Two-Dimensional Mammography Imaging Techniques for Screening Women with Silicone Breast Implants: A Pilot Phantom Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Case Illustrating the Different Acquisition Techniques
2.1.1. Clinical Context and Characteristics of Breast Implants
2.1.2. Follow-Up by Mammography Imaging
2.2. Digital Mammography System
2.3. Characterization of the Automatic Exposure Control
2.3.1. Description of the Automatic Exposure Control
2.3.2. Tests of Automatic Exposure Control Sensitivity
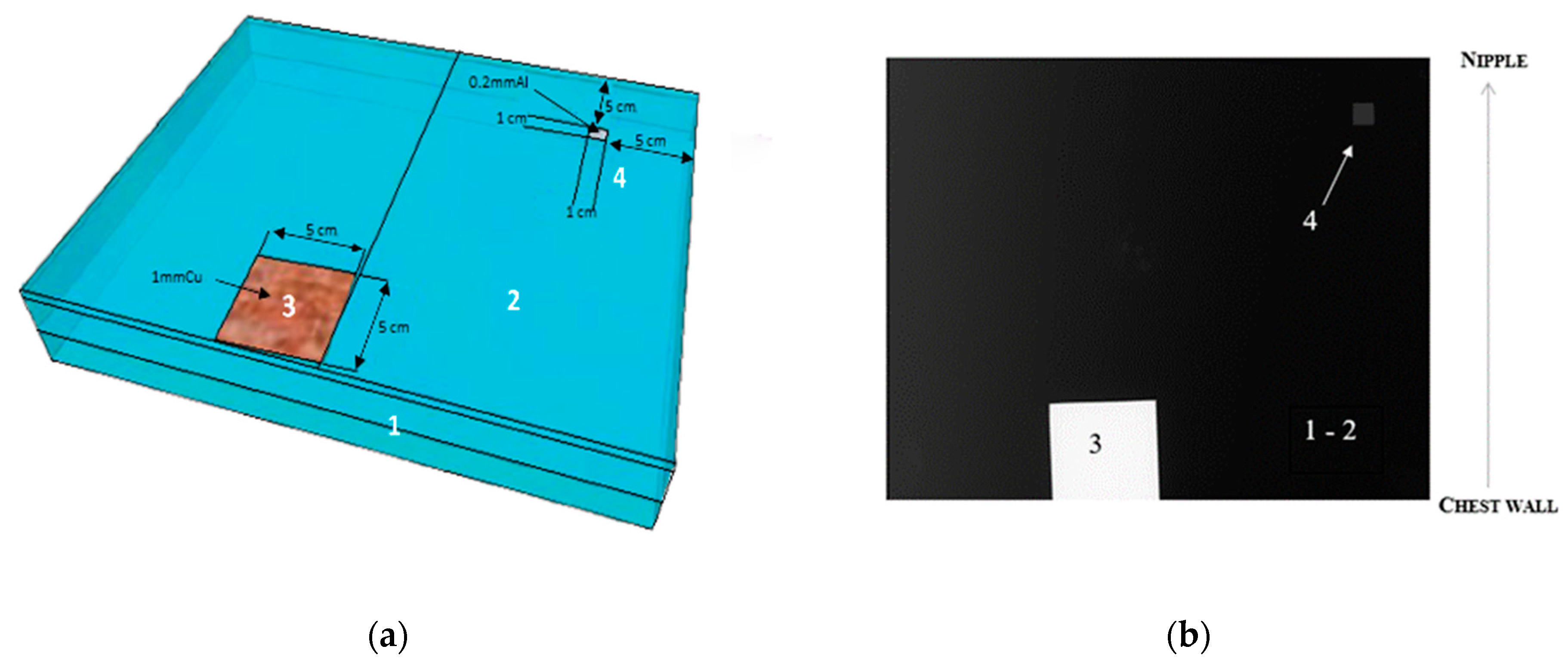
2.4. Description of the IAEA Phantom for Mammography
2.5. Description of 2D Mammographic Imaging Techniques for Breast Implants and Assimilates
2.5.1. Image Acquisition Techniques in Patients with Breast Implants
2.5.2. Replication of Clinical Acquisition Techniques with the IAEA Phantom
Image Acquisition in Automatic Mode with Various Positions of the AEC Sensor
- Case with AEC sensor partly positioned below the copper plate:
- Case with AEC sensor positioned outside the copper plate:
Image Acquisition According to Several Settings in Manual Mode
2.6. Image Quality Evaluation of Breast Implants and Assimilates
2.6.1. Image Quality Evaluation in Patients with Breast Implants
2.6.2. Image Metrics and Task-Based Image Quality Assessment of the IAEA Phantom
- Signal-Difference-to-Noise Ratio
- Detectability Index
2.7. Data Analysis
3. Results
3.1. Clinical Case Illustrating the Different Acquisition Techniques
3.1.1. Impact of Breast Implant Radiopacity According to Acquisition Technique Based on the Recommended Settings Compared to the Reference Settings

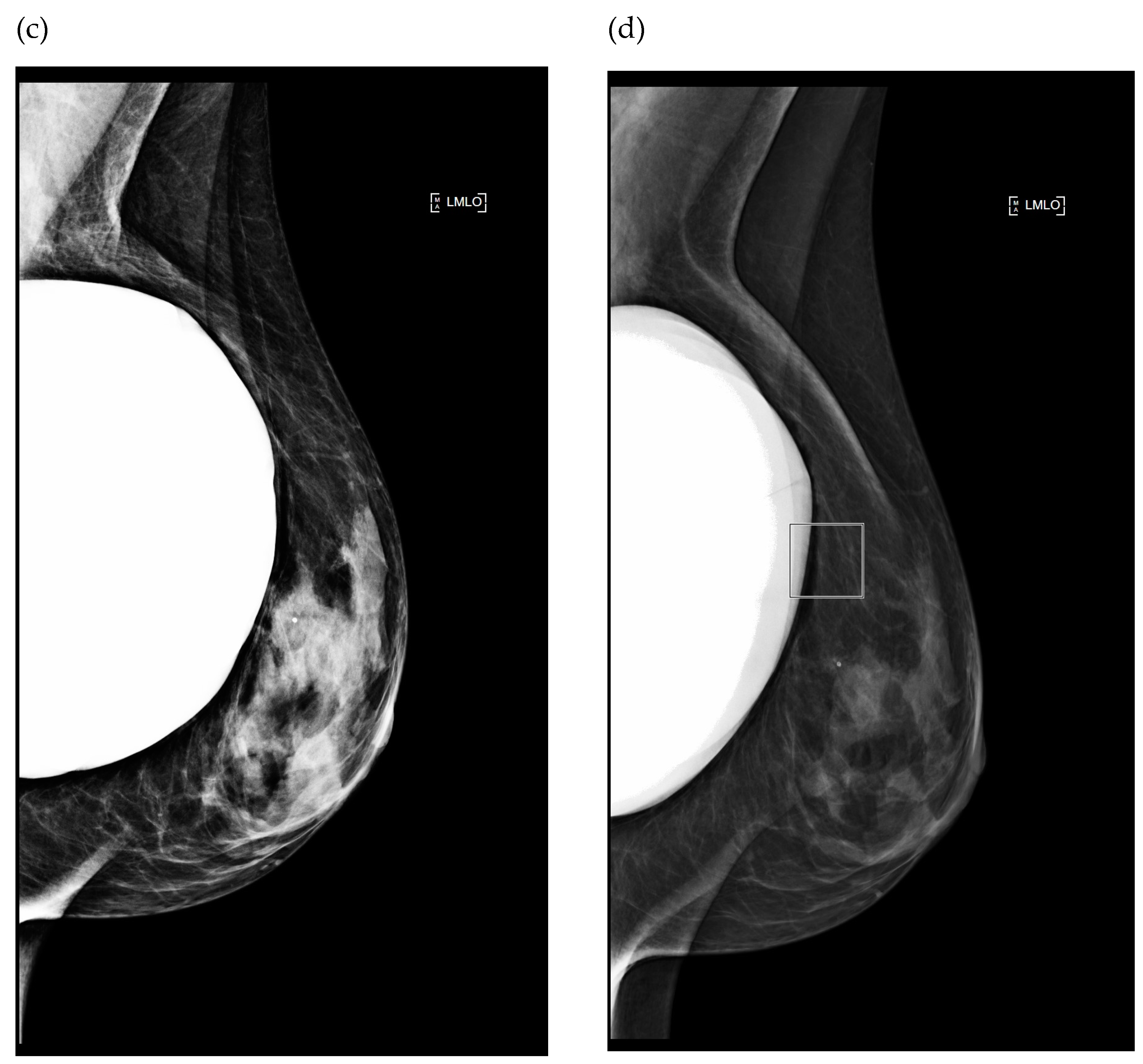
3.1.2. Impact of Breast Implant Radiopacity According to Acquisition Technique Based on the Recommended Settings Compared to the Misused Settings
3.2. Digital Mammography System: Tests of Automatic Exposure Control Sensitivity
3.3. Replication of Clinical Acquisition Techniques with the IAEA Phantom
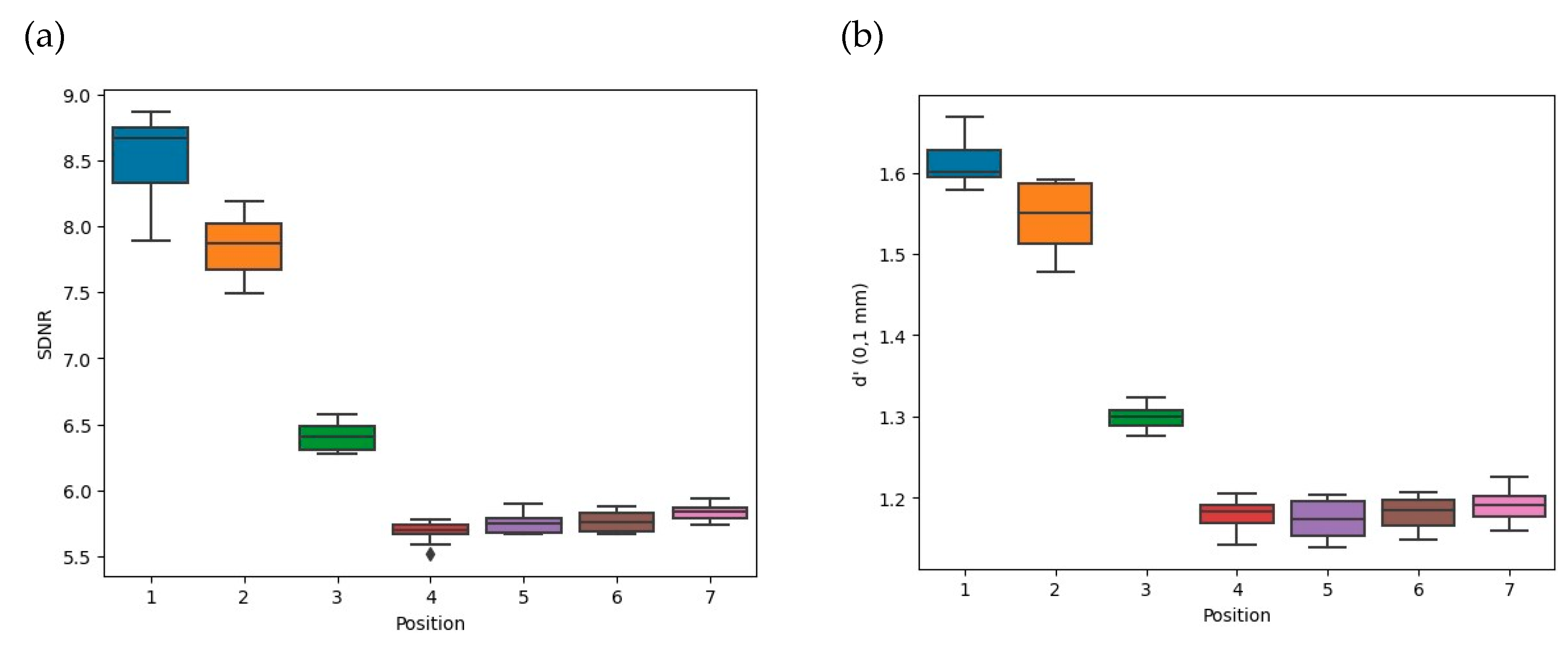
3.3.1. Comparison of Acquisition Techniques in Automatic Mode: Reference versus Misused Settings with Various Positions of the AEC Sensor
3.3.2. Comparison of Several Settings in Manual Mode
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency (IAEA). Worldwide Implementation of Digital Mammography Imaging; IAEA: Vienna, Austria, 2022. [Google Scholar]
- Benitez Fuentes, J.D.; Morgan, E.; De Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.-H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency (IAEA). Quality Assurance Programme for Screen Film Mammography; Human Health Series No. 2; IAEA: Vienna, Austria, 2009. [Google Scholar]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early Breast Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency (IAEA). Worldwide Implementation of Digital Imaging in Radiology; IAEA: Vienna, Austria, 2015. [Google Scholar]
- Salibian, A.A.; Karp, N.S. Modern Approaches to Implant-Based Breast Reconstruction. Clin. Plast. Surg. 2023, 50, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zeng, J.; Tian, X.; Zheng, H.; Wu, X. A Review of Different Breast Reconstruction Methods. Am. J. Transl. Res. 2023, 15, 3846–3855. [Google Scholar]
- Daskalaki, A.; Bliznakova, K.; Pallikarakis, N. Evaluation of the Effect of Silicone Breast Inserts on X-ray Mammography and Breast Tomosynthesis Images: A Monte Carlo Simulation Study. Phys. Medica 2016, 32, 353–361. [Google Scholar] [CrossRef]
- Silva, F.A.R.; Souza, L.F.; Salmon, C.E.G.; Souza, D.N. Breast Phantom with Silicone Implant for Evaluation in Conventional Mammography. J. Appl. Clin. Med. Phys. 2011, 12, 199–206. [Google Scholar] [CrossRef]
- Deandrea, S.; Cavazzana, L.; Principi, N.; Luconi, E.; Campoleoni, M.; Bastiampillai, A.J.; Bracchi, L.; Bucchi, L.; Pedilarco, S.; Piscitelli, A.; et al. Screening of Women with Aesthetic Prostheses in Dedicated Sessions of a Population-Based Breast Cancer Screening Programme. Radiol. Med. 2021, 126, 946–955. [Google Scholar] [CrossRef]
- Sá dos Reis, C.; Gremion, I.; Richli Meystre, N. Study of Breast Implants Mammography Examinations for Identification of Suitable Image Quality Criteria. Insights Imaging 2020, 11, 3. [Google Scholar] [CrossRef]
- Sá dos Reis, C.; Gremion, I.; Richli Meystre, N. Consensus about Image Quality Assessment Criteria of Breast Implants Mammography Using Delphi Method with Radiographers and Radiologists. Insights Imaging 2020, 11, 56. [Google Scholar] [CrossRef]
- Park, J.; Ko, E.Y.; Han, B.-K.; Ko, E.S.; Choi, J.S.; Kim, H. Appropriate Screening Mammography Method for Patients with Breast Implants. Sci. Rep. 2023, 13, 1811. [Google Scholar] [CrossRef]
- Young, V.L.; Diehl, G.J.; Eichling, J.; Monsees, B.S.; Destouet, J. The Relative Radiolucencies of Breast Implant Filler Materials. Plast. Reconstr. Surg. 1993, 91, 1066–1072. [Google Scholar] [CrossRef]
- Jewell, M.L.; Bengtson, B.P.; Smither, K.; Nuti, G.; Perry, T. Physical Properties of Silicone Gel Breast Implants. Aesthetic Surg. J. 2019, 39, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Foroushani, F.T.; Dzobo, K.; Khumalo, N.P.; Mora, V.Z.; De Mezerville, R.; Bayat, A. Advances in Surface Modifications of the Silicone Breast Implant and Impact on Its Biocompatibility and Biointegration. Biomater. Res. 2022, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J.; Bloomquist, A.K.; Hunter, D.M.; Mawdsley, G.E.; Chiarelli, A.M.; Muradali, D.; Mainprize, J.G. Comparative Performance of Modern Digital Mammography Systems in a Large Breast Screening Program: Comparative Performance of CR and DR Mammography Systems. Med. Phys. 2013, 40, 121915. [Google Scholar] [CrossRef]
- European Commission; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency; International Labour Organization; OECD Nuclear Energy Agency; Pan American Health Organization; United Nations Environment Programme; World Health Organization. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; General Safety Requirements Part 3; International Atomic Energy Agency: Vienna, Austria, 2014. [Google Scholar]
- International Atomic Energy Agency (IAEA). Radiation Protection and Safety in Medical Uses of Ionizing Radiation; IAEA Safety Standards Series No. SSG-46; IAEA: Vienna, Austria, 2018. [Google Scholar]
- Tsapaki, V. Radiation Dose Optimization in Diagnostic and Interventional Radiology: Current Issues and Future Perspectives. Phys. Medica 2020, 79, 16–21. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Implementation of a Remote and Automated Quality Control Programme for Radiography and Mammography Equipment; IAEA: Vienna, Austria, 2021. [Google Scholar]
- International Atomic Energy Agency (IAEA). Handbook of Basic Quality Control Tests for Diagnostic Radiology; IAEA: Vienna, Austria, 2022. [Google Scholar]
- European Commission (EC). Criteria for Acceptability of Medical Radiological Equipment Used in Diagnostic Radiology, Nuclear Medicine and Radiotherapy; 2012. Available online: https://data.europa.eu/doi/10.2768/22561 (accessed on 2 January 2013).
- American Association of Physicists in Medicine (AAPM). Report of Task Group #12 Diagnostic X-ray Imaging Committee. In Quality Control in Diagnostic Radiology; AAPM: Madison, WI, USA, 2002. [Google Scholar]
- Institute of Physicists and Engineers in Medicine (IPEM). The Commissioning and Routine Testing of Mammographic X-ray Systems; IPEM: New York, NY, USA, 2005. [Google Scholar]
- European Federation of Organizations for Medical Physics (EFOMP). Quality Control in Digital Mammography; EFOMP: Utrecht, The Netherlands, 2015. [Google Scholar]
- International Atomic Energy Agency (IAEA). Quality Assurance Programme for Digital Mammography; Human Health Series No. 17; IAEA: Vienna, Austria, 2011. [Google Scholar]
- Fitton, I.; Noël, A.; Minassian, J.; Zerhouni, M.; Wojak, J.; Adel, M.; Fournier, L. Technical Note: Design and Initial Evaluation of a Novel Physical Breast Phantom to Monitor Image Quality in Digital Breast Tomosynthesis. Med. Phys. 2022, 49, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Fitton, I. Image Quality Comparison between Digital Breast Tomosynthesis Associated to Synthesised Image versus 2D FFDM. In Proceedings of the 2017 European Congress of Radiology, Vienna, Austria, 1–5 March 2017. 1113 words. [Google Scholar] [CrossRef]
- IAEA. Remote/Automated Quality Control in Radiology; IAEA: Vienna, Austria, 2023. [Google Scholar]
- Mora, P.; Pfeiffer, D.; Zhang, G.; Bosmans, H.; Delis, H.; Razi, Z.; Arreola, M.; Tsapaki, V. The IAEA Remote and Automated Quality Control Methodology for Radiography and Mammography. J. Appl. Clin. Med. Phys. 2021, 22, 126–142. [Google Scholar] [CrossRef]
- Tsalafoutas, I.A.; AlKhazzam, S.; Tsapaki, V.; AlNaemi, H.; Kharita, M.H. Digital Radiography Image Quality Evaluation Using Various Phantoms and Software. J. Appl. Clin. Med. Phys. 2022, 23, e13823. [Google Scholar] [CrossRef]
- IAEA. Advanced Tools for Quality and Dosimetry of Digital Imaging in Radiology; IAEA: Vienna, Austria, 2023. [Google Scholar]
- French Agency for the Safety of Health Products. Décision du 15 Janvier 2020 Fixant les Modalités du Contrôle de Qualité des Installations de Mammographie Numérique. 2020. Available online: https://ansm.sante.fr/actualites/decision-du-15-01-2020-fixant-les-modalites-du-controle-de-qualite-des-installations-de-mammographie-numerique (accessed on 21 January 2020).
- EUREF. Protocol for the Quality Control of the Physical and Technical Aspects of Digital Breast Tomosynthesis Systems; EUREF: 2018. Available online: https://euref.org/european-guidelines/physico-technical-protocol/ (accessed on 28 September 2018).
- Kotre, C.J.; Robson, K.J.; Simpson, W. Improving the Visibility of Radio-Opaque Markers in Mammography. Br. J. Radiol. 1999, 72, 799–801. [Google Scholar] [CrossRef]
- Eklund, G.; Busby, R.; Miller, S.; Job, J. Improved Imaging of the Augmented Breast. Am. J. Roentgenol. 1988, 151, 469–473. [Google Scholar] [CrossRef]
- Chetlen, A.; Niell, B.L.; Brown, A.; Baskies, A.M.; Battaglia, T.; Chen, A.; Jochelson, M.S.; Klein, K.A.; Malak, S.F.; Mehta, T.S.; et al. ACR Appropriateness Criteria® Breats Implant Evaluation. J. Am. Coll. Radiol. 2023, 20, S329–S350. [Google Scholar] [CrossRef]
- Raj, S.D.; Karimova, E.J.; Fishman, M.D.C.; Fein-Zachary, V.; Phillips, J.; Dialani, V.; Slanetz, P.J. Imaging of Breast Implant–Associated Complications and Pathologic Conditions: Breast Imaging. RadioGraphics 2017, 37, 1603–1604. [Google Scholar] [CrossRef]
- Shah, A.T.; Jankharia, B.B. Imaging of Common Breast Implants and Implant-Related Complications: A Pictorial Essay. Indian J. Radiol. Imaging 2016, 26, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Spuur, K.; Webb, J.; Poulos, A.; Nielsen, S.; Robinson, W. Mammography Image Quality and Evidence Based Practice: Analysis of the Demonstration of the Inframammary Angle in the Digital Setting. Eur. J. Radiol. 2018, 100, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Monnin, P.; Marshall, N.W.; Bosmans, H.; Bochud, F.O.; Verdun, F.R. Image Quality Assessment in Digital Mammography: Part II. NPWE as a Validated Alternative for Contrast Detail Analysis. Phys. Med. Biol. 2011, 56, 4221–4238. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.M.; Mackenzie, A.; Cooke, J.; Given-Wilson, R.M.; Wallis, M.G.; Chakraborty, D.P.; Dance, D.R.; Bosmans, H.; Young, K.C. Effect of Image Quality on Calcification Detection in Digital Mammography: Image Quality and Calcification Detection in Digital Mammography. Med. Phys. 2012, 39, 3202–3213. [Google Scholar] [CrossRef]
- Zanca, F.; Van Ongeval, C.; Marshall, N.; Meylaers, T.; Michielsen, K.; Marchal, G.; Bosmans, H. The Relationship between the Attenuation Properties of Breast Microcalcifications and Aluminum. Phys. Med. Biol. 2010, 55, 1057–1068. [Google Scholar] [CrossRef]
- IEC 62220-1-1:2015; Medical Electrical Equipment—Characteristics of Digital X-ray Imaging Devices—Part 1-1: Determination of the Detective Quantum Efficiency—Detectors Used in Radiographic Imaging. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2015.
- IEC 62220-1-2:2007; Medical Electrical Equipment-Characteristics of Digital X-ray Imaging Devices-Part 1–2: Determination of Detective Quantum Efficiency Detectors Used in Mammography. International Electrotechnical Commission (IEC): Geneva, Switzerland, 2007.
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Hotelling, H., Eds.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Jafari, M.; Ansari-Pour, N. Why, When and How to Adjust Your P Values? Cell J. 2018, 20, 604–607. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Radiologic Technologist Mammography Specific Training. In Policy Guidance Help System; United States of America, Food & Drug Administration: Washington, DC, USA, 2017. Available online: https://www.accessdata.fda.gov/cdrh_docs/presentations/pghs/Radiologic_Technologist_Mammography_Specific_Training.htm (accessed on 21 January 2020).
- Brown, S.L.; Todd, J.F.; Luu, H.-M.D. Breast Implant Adverse Events during Mammography: Reports to the Food and Drug Administration. J. Women’s Health 2004, 13, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sechopoulos, I.; Dance, D.R.; Boone, J.M.; Bosmans, H.T.; Caballo, M.; Diaz, O.; Van Engen, R.; Fedon, C.; Glick, S.J.; Hernandez, A.M.; et al. Joint AAPM Task Group 282/EFOMP Working Group Report: Breast Dosimetry for Standard and Contrast-enhanced Mammography and Breast Tomosynthesis. Med. Phys. 2023, 51, 712–739. [Google Scholar] [CrossRef] [PubMed]




| LCC Projection with Eklund Maneuver | LMLO Projection | |||
|---|---|---|---|---|
| Manual-Mode Recommended Settings | Automatic-Mode Reference Settings | Manual-Mode Recommended Settings | Automatic-Mode Misused Settings | |
| kVp | 28 | 29 | 28 | 32 |
| mAs | 120 | 108 | 120 | 159 |
| Breast thickness (mm) | 46 | 50 | 51 | 66 |
| Compression Force (N) | 20.1 | 63.6 | 38.4 | 54.7 |
| Anode/Filter | W/Rh | W/Rh | W/Rh | W/Rh |
| SNRBT | 12.1 ± 6.2 | 4.6 ± 1.7 | 8.7 ± 6.4 | 17.2 ± 10.2 |
| AGD (mGy) | 1.7 | 1.4 | 1.4 | 2.5 |
| Criteria | LCC Projection with Eklund Maneuver | LMLO Projection | ||
|---|---|---|---|---|
| Manual-Mode Recommended Settings | Automatic-Mode Reference Settings | Manual-Mode Recommended Settings | Automatic-Mode Misused Settings | |
| Positioning | ||||
| Breast centrally placed |  |  |  |  |
| Visualization of retroglandular adipose tissue |  |  | NA | NA |
| Inframammary angle clearly demonstrated | NA | NA |  |  |
| Full visualization of inferior breast tissue | NA | NA |  |  |
| Pectoral muscle visualized | NA | NA | NA | NA |
| Medial border of the breast included on the image |  |  | NA | NA |
| Nipple in the midline (+/−10°) |  |  | NA | NA |
| Nipple in profile or transected by skin |  |  |  |  |
| Visibility of implant edge in the image |  |  |  |  |
| Maximum “retropulsion” of the implant |  |  | NA | NA |
| Artefacts | ||||
| No skin folds |  |  |  |  |
| No artefacts |  |  |  |  |
| Skin edges visualized |  |  | NA | NA |
| Sharpness | ||||
| Spread of breast tissue to differentiate adipose from fibroglandular tissue |  |  |  |  |
| Sharpness of glandular tissue |  |  |  |  |
| Appropriate contrast |  |  |  |  |
 : Correct;
: Correct;  : Incorrect; NA: not applicable.
: Incorrect; NA: not applicable.| Position of the AEC Sensor | All AEC Positions | COV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| kVp | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | 28 ± 0 | 0.00 |
| mAs | 94.6 ± 0.8 | 94.2 ± 0.4 | 93.8 ± 0.4 | 93.8 ± 0.4 | 94.0 ± 0.0 | 93.8 ± 0.4 | 94.4 ± 0.5 | 94.1 ± 0.2 | 0.01 |
| Compressed thickness (mm) | 42.2 ± 0.4 | 42.2 ± 0.4 | 42.2 ± 0.4 | 42.0 ± 0.0 | 42.0 ± 0.0 | 42.0 ± 0.0 | 42.0 ± 0.0 | 42.1 ± 0.1 | 0.01 |
| AGD (mGy) | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 | 0.01 |
| SNRC | 55.4 ± 0.7 | 54.8 ± 0.6 | 54.6 ± 0.2 | 54.7 ± 0.5 | 54.7 ± 0.4 | 54.8 ± 0.3 | 54.7 ± 0.1 | 54.8 ± 0.2 | 0.01 |
| SNRCW1 | 57.8 ± 1.0 | 57.4 (0.8) | 56.9 ± 0.2 | 56.9 ± 0.1 | 57.0 ± 0.4 | 56.8 ± 0.2 | 56.7 ± 0.2 | 57.1 ± 0.2 | 0.01 |
| SNRN1 | 39.8 ± 1.0 | 39.5 (0.3) | 39.2 ± 0.4 | 39.3 ± 0.2 | 39.3 ± 0.3 | 39.2 ± 0.3 | 38.7 ± 0.2 | 39.3 ± 0.2 | 0.01 |
| SNRN2 | 41.7 ± 1.2 | 41.0 (0.5) | 40.7 ± 0.3 | 40.9 ± 0.1 | 40.7 ± 0.2 | 40.2 ± 0.5 | 39.9 ± 0.4 | 40.7 ± 0.3 | 0.02 |
| SNRCW2 | 60.4 ± 1.0 | 58.7 (0.9) | 58.2 ± 0.2 | 58.2 ± 0.4 | 58.4 ± 0.4 | 58.2 ± 0.2 | 58.2 ± 0.2 | 58.5 ± 0.2 | 0.01 |
| Automatic Mode | SDNR | d’ (0.1 mm) | d’ (0.25 mm) | AGD (mGy) |
|---|---|---|---|---|
| Misused settings | 7.9 (1.8) | 1.6 (0.3) | 9.2 (1.7) | 2.2 (0.8) |
| Reference settings | 5.8 (0.1) | 1.2 (0.0) | 5.8 (0.1) | 1.2 (0.0) |
| p value | <0.03 1 | <0.03 1 | <0.03 1 | <0.03 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitton, I.; Tsapaki, V.; Zerbib, J.; Decoux, A.; Kumar, A.; Stembert, A.; Malchair, F.; Van Ngoc Ty, C.; Fournier, L. Two-Dimensional Mammography Imaging Techniques for Screening Women with Silicone Breast Implants: A Pilot Phantom Study. Bioengineering 2024, 11, 884. https://doi.org/10.3390/bioengineering11090884
Fitton I, Tsapaki V, Zerbib J, Decoux A, Kumar A, Stembert A, Malchair F, Van Ngoc Ty C, Fournier L. Two-Dimensional Mammography Imaging Techniques for Screening Women with Silicone Breast Implants: A Pilot Phantom Study. Bioengineering. 2024; 11(9):884. https://doi.org/10.3390/bioengineering11090884
Chicago/Turabian StyleFitton, Isabelle, Virginia Tsapaki, Jonathan Zerbib, Antoine Decoux, Amit Kumar, Aude Stembert, Françoise Malchair, Claire Van Ngoc Ty, and Laure Fournier. 2024. "Two-Dimensional Mammography Imaging Techniques for Screening Women with Silicone Breast Implants: A Pilot Phantom Study" Bioengineering 11, no. 9: 884. https://doi.org/10.3390/bioengineering11090884
APA StyleFitton, I., Tsapaki, V., Zerbib, J., Decoux, A., Kumar, A., Stembert, A., Malchair, F., Van Ngoc Ty, C., & Fournier, L. (2024). Two-Dimensional Mammography Imaging Techniques for Screening Women with Silicone Breast Implants: A Pilot Phantom Study. Bioengineering, 11(9), 884. https://doi.org/10.3390/bioengineering11090884







