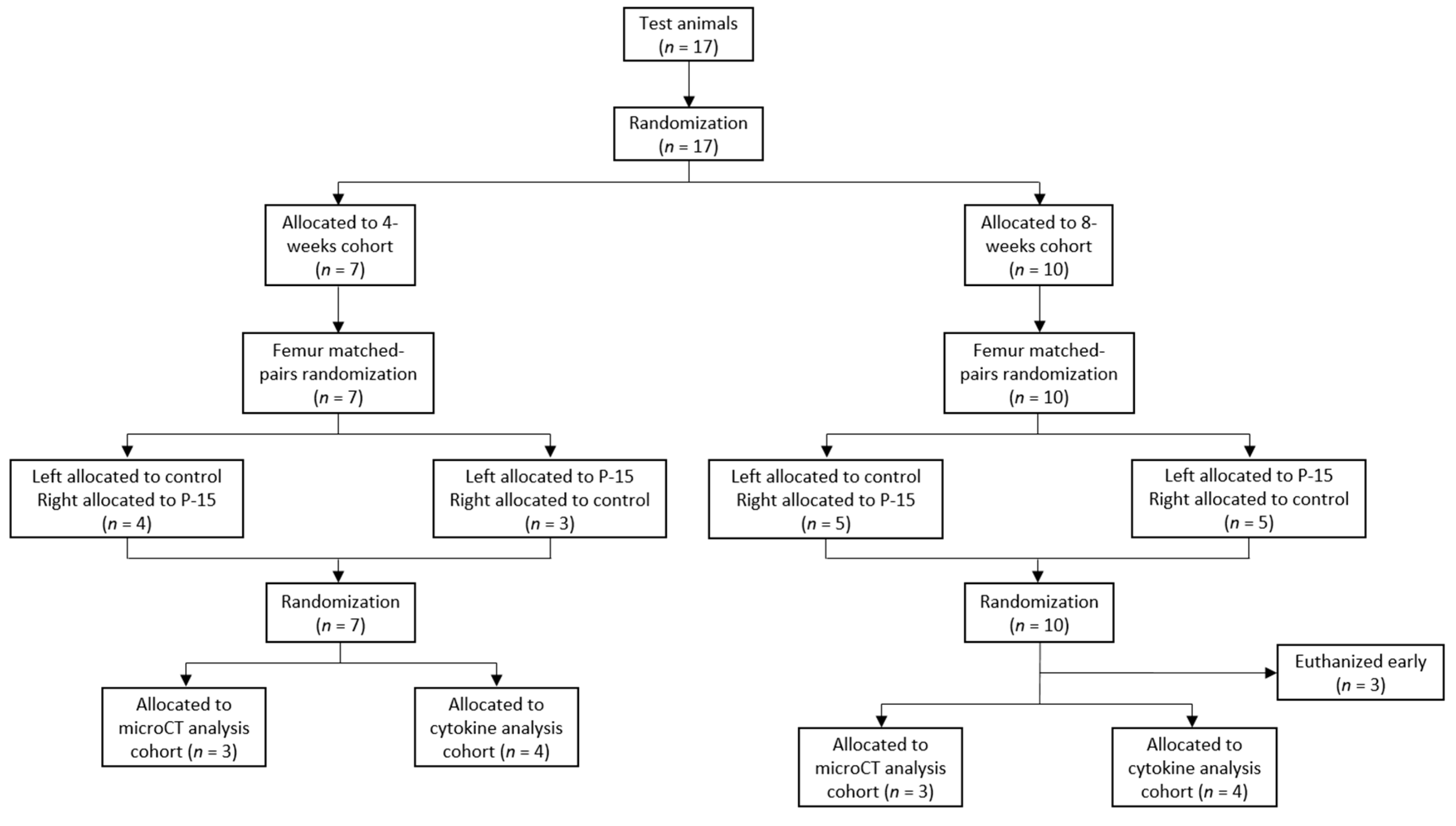
2.2. Animal Model
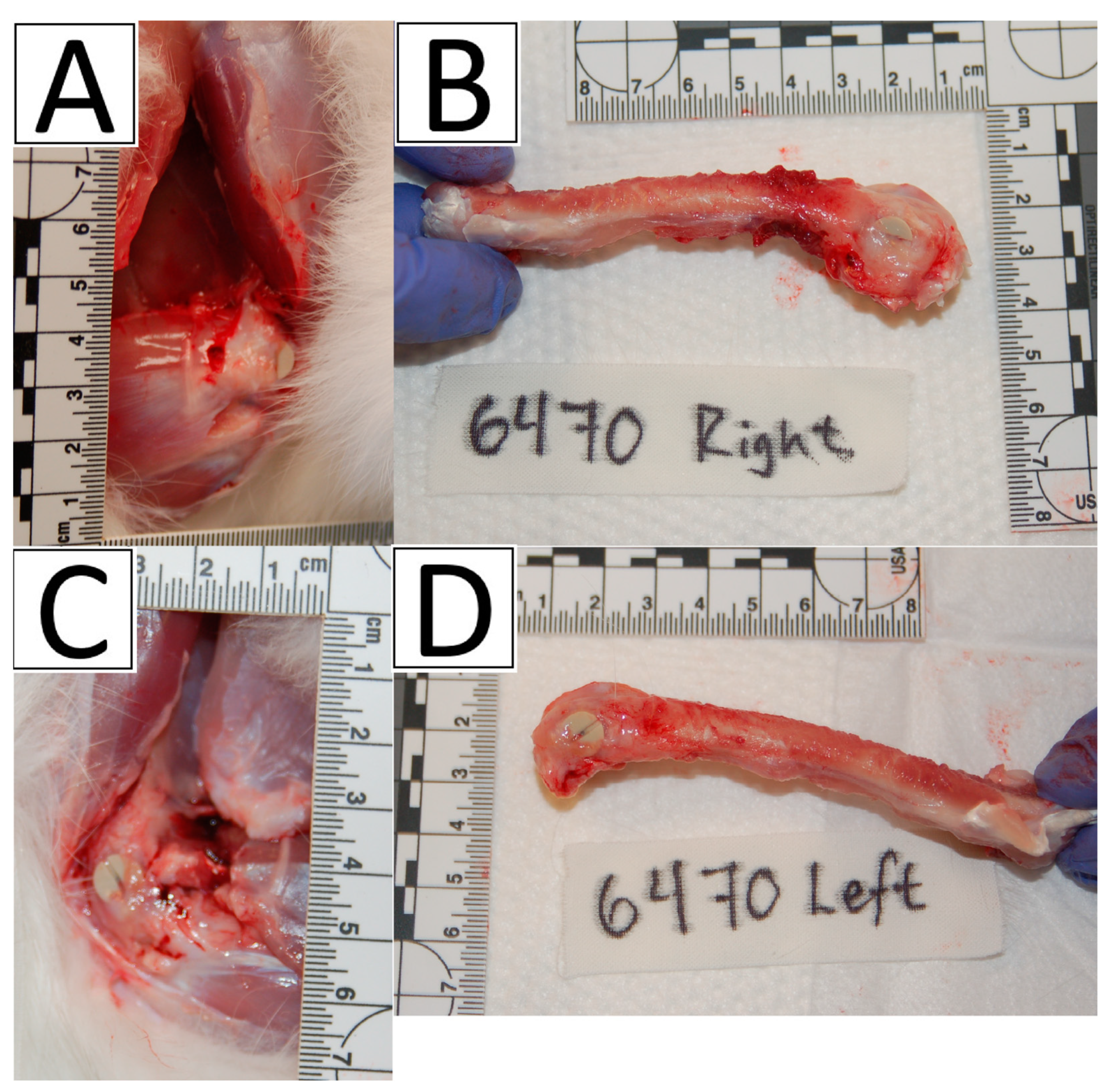
2.3. Surgical Procedures
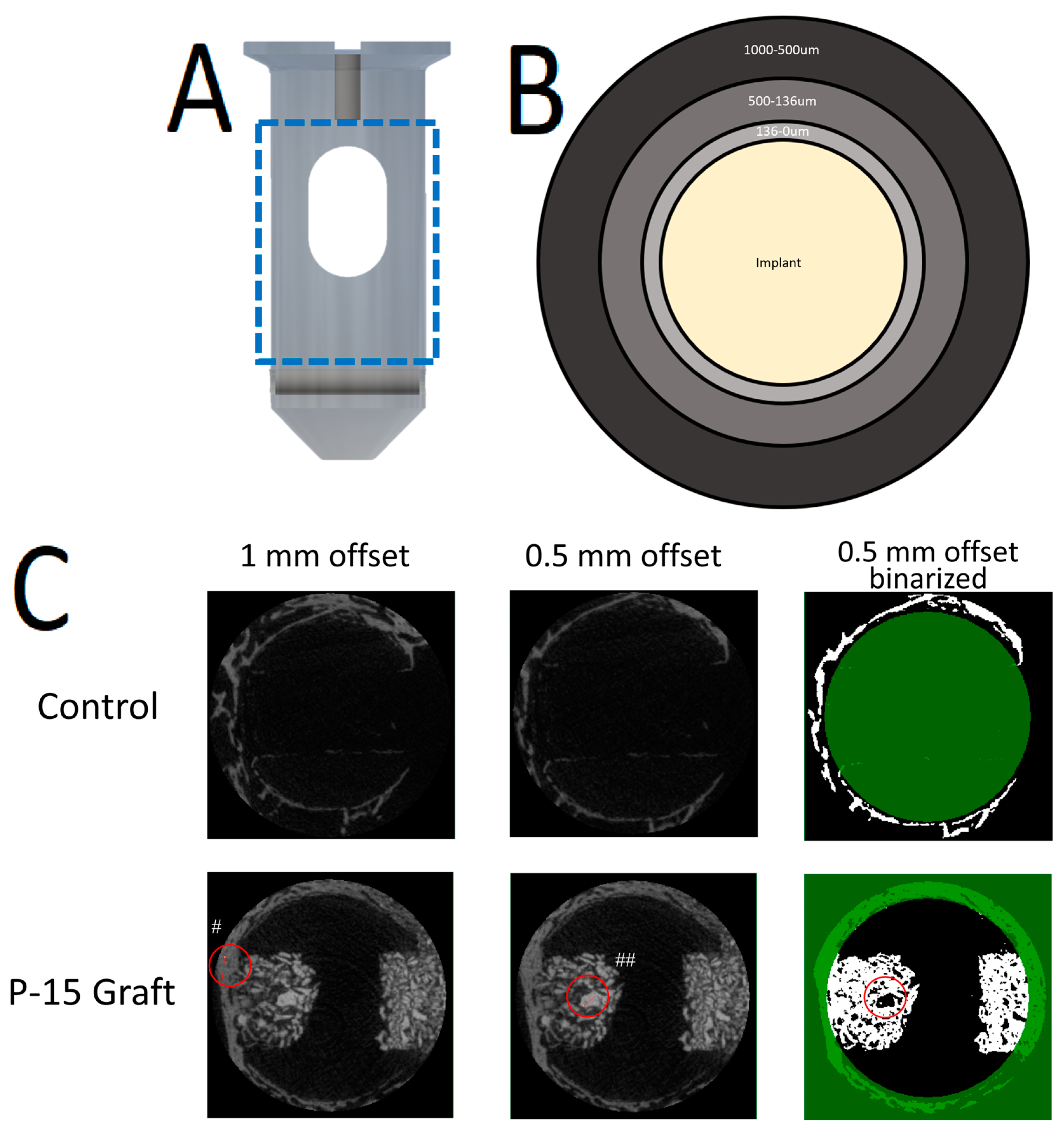
2.4. MicroCT Analysis
2.4.1. Scanning Procedures
2.4.2. Analysis Procedures
2.5. Cytokine Analysis
2.6. Statistical Analysis
3. Results
3.1. Surgical Procedures
3.2. MicroCT Analysis
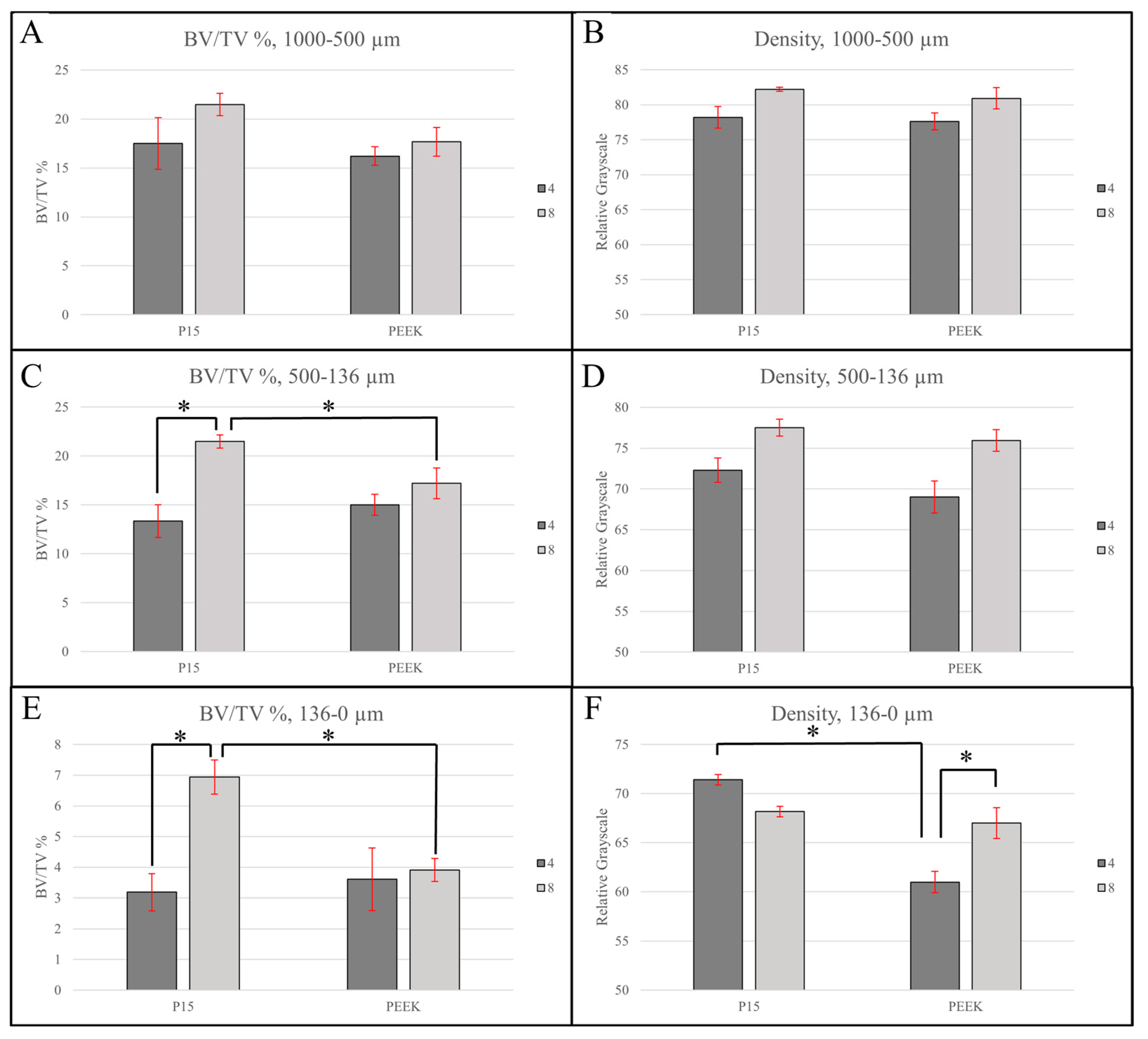
3.2.1. Large Region of Interest (1000–500 µm)
3.2.2. Intermediate Region of Interest (500–136 µm)
3.2.3. Small Region of Interest (136–0 µm)
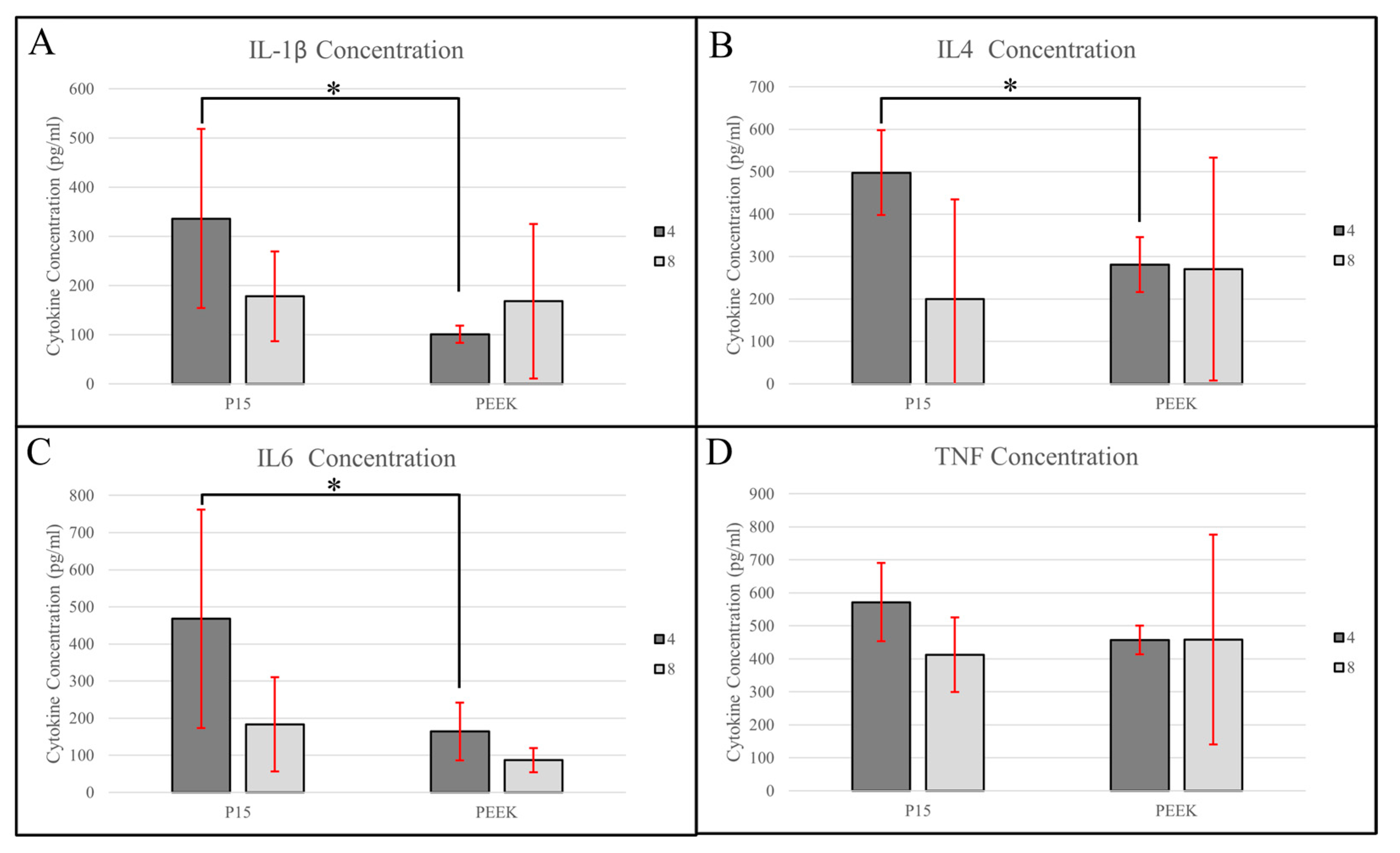
3.3. Cytokine Analysis
3.3.1. IL-1β
3.3.2. IL-4
3.3.3. IL-6
3.3.4. TNF-α
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.C.; Mahon, O.R.; Kelly, D.J.; Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: Considering more than macrophages. Acta Biomater. 2021, 133, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Wei, R.; Wu, J.; Li, Y. Macrophage polarization following three-dimensional porous PEEK. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109948. [Google Scholar] [CrossRef]
- Yoon, S.T.; Konopka, J.A.; Wang, J.C.; Youssef, J.A.; Meisel, H.J.; Brodke, D.S.; Park, J.B. ACDF Graft Selection by Surgeons: Survey of AOSpine Members. Glob. Spine J. 2017, 7, 410–416. [Google Scholar] [CrossRef]
- Walsh, W.R.; Pelletier, M.H.; Christou, C.; He, J.; Vizes, F.; Boden, S.D. The in vivo response to a novel Ti coating compared with polyether ether ketone: Evaluation of the periphery and inner surfaces of an implant. Spine J. 2018, 18, 1231–1240. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone Immune Response to Materials, Part II: Copper and Polyetheretherketone (PEEK) Compared to Titanium at 10 and 28 Days in Rabbit Tibia. J. Clin. Med. 2019, 8, 814. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant materials generate different peri-implant inflammatory factors: Poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine 2015, 40, 399–404. [Google Scholar] [CrossRef]
- Heary, R.F.; Parvathreddy, N.; Sampath, S.; Agarwal, N. Elastic modulus in the selection of interbody implants. J. Spine. Surg. 2017, 3, 163–167. [Google Scholar] [CrossRef]
- Cabraja, M.; Oezdemir, S.; Koeppen, D.; Kroppenstedt, S. Anterior cervical discectomy and fusion: Comparison of titanium and polyetheretherketone cages. BMC Musculoskelet. Disord. 2012, 14, 172. [Google Scholar] [CrossRef]
- Krause, K.L.; Obayashi, J.T.; Bridges, K.J.; Raslan, A.M.; Than, K.D. Fivefold higher rate of pseudarthrosis with polyetheretherketone interbody device than with structural allograft used for 1-level anterior cervical discectomy and fusion. J. Neurosurg. Spine 2018, 30, 46–51. [Google Scholar] [CrossRef]
- Maharaj, M.M.; Phan, K.; Mobbs, R.J. Anterior cervical discectomy and fusion (ACDF) autograft versus graft substitutes: What do patients prefer?-A clinical study. J. Spine. Surg. 2016, 2, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Xiong, C.; Daubs, M.D.; Montgomery, S.R.; Aghdasi, B.; Inoue, H.; Tian, H.; Suzuki, A.; Tan, Y.; Hayashi, T.; Ruangchainikom, M.; et al. BMP-2 adverse reactions treated with human dose equivalent dexamethasone in a rodent model of soft-tissue inflammation. Spine 2013, 38, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.S.; Qian, J.J.; Gough, C.A. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: Structural and biological evidence for conformational tautomerism on fiber surface. J. Biomol. Struct. Dyn. 1997, 14, 547–560. [Google Scholar] [CrossRef]
- Cheng, C.T.; Vyas, P.S.; McClain IV, E.J.; Hoelen, T.-C.A.; Arts, J.J.C.; McLaughlin, C.; Altman, D.T.; Yu, A.K.; Cheng, B.C. The Osteogenic Peptide P-15 for Bone Regeneration: A Narrative Review of the Evidence for a Mechanism of Action. Bioengineering 2024, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone Immune Response to Materials, Part I: Titanium, PEEK and Copper in Comparison to Sham at 10 Days in Rabbit Tibia. J. Clin. Med. 2018, 7, 526. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, Z.; Wang, Y. The degree of peri-implant osteolysis induced by PEEK, CoCrMo, and HXLPE wear particles: A study based on a porous Ti6Al4V implant in a rabbit model. J. Orthop. Surg. Res. 2018, 13, 23. [Google Scholar] [CrossRef]
- Li, D.J.; Ohsaki, K.; Ii, K.; Cui, P.-C.; Ye, Q.; Baba, K.; Wang, Q.-C.; Tenshin, S.; Takano-Yamamoto, T. Thickness of fibrous capsule after implantation of hydroxyapatite in subcutaneous tissue in rats. J. Biomed. Mater. Res. 1999, 45, 322–326. [Google Scholar] [CrossRef]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef]
- Kudo, O.; Fujikawa, Y.; Itonaga, I.; Sabokbar, A.; Torisu, T.; Athanasou, N.A. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J. Pathol. 2002, 198, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-alpha on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, T.S.; Choi, Y.; Lorenzo, J. Osteoimmunology: Cytokines and the skeletal system. BMB Rep. 2008, 41, 495–510. [Google Scholar] [CrossRef]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Histiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel. Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Lin, S.; Qiu, M.; Chen, J. IL-4 Modulates Macrophage Polarization in Ankylosing Spondylitis. Cell. Physiol. Biochem. 2015, 35, 2213–2222. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]

| Animal | Cohort | Left | Right | Analysis |
|---|---|---|---|---|
| 1 | 8 | P-15 | Control | MicroCT |
| 2 | 8 | Control | P-15 | Cytokine |
| 3 | 8 | P-15 | Control | MicroCT |
| 4 | 8 | Control | P-15 | Cytokine |
| 5 | 8 | P-15 | Control | MicroCT |
| 6 | N/A | Control | P-15 | Cytokine |
| 7 | N/A | P-15 | Control | Cytokine |
| 8 | N/A | Control | P-15 | Cytokine |
| 9 | 8 | P-15 | Control | Cytokine |
| 10 | 8 | Control | P-15 | Cytokine |
| 11 | 4 | Control | P-15 | MicroCT |
| 12 | 4 | Control | P-15 | Cytokine |
| 13 | 4 | P-15 | Control | MicroCT |
| 14 | 4 | P-15 | Control | Cytokine |
| 15 | 4 | Control | P-15 | MicroCT |
| 16 | 4 | Control | P-15 | Cytokine |
| 17 | 4 | P-15 | Control | Cytokine |
| Region of Interest | Timepoint (Week) | Mean Bone Volume Fraction (%) | Adjusted Mean Bone Volume Fraction (%) with 95% CI * | Adjusted p Value **,† | |||||
|---|---|---|---|---|---|---|---|---|---|
| PEEK and P-15 at Week 4 | PEEK and P-15 at Week 8 | PEEK from Week 4 to 8 | P-15 from Week 4 to 8 | ||||||
| PEEK | P-15 | PEEK | P-15 | ||||||
| Large (1000–500 µm) | 4 (n = 3) | 16.2 ± 2.3 | 17.5 ± 6.4 | 16.2 ± 2.4 (10.7–21.7) | 17.5 ± 2.4 (12.0–23.0) | N/A | N/A | N/A | N/A |
| 8 (n = 3) | 17.7 ± 3.6 | 21.5 ± 2.8 | 17.7 ± 2.4 (12.2–23.1) | 21.5 ± 2.4 (16.0–26.9) | |||||
| Intermediate (500–136 µm) | 4 (n = 3) | 15.0 ± 2.6 | 13.3 ± 4.1 | 15.0 ± 1.9 (10.2–19.8) | 13.3 ± 1.9 (8.5–18.2) | 0.22 | 0.020 | 0.44 | 0.028 |
| 8 (n = 3) | 17.2 ± 3.9 | 21.5 ± 1.6 | 17.2 ± 1.9 (12.4–22.0) | 21.5 ± 1.9 (16.7–26.3) | |||||
| Small (136–0 µm) | 4 (n = 3) | 3.6 ± 2.5 | 3.2 ± 1.5 | 3.6 ± 1.0 (1.4–5.9) | 3.2 ± 1.0 (0.9–5.5) | 0.71 | 0.047 | 0.83 | 0.028 |
| 8 (n = 3) | 3.9 ± 0.9 | 6.9 ± 1.4 | 3.9 ± 1.0 (1.6–6.2) | 6.9 ± 1.0 (4.7–9.2) | |||||
| Region of Interest | Timepoint (Week) | Mean Density (GV *) | Adjusted Mean Density (GV) with 95% CI ** | Adjusted p Value †,†† | |||||
|---|---|---|---|---|---|---|---|---|---|
| PEEK and P-15 at Week 4 | PEEK and P-15 at Week 8 | PEEK from Week 4 to 8 | P-15 from Week 4 to 8 | ||||||
| PEEK | P-15 | PEEK | P-15 | ||||||
| Large (1000–500 µm) | 4 (n = 3) | 77.6 ± 3.0 | 78.2 ± 3.8 | 77.6 ± 1.8 (73.3–81.9) | 78.2 ± 1.8 (73.9–82.5) | N/A | N/A | N/A | N/A |
| 8 (n = 3) | 80.9 ± 3.7 | 82.2 ± 0.7 | 80.9 ± 1.8 (76.6–85.2) | 82.2 ± 1.8 (77.9–86.5) | |||||
| Intermediate (500–136 µm) | 4 (n = 3) | 69.0 ± 4.8 | 72.3 ± 3.7 | 69.0 ± 2.1 (63.7–74.3) | 72.3 ± 2.1 (67.0–77.6) | N/A | N/A | 0.064 | 0.14 |
| 8 (n = 3) | 75.9 ± 3.3 | 77.5 ± 2.5 | 75.9 ± 2.1 (70.6–81.2) | 77.5 ± 2.1 (72.2–82.8) | |||||
| Small (136–0 µm) | 4 (n = 3) | 61.0 ± 2.7 | 71.4 ± 1.3 | 61.0 ± 1.5 (57.6–64.4) | 71.4 ± 1.5 (68.0–74.8) | 0.0036 | 0.53 | 0.021 | 0.16 |
| 8 (n = 3) | 67.0 ± 3.8 | 68.2 ± 1.3 | 67.0 ± 1.5 (63.6–70.4) | 68.2 ± 1.5 (64.8–71.6) | |||||
| Cytokine | Timepoint (Week) | Mean Cytokine Level (pg/mL) | Adjusted Mean Cytokine Level (pg/mL) with 95% CI * | Adjusted p Value **,† | |||||
|---|---|---|---|---|---|---|---|---|---|
| PEEK and P-15 at Week 4 | PEEK and P-15 at Week 8 | PEEK from Week 4 to 8 | P-15 from Week 4 to 8 | ||||||
| PEEK | P-15 | PEEK | P-15 | ||||||
| IL-1β | 4 (n = 4) | 101.1 ± 17.6 | 336.2 ± 182.3 | 101.0 ± 64.5 (−40.8–243.0) | 336.0 ± 64.5 (194.3–478.0) | 0.023 | 0.90 | 0.48 | 0.11 |
| 8 (n = 4) | 167.9 ± 157.4 | 178.1 ± 91.3 | 168.0 ± 64.5 (26.1–310.0) | 178.0 ± 64.5 (36.3–320.0) | |||||
| IL-4 | 4 (n = 4) | 281.2 ± 64.6 | 497.5 ± 100.0 | 281.0 ± 92.9 (57.7–505.0) | 497.0 ± 92.9 (274.0–721.0) | 0.0009 | 0.095 | 0.94 | 0.061 |
| 8 (n = 4) | 270.4 ± 262.4 | 200.1 ± 234.6 | 270.0 ± 92.9 (46.8–494.0) | 200.0 ± 92.9 (−23.4–424.0) | |||||
| IL-6 | 4 (n = 4) | 164.2 ± 78.0 | 467.6 ± 293.9 | 164.0 ± 82.8 (−16.1–344.0) | 468.0 ± 82.8 (287.3–648.0) | 0.041 | 0.44 | 0.52 | 0.032 |
| 8 (n = 4) | 87.0 ± 32.8 | 183.2 ± 126.6 | 87.0 ± 82.8 (−93.3–267.0) | 183.0 ± 82.8 (2.9–364.0) | |||||
| TNF-α | 4 (n = 4) | 457.5 ± 43.5 | 571.6 ± 118.6 | 458.0 ± 90.1 (257.0–658.0) | 572.0 ± 90.1 (371.0–772.0) | N/A | N/A | N/A | N/A |
| 8 (n = 4) | 458.6 ± 317.8 | 412.3 ± 113.3 | 459.0 ± 90.1 (258.0–659.0) | 412.0 ± 90.1 (212.0–613.0) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.C.; Swink, I.R.; Cheng, C.T.; Corcoran, O.G.; Wang, V.Z.; McClain, E.J., IV; Vyas, P.S.; Owen, I.; Xu, C.; Altman, D.T.; et al. Comparing the Immune Response to PEEK as an Implant Material with and without P-15 Peptide as Bone Graft Material in a Rabbit Long Bone Model. Bioengineering 2024, 11, 898. https://doi.org/10.3390/bioengineering11090898
Cheng BC, Swink IR, Cheng CT, Corcoran OG, Wang VZ, McClain EJ IV, Vyas PS, Owen I, Xu C, Altman DT, et al. Comparing the Immune Response to PEEK as an Implant Material with and without P-15 Peptide as Bone Graft Material in a Rabbit Long Bone Model. Bioengineering. 2024; 11(9):898. https://doi.org/10.3390/bioengineering11090898
Chicago/Turabian StyleCheng, Boyle C., Isaac R. Swink, Cooper T. Cheng, Owen G. Corcoran, Vicki Z. Wang, Edward J. McClain, IV, Praveer S. Vyas, Izzy Owen, Chen Xu, Daniel T. Altman, and et al. 2024. "Comparing the Immune Response to PEEK as an Implant Material with and without P-15 Peptide as Bone Graft Material in a Rabbit Long Bone Model" Bioengineering 11, no. 9: 898. https://doi.org/10.3390/bioengineering11090898
APA StyleCheng, B. C., Swink, I. R., Cheng, C. T., Corcoran, O. G., Wang, V. Z., McClain, E. J., IV, Vyas, P. S., Owen, I., Xu, C., Altman, D. T., & Yu, A. K. (2024). Comparing the Immune Response to PEEK as an Implant Material with and without P-15 Peptide as Bone Graft Material in a Rabbit Long Bone Model. Bioengineering, 11(9), 898. https://doi.org/10.3390/bioengineering11090898






