Abstract
Autism spectrum disorder (ASD) is a collection of neurodevelopmental disorders whose pathobiology remains elusive. This study aimed to investigate the possible neural mechanisms underlying ASD using a dynamic brain network model and a relatively large-sample, multi-site dataset. Resting-state functional magnetic resonance imaging data were acquired from 208 ASD patients and 227 typical development (TD) controls, who were drawn from the multi-site Autism Brain Imaging Data Exchange (ABIDE) database. Brain network flexibilities were estimated and compared between the ASD and TD groups at both global and local levels, after adjusting for sex, age, head motion, and site effects. The results revealed significantly increased brain network flexibilities (indicating a decreased stability) at the global level, as well as at the local level within the default mode and sensorimotor areas in ASD patients than TD participants. Additionally, significant ASD-related decreases in flexibilities were also observed in several occipital regions at the nodal level. Most of these changes were significantly correlated with the Autism Diagnostic Observation Schedule (ADOS) total score in the entire sample. These results suggested that ASD is characterized by significant changes in temporal stabilities of the functional brain network, which can further strengthen our understanding of the pathobiology of ASD.
1. Introduction
Autism spectrum disorder (ASD) is a collection of neurodevelopmental disorders that are characterized by early social communication deficits and impaired repetitive behaviors/interests [1,2]. The prevalence of ASD has been growing in the past decades, leading to high economic burdens globally [1,3]. However, the pathobiology of ASD remains elusive; multiple genetic mutations, maternal immune activations, and environmental factors are thought to be involved in the development of ASD [2,4].
Functional magnetic resonance imaging (fMRI) has been widely used in clinical studies, as a non-invasive and convenient method, to investigate the neural mechanisms underlying many common mental disorders (e.g., major depressive disorder and schizophrenia) [5,6,7]. Past fMRI studies have demonstrated that ASD is associated with aberrant brain functions such as significantly decreased/increased functional connectivity (FC) within the visual, frontoparietal (cognition), and language-related subnetworks in the brain [8,9,10,11]. These studies have significantly improved our understanding of the complex pathobiology of ASD.
These traditional fMRI studies were usually performed under the assumption that FCs within or between different brain networks would be “static (never change)” over time during the fMRI scans. In recent years, however, it has been suggested that FCs within/between different brain networks might actually fluctuate over time and that constructing a “dynamic” rather than “static” brain network model may be valuable in capturing important information ignored by traditional brain network models [12,13,14]. Research on the properties of a “dynamic brain network” under such a framework is emerging, with various measures of temporal variability/stability of the brain networks proposed by different researchers [15,16,17,18]. Specifically, the “flexibility” (also named the “switching rate” by some researchers) of a dynamic functional brain network, which estimates the temporal stability of a brain network according to its changing frequencies of modularity structures, has been proved to be valid [19] and has been widely used in recent studies [20,21,22,23,24,25,26,27,28]. In these studies, the flexibility of the brain network is associated with cognition [20,21], emotion [26], and aging [24], as well as many common neuropsychiatric disorders such as Parkinson’s disease [27], attentiondeficit/hyperactivity disorder [25], schizophrenia [22], anxiety disorder [28], and major depressive disorder [23]. Notably, a published study by Harlalka et al. [29] has reported that atypical flexibility of the dynamic functional brain network can quantify (positively associated with) illness severity in ASD patients. A significantly increased brain network flexibility when compared to matched normal controls has been thought to be reflective of brain dysfunction, which indicates a decreased stability of the brain network organization [30,31]. Therefore, the observed positive correlation of symptom severity with flexibility in ASD patients [29] has suggested that disrupted stability of the brain network may be related to the pathobiology of ASD.
Nevertheless, there are still some notable limitations in the above-mentioned earlier work by Harlalka et al. [29]. First, their sample size is relatively small: only 72 patients with ASD and 72 typical development (TD) controls from a single study site were included [29]. Previous researchers have suggested that for human connectome studies, satisfactory statistical power and reliability can be achieved when the sample size is larger than a cutoff of approximately 250 subjects [32]. Therefore, repeating the study with a larger sample size might be necessary to obtain a more reliable conclusion. Second, besides brain network flexibility at the global level, flexibility can be also estimated at the subnetwork level for particular subsystems of the brain (e.g., for the default mode, visual, and frontoparietal subnetworks). For instance, using the measure of brain network flexibility, a study by Huang et al. [30] suggested that childhood trauma experiences are linked to decreased temporal stabilities within the default mode, fronto-parietal, cingulo-opercular, and occipital subnetworks in the brain network. Beyond the global-level brain network flexibility, such results could further strengthen our understanding of the relationship between brain network stability and psychiatric symptoms/disorders at the local level. However, in the earlier study by Harlalka et al. [29], there were no results reported on the possible relationship between brain network flexibility and ASD at the subnetwork level, which deserves further investigation. Third, when constructing the dynamic functional brain network, an anatomically based automated anatomical labeling (AAL) atlas with 90 regions of interest (ROIs) was used in the study by Harlalka et al. [29]. However, it has been well documented that in studies on human functional brain networks, the anatomical-based AAL atlas would have a poorer performance compared to those higher-resolution functional atlases, such as the Dosenbach atlas (with 160 ROIs) and the Power atlas (with 264 ROIs) [13,32,33]. Thus, performing the analyses based on a functional atlas might be also meaningful for obtaining more accurate results.
To fill the gaps mentioned above, this study aimed to investigate the possible associations between changes in the brain network flexibility and ASD using a multi-site dataset, with the following advantages to the prior work:
- A multi-site resting-state fMRI dataset of ASD patients was used, which had a much larger sample (~500 participants in total) than most of the previous studies on ASD-related alterations in dynamic FCs.
- A widely used, validated functional atlas (Dosenbach atlas) was used during the construction of the brain networks, which may provide more accurate brain parcellations and more reliable results.
- Brain network flexibility was compared between patients with ASD and TD controls at both the global and local (subnetwork) levels, which will contribute to a deeper understanding of the pathobiology of ASD at the level of brain subnetworks.
2. Materials and Methods
2.1. Study Steps
The major steps of the present study include selecting participants from the dataset, finishing neuroimaging data acquisition and preprocessing, constructing dynamic brain networks, and estimating brain network flexibility, as well as performing statistical analyses (including several supplementary analyses), as presented in the flow chart (Figure 1).
Figure 1.
Flow chart of the present study. Abbreviations: ABIDE = “Autism Brain Imaging Data Exchange”.
2.2. Participants
The analyzed sample in this study consisted of 435 subjects (208 ASD patients and 227 healthy TD participants) from 9 study sites. Such a sample was drawn from the open-access, multi-site Autism Brain Imaging Data Exchange (ABIDE)-Preprocessed database at: http://preprocessed-connectomes-project.org/abide/ (accessed on 1 April 2024) [34,35]. Details about the participant recruitment, assessment, resting-state fMRI neuroimaging data acquisition, and data preprocessing protocols can be found on the ABIDE website and prior publications [34,35]. Most participants completed the Autism Diagnostic Observation Schedule (ADOS) [36] to assess the severity of ASD symptoms.
The original ABIDE-Preprocessed database includes more than one thousand participants recruited from 16 different study sites. In this study, we selected the participants in the analyzed sample based on the following steps: (1) the participants whose demographic information was incomplete (e.g., sex and age) were first excluded; (2) participants were excluded when the repetition time (TR) ≠ 2 s during the fMRI scans, to prevent biases caused by different temporal resolutions of the dynamic brain networks [37,38]; (3) participant with poor fMRI data quality were excluded, as defined by a mean framewise displacement (FD) > 0.2 mm [38,39], bad image coverage (signal loss in any ROI in the Dosenbach atlas), or any of the 3 independent raters gave a “Fail” or “Maybe” rating when performing manual data checking during the ABIDE preprocessing pipeline [29]; (4) finally, the sites with fewer than 10 individuals left after the above steps were excluded from the analysis, as was done in some other multi-site fMRI studies [39,40]. The final analyzed sample of 435 subjects were from the following nine study sites: one from New York University (NYU), one from the Olin Center (OLIN), one from San Diego State University (SDSU), one from the Trinity Centre for Health Sciences (TRINITY), one from the University of California Los Angeles (UCLA), two from the University of Miami (UM_1 and UM_2), one from the University of Utah School of Medicine (USM), and one from the Yale Child Study Center (YALE) (see Table 1 for detailed number of participants in each site).

Table 1.
Number of participants from each site in the analyzed sample.
The ABIDE-Preprocessed database is open-shared, and institutional review board (IRB) approval was provided by each site (data contributor) in the ABIDE database.
2.3. Neuroimaging Data Acquisition and Preprocessing
The resting-state fMRI data downloaded from the ABIDE database were preprocessed using pipelines whose details can be found at http://preprocessed-connectomes-project.org/abide/Pipelines.html (accessed on 1 April 2024). For all participants, the fMRI scans were acquired and preprocessed at each study site independently. The scans were acquired using SIEMENS, PHILIPS, or GE magnetic resonance imaging machines; the TR was 2 s for all scans, but there might be differences in other scanning parameters (e.g., echo time and total scan duration). The preprocessing pipeline includes slice timing correction, motion realignment, nuisance signal (motion and tissue signal) removal, and registration. Notably, the ABIDE database provided both the preprocessed data with and without the step of global signal regression (GSR). In the current study, we used the data without GSR since the usage of GSR is still controversial [41,42]. Furthermore, to control for confounding effects caused by differences in data acquisition and preprocessing procedures between different sites, we included “site” as covariates (using dummy coding) in all analyses (see details in the following sections).
2.4. Construction of Dynamic Brain Networks
The steps of constructing dynamic brain networks and estimating brain network flexibility are summarized in Figure 2. A dynamic functional brain network is composed of a set of nodes (ROIs) and connections (FCs) between nodes, where the strengths of connections change over time [12]. In this study, nodes in the dynamic brain network were defined using the 160 ROIs from the Dosenbach atlas [43], which has been validated and widely used in clinical studies [30,44,45,46]. For each participant, the mean time series were extracted from each ROI and then divided into a number of partly overlapping time windows using the “sliding window” method, which is commonly used in dynamic brain network studies (Figure 2A) [47,48,49]. Here, a fixed window width of 100 s (50 TRs) and a sliding step length of 2 s (1 TR) were used, according to the recommendations in previous works, to balance the reliability of results and computational complexity [12,30,38,50]. Within each time window, the FC strengths between all possible pairs of ROIs were computed using Pearson correlations, resulting in a “snapshot” of the brain network organization as shown by a 160 × 160 FC matrix. These time-ordered “snapshots” (matrices) then formed a multilayer dynamic brain network G = (Gt)t = 1, 2, 3, …, T, where the tth “snapshot”/matrix (Gt) represents the brain network organization at the tth time window (Figure 2B). Note that the total number of time windows (T) was determined by the length of fMRI scanning and was thus different between different study sites.
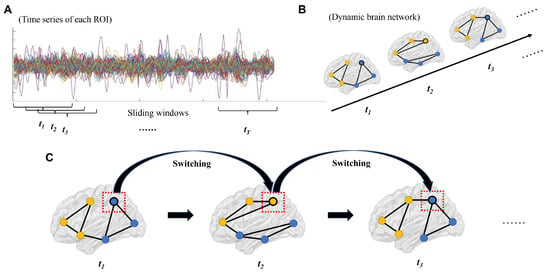
Figure 2.
Steps of constructing dynamic brain networks and calculating flexibility (see details in Section 2.4 and Section 2.5). (A) The time series of each region of interest (ROI) were divided into a number of time windows using the sliding window approach. (B) Brain network organizations were constructed for each time window, which formed a dynamic brain network. (C) Flexibilities (switching rates) of each ROI were then estimated by the number of times for which it switched from one “community” to another. Note: the lines with different colors in subfigure A represent time series of different nodes, and the dots with different colors in subfigures B/C represent nodes in different communities.
2.5. Estimating Brain Network Flexibility
After constructing the dynamic brain network for each participant, brain network flexibility was then computed strictly following some previous publications [20,30,45,51,52,53]. Briefly, a dynamic community detection algorithm as described by Mucha et al. [52] was implemented in Matlab (version R2022a) using an open-source code package, which can be found at https://github.com/GenLouvain/GenLouvain (accessed on 1 April 2024) [54]. Based on this algorithm, all nodes (ROIs) in the dynamic brain network were assigned into several communities at each time window, and this resulted in different community assignments for different time windows. The “flexibility/switching rate” of a node i (fi) can be then computed based on its switching frequency between different communities over time as
where Ni is the number of times for which the node i “switched” from one community to another (Figure 2C) [55]. The calculation was performed with the assistance of the Network Community Toolbox at: http://commdetect.weebly.com/ (accessed on 1 April 2024) [55]. Notably, since individual runs of the algorithm could lead to slightly different community assignments, the algorithm and flexibility calculation were repeated a total of 100 times, and the final flexibility values were averaged across the 100 runs [30,45,53,56]. After that, the flexibility of the whole brain network (global-level flexibility) was obtained by averaging the flexibilities of all the 160 ROIs.
fi = Ni/(T − 1),
According to previous research [43], all ROIs from the Dosenbach atlas can be assigned to six subnetworks including the default mode, occipital, cingulo-opercular, fronto-parietal, sensorimotor, and cerebellar subnetworks. On the basis of such an assignment, flexibilities for each of the six subnetworks were further obtained by averaging all nodes belonging to each particular subnetwork [22,30,45].
2.6. Statistics
Demographic and clinical characteristics were compared between the ASD and TD groups using two-sample t tests or chi-square tests as appropriate. All brain network measures (flexibilities at the global, subnetwork, and nodal levels) were compared between the ASD and TD groups by the analysis of covariance (ANCOVA) covarying for age, sex, head motion (mean FD), and site (dummy coded). False discovery rate (FDR) corrections were performed to correct for multiple comparisons across the six subnetworks or across the 160 nodes. Significance was set at a FDR-corrected p < 0.05.
When significant between-group differences were found on any brain network measures, post hoc correlation tests were further performed to investigate their possible relationships with the severity of clinical symptoms (measured by the total ADOS score) in the ASD patients. Here, partial Pearson correlations adjusted for age, sex, head motion, and site effects were performed between the brain network measures and ADOS total score. The correlation analyses were performed in the entire sample and the ASD group independently, respectively. Similar to the between-group comparisons, FDR corrections were performed to correct for multiple correlation tests across multiple subnetworks or nodes. Significance was set at a FDR-corrected p < 0.05, too.
2.7. Supplementary Analyses
When significant group differences were found in any brain network measures, supplementary analyses were further performed to rule out possible confounding effects on them. In short, since sex ratio and head motion were not well matched between the ASD and TD groups in the main analyses, we drew a subset where sex and head motion were matched between the ASD and TD groups by excluding a number of the participants (male TD participants with a mean FD > 0.05 as well as female TD participants with a mean FD > 0.06 were excluded). Post hoc group comparisons (between the ASD and TD participants) on the brain network measures showing significant results in the entire sample were then repeated in such a subset. Significance was still set at a corrected p < 0.05 after FDR corrections across multiple tests.
3. Results
3.1. The Structures of Results
Results of the following analyses will be presented in Section 3: group comparisons on sample characteristics, group comparisons on flexibilities, correlation analyses, and supplementary analyses.
3.2. Sample Characteristics
Demographic and clinical characteristics of the ASD and TD groups are presented in Table 2. There was no significant group difference in age (t = 0.847, p = 0.398); nevertheless, the ASD group had a significantly higher proportion of males and a higher mean FD value than the TD group (both p < 0.05). Furthermore, the ASD group had a significantly higher mean ADOS total score than the TD group (t = 20.385, p < 0.001), which is not surprising.

Table 2.
Comparisons of demographic and clinical characteristics between the ASD and TD groups.
3.3. Group Comparisons on Flexibilities
Compared to the TD group, the ASD patients showed a significantly higher brain network flexibility at the global level (F = 4.280, p = 0.039) (Figure 3A). Furthermore, at the subnetwork level, the ASD patients showed significantly higher flexibilities in the default mode and sensorimotor subnetworks than TD participants (F = 11.404/7.730, FDR-corrected p = 0.006/0.018 for the default mode/sensorimotor subnetworks, respectively) (Figure 3B).
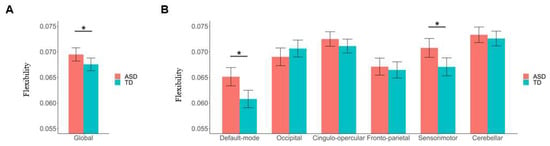
Figure 3.
Results of comparisons of global and subnetwork-level brain network flexibilities between the autism spectrum disorder (ASD) and typical development (TD) groups. (A) Comparison of flexibility at the global level. (B) Comparisons of flexibilities at the subnetwork level. The “*” indicates a significant between-group difference with corrected-p < 0.05. Details of the definition of “flexibility” can be found in Section 2.5.
Compared to the TD group, significantly higher nodal level flexibilities in ASD patients were found in several ROIs belonging to the default mode subnetwork, including the precuneus, angular gyrus, and post-cingulate cortex (all corrected—p < 0.05, as marked in red in Figure 4); meanwhile, significantly lower nodal level flexibilities in ASD patients were found in two ROIs within the occipital subnetwork, which are located in the occipital and post-occipital regions, respectively (both corrected—p < 0.05, as marked in yellow in Figure 4). More details about the coordinates of the nodes showing significant between-group differences as well as the statistical results can be found in Table 3.
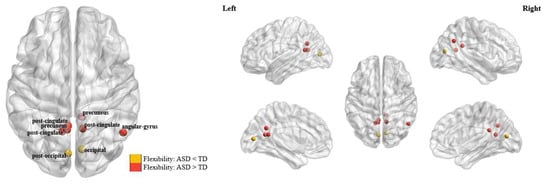
Figure 4.
The brain nodes which showed significant differences in flexibility (corrected—p < 0.05) between the autism spectrum disorder (ASD) and typical development (TD) groups.

Table 3.
Details of coordinates of the nodes showing significant between-group differences as well as the statistical results.
3.4. Correlation Analyses
When testing correlations in the entire sample, the AODS total score was found to be significantly positively associated with flexibilities in the default mode and sensorimotor subnetworks flexibility after adjusting for sex, age, head motion, and site effects (r = 0.109/0.134, FDR-corrected p = 0.024/0.010 for the default mode/sensorimotor subnetworks, respectively) (Figure 5). Moreover, the AODS total score was found to be significantly associated with flexibilities in several nodes belonging to the default mode and occipital subnetworks (Table 4). Nevertheless, no significant results were found when correlations were tested in the ASD group independently (corrected p > 0.05).
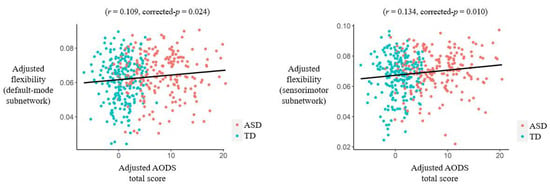
Figure 5.
Results of partial correlations (adjusted for sex, age, head motion, and site effects) between the Autism Diagnostic Observation Schedule (AODS) total score and subnetwork-level flexibilities in the entire sample. Abbreviations: ASD = autism spectrum disorder; TD = typical development.

Table 4.
Results of partial correlations (adjusted for sex, age, head motion, and site effects) between the AODS total score and nodal-level flexibilities in the entire sample.
3.5. Results of Supplementary Analyses
After excluding a number of the TD participants, there were 115 TD participants left in the subset where sex ratio and head motion were well matched between ASD and TD groups (both p > 0.05 for group comparisons; see Table 5). Most of the findings in the current study were still significant in such a drawn subset. Compared to the TD group, the ASD patients still showed significantly higher brain network flexibility at the global level (F = 3.882, p = 0.049), as well as significantly higher flexibilities in the default mode and sensorimotor subnetworks (F = 9.595/5.824, FDR-corrected p = 0.004/0.016 for the default mode/sensorimotor subnetworks, respectively) in the subset. Most of the findings at the nodal level also kept significant in the subset (corrected—p < 0.05 for most nodes, Table 6).

Table 5.
Comparisons on demographic and clinical characteristics between groups in the subset (where sex and head motion were matched between the ASD and TD groups).

Table 6.
Results of post hoc supplementary group comparisons (performed on the subset where sex and head motion were matched between the ASD and TD groups) of flexibilities of the nodes which showed significant between-group differences in the main analyses.
4. Discussion
In the present study, we explored the possible neural mechanisms underlying ASD using a measure of “flexibility” based on the dynamic brain network model, and a relatively large-sample, multi-site dataset. When compared to TD participants, the most significant findings in ASD patients included a higher brain network flexibility at the global level, as well as higher flexibilities within the default mode/sensorimotor areas and lower flexibilities within the occipital areas at the local levels. These findings may strengthen our understanding of the pathobiology of ASD from the perspective of brain network stability.
At the global level, we observed a significantly higher flexibility (switching rate) in the ASD patients than in the TD controls (Figure 3A), which is in line with an earlier study with a relatively small sample size by Harlalka et al. [29]. It was thought that the human brain network needs to be “flexible” (changing the community structures over time) for cognitive and affective processes; however, an observed excessively increased flexibility than normal controls may also be abnormal, indicating a decreased temporal stability of the brain functional organizations [26,31,51,55]. Therefore, our results could further support the opinion by Harlalka et al. that ASD is accompanied by decreased stability in the brain network [29]. Some previous studies have reported both decreased and increased temporal stabilities of functional brain network in ASD patients based on other measures (other than flexibility) under a framework of dynamic brain network. For example, using resting-state fMRI data collected from 62 ASD patients/57 TD participants and the “transient connectivity pattern/state”-based analyses, Mash et al. [57] found an increased variability over time (decreased stability) of brain network in ASD patients, which is in line with the results in the current study. Another study by Xie et al. [58] also reported a significantly higher global mean of “module dynamics” in ASD patients than healthy controls, which indicates a decreased temporal stability of brain network in ASD. Notably, although module dynamics were not compared at the subnetwork level, the above study by Xie et al. [58] reported significant ASD-related increases in module dynamics within several sensorimotor (e.g., prefrontal cortex) and default mode (e.g., posterior cingulate gyrus and angular gyrus) areas at the nodal level, which are partly in line with our findings at the subnetwork level. However, using fMRI data from 24 ASD patients and 26 TD individuals, an opposite conclusion was reported by Watanabe et al. [59] that ASD is associated with “overly stable neural dynamics” (increased stability) in functional brain network. Notably, by analyzing a multi-site dataset, the sample size in the current study is much larger when compared to most of the above published studies, which could lead to more reliable results [32]. Therefore, our results may offer more solid evidence to demonstrate that ASD is associated with a “less stable” functional brain network.
At the local (subnetwork and nodal) levels, it was observed that ASD-related decreases in stabilities of the brain network (increases in brain network flexibilities) were mainly found in the default mode and sensorimotor areas (Figure 3B and Figure 4). The default mode subnetwork in the brain is known to mediate one’s self-referential and internally directed processing [60], and a decreased stability of it has been linked to multiple common mental illnesses such as depression [38,61]. The sensorimotor areas in the brain play important roles in sensorimotor control [62], whose dysfunctions have also been associated with multiple illnesses such as depression and schizophrenia [17,63]. Actually, the key functions of these brain systems are well known to be disrupted in ASD, supported by previous studies. For example, there has been ample evidence that ASD patients are impaired in one of the key functions of the default mode subnetwork: self-referential cognition, which is the ability to process social information relative to oneself, and such impairments may be closely related to the social cognitive dysfunction in ASD [64,65,66,67]. On the other hand, it has been widely reported that sensorimotor skills are atypical in individuals with ASD, and such deficits are associated with severity of ASD symptoms [68,69,70]. Therefore, the results of the current study may point to ASD-related dysfunctions in the default mode and sensorimotor systems from a perspective of brain functional dynamics, which may underlie the clinical features of ASD such as impaired self-referential cognition and sensorimotor difficulties.
At the nodal level, significant decreases in flexibilities (increased stability) in ASD patients were additionally observed in several occipital regions (Figure 4). Such alterations, interestingly, are opposite to the observed ASD-related changes at the global level, as well as the changes in other subnetworks. While a decreased stability may suggest abnormal changes in the brain network as mentioned earlier, an excessively “decreased variability” (increased stability) may be also indicative of brain dysfunctions and a decreased ability to adapt to changing environmental demands [31,51]. The occipital cortex is known to be involved in the visual processing of the brain [51,71]; previous research has reported significant ASD-related alterations in both structures and functions in the occipital cortex, which were associated with visual processing and social communication deficits in ASD patients [72,73]. Our results thus again highlighted the critical importance of focusing on the occipital areas in research on ASD. Furthermore, combining such results with findings in the default mode/sensorimotor areas, it might be concluded that both excessively increased and decreased functional stabilities (in different brain systems) are involved in the pathobiology of ASD.
Our study has some limitations. First, although positive correlations were shown between the brain network flexibilities and ASD symptom severity in the entire sample (Figure 5), no significant results were obtained when testing the correlations in the ASD group independently to support our clinical symptoms-related hypotheses. Second, the current study used cross-sectional data and thus we were unable to ascertain the participants’ developmental trajectories; future studies will benefit from longitudinal designs. Third, sex and head motion were not completely matched between the ASD and TD groups in the main analyses. Nevertheless, we have controlled possible sex, age, head motion, and site effects in all analyses; moreover, we have repeated the analyses in a subset where sex and head motion were matched between groups, and most of the findings were still significant in such a subset. Therefore, it is unlikely that our findings were largely driven by these factors. Fourth, most of the participants included in the current study are adolescents or young adults (with an average age = 16.35 years for ASD patients). Future studies including participants with a relatively wider range of age (e.g., including more elderly patients) may be meaningful for further investigating the possible age-related heterogeneity in brain dysfunctions in ASD patients.
In conclusion, this study investigated the possible relationships between ASD and changes in functional brain network dynamics using a multi-site dataset. The results suggested significantly increased brain network flexibilities (indicating a decreased stability) at the global level, as well as at the local level within the default mode and sensorimotor areas in ASD patients than TD participants. Additionally, significant ASD-related decreases in flexibilities (indicating excessively increased stability) were also observed in several occipital regions at the nodal level. These results also pointed to ASD-related dysfunctions in the default mode, sensorimotor, and occipital systems from a perspective of brain network stability, which can further strengthen our understanding of the pathobiology of ASD.
Author Contributions
H.Z., S.T. and Y.L. conceived the idea and structured the manuscript. Y.L. performed the data analysis. H.Z. and Y.L. drafted the manuscript. D.P., S.T. and A.B. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hunan Province, China (2023JJ60438 to Hui Zhang), the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University (HQ20230261 to Bian Yao and to Yicheng Long), the Health Research Project of Hunan Provincial Health Commission (W20243225 to Yicheng Long), and the National Natural Science Foundation of China (82201692 to Yicheng Long).
Institutional Review Board Statement
The Autism Brain Imaging Data Exchange (ABIDE)-Preprocessed data is open shared, and Institutional Review Board (IRB) approval was provided by each site (data contributor) in the ABIDE database.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The Autism Brain Imaging Data Exchange (ABIDE)-Preproceesed database is available at: http://preprocessed-connectomes-project.org/abide/ (accessed on 1 April 2024). The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hirota, T.; King, B.H. Autism Spectrum Disorder. JAMA 2023, 329, 157. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef] [PubMed]
- Talantseva, O.I.; Romanova, R.S.; Shurdova, E.M.; Dolgorukova, T.A.; Sologub, P.S.; Titova, O.S.; Kleeva, D.F.; Grigorenko, E.L. The Global Prevalence of Autism Spectrum Disorder: A Three-Level Meta-Analysis. Front. Psychiatry 2023, 14, 1071181. [Google Scholar] [CrossRef] [PubMed]
- Willsey, H.R.; Willsey, A.J.; Wang, B.; State, M.W. Genomics, Convergent Neuroscience and Progress in Understanding Autism Spectrum Disorder. Nat. Rev. Neurosci. 2022, 23, 323–341. [Google Scholar] [CrossRef]
- Long, Y.; Li, X.; Cao, H.; Zhang, M.; Lu, B.; Huang, Y.; Liu, M.; Xu, M.; Liu, Z.; Yan, C.; et al. Common and Distinct Functional Brain Network Abnormalities in Adolescent, Early-Middle Adult, and Late Adult Major Depressive Disorders. Psychol. Med. 2024, 54, 582–591. [Google Scholar] [CrossRef]
- Lin, Z.; Long, Y.; Wu, Z.; Xiang, Z.; Ju, Y.; Liu, Z. Associations between Brain Abnormalities and Common Genetic Variants for Schizophrenia: A Narrative Review of Structural and Functional Neuroimaging Findings. Ann. Palliat. Med. 2021, 10, 10031–10052. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Liu, M.; Zhang, M.; Liu, Y.; Teng, T.; Liu, X.; Yu, Y.; Jiang, Y.; Ouyang, X.; et al. Childhood Trauma Is Linked to Abnormal Static-Dynamic Brain Topology in Adolescents with Major Depressive Disorder. Int. J. Clin. Health Psychol. 2023, 23, 100401. [Google Scholar] [CrossRef]
- Hua, Z.; Hu, J.; Zeng, H.; Li, J.; Cao, Y.; Gan, Y. Auditory Language Comprehension among Children and Adolescents with Autism Spectrum Disorder: An ALE Meta-analysis of FMRI Studies. Autism Res. 2024, 17, 482–496. [Google Scholar] [CrossRef]
- Larson, C.; Thomas, H.R.; Crutcher, J.; Stevens, M.C.; Eigsti, I.-M. Language Networks in Autism Spectrum Disorder: A Systematic Review of Connectivity-Based FMRI Studies. Rev. J. Autism Dev. Disord. 2023, 1–28. [Google Scholar] [CrossRef]
- Lee, Y.; Park, B.; James, O.; Kim, S.-G.; Park, H. Autism Spectrum Disorder Related Functional Connectivity Changes in the Language Network in Children, Adolescents and Adults. Front. Hum. Neurosci. 2017, 11, 418. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Yang, C.; Fang, X.; Ye, M.; Wei, L.; Liu, J.; Li, B.; Gan, Y.; Yang, B.; et al. Altered Functional Connectivity in Children With Low-Function Autism Spectrum Disorders. Front. Neurosci. 2019, 13, 806. [Google Scholar] [CrossRef] [PubMed]
- Sizemore, A.E.; Bassett, D.S. Dynamic Graph Metrics: Tutorial, Toolbox, and Tale. Neuroimage 2018, 180, 417–427. [Google Scholar] [CrossRef]
- Long, Y.; Ouyang, X.; Yan, C.; Wu, Z.; Huang, X.; Pu, W.; Cao, H.; Liu, Z.; Palaniyappan, L. Evaluating Test–Retest Reliability and Sex-/Age-Related Effects on Temporal Clustering Coefficient of Dynamic Functional Brain Networks. Hum. Brain Mapp. 2023, 44, 2191–2208. [Google Scholar] [CrossRef]
- Rossi, A.; Deslauriers-Gauthier, S.; Natale, E. On Null Models for Temporal Small-Worldness in Brain Dynamics. Netw. Neurosci. 2024, 8, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, X.; Long, Y.; Xiang, Z.; Wu, Z.; Liu, Z.; Bian, D.; Tang, S. Problematic Smartphone Use Is Associated with Differences in Static and Dynamic Brain Functional Connectivity in Young Adults. Front. Neurosci. 2022, 16, 1010488. [Google Scholar] [CrossRef]
- Ouyang, X.; Long, Y.; Wu, Z.; Liu, D.; Liu, Z.; Huang, X. Temporal Stability of Dynamic Default Mode Network Connectivity Negatively Correlates with Suicidality in Major Depressive Disorder. Brain Sci. 2022, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Liu, Z.; Chan, C.K.Y.; Wu, G.; Xue, Z.; Pan, Y.; Chen, X.; Huang, X.; Li, D.; Pu, W. Altered Temporal Variability of Local and Large-Scale Resting-State Brain Functional Connectivity Patterns in Schizophrenia and Bipolar Disorder. Front. Psychiatry 2020, 11, 422. [Google Scholar] [CrossRef]
- Han, S.; Cui, Q.; Wang, X.; Li, L.; Li, D.; He, Z.; Guo, X.; Fan, Y.S.; Guo, J.; Sheng, W.; et al. Resting State Functional Network Switching Rate Is Differently Altered in Bipolar Disorder and Major Depressive Disorder. Hum. Brain Mapp. 2020, 41, 3295–3304. [Google Scholar] [CrossRef]
- Yang, Z.; Telesford, Q.K.; Franco, A.R.; Lim, R.; Gu, S.; Xu, T.; Ai, L.; Castellanos, F.X.; Yan, C.G.; Colcombe, S.; et al. Measurement Reliability for Individual Differences in Multilayer Network Dynamics: Cautions and Considerations. Neuroimage 2021, 225, 117489. [Google Scholar] [CrossRef]
- Pedersen, M.; Zalesky, A.; Omidvarnia, A.; Jackson, G.D. Multilayer Network Switching Rate Predicts Brain Performance. Proc. Natl. Acad. Sci. USA 2018, 115, 13376–13381. [Google Scholar] [CrossRef]
- He, L.; Zhuang, K.; Li, Y.; Sun, J.; Meng, J.; Zhu, W.; Mao, Y.; Chen, Q.; Chen, X.; Qiu, J. Brain Flexibility Associated with Need for Cognition Contributes to Creative Achievement. Psychophysiology 2019, 56, e13464. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schäfer, A.; Bassett, D.S.; Rausch, F.; Schweiger, J.I.; Bilek, E.; Erk, S.; Romanczuk-Seiferth, N.; Grimm, O.; Geiger, L.S.; et al. Dynamic Brain Network Reconfiguration as a Potential Schizophrenia Genetic Risk Mechanism Modulated by NMDA Receptor Function. Proc. Natl. Acad. Sci. USA 2016, 113, 12568–12573. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, S.; Mo, Z.; Chattun, M.R.; Wang, Q.; Wang, L.; Zhu, R.; Shao, J.; Wang, X.; Yao, Z.; et al. Antidepressants Normalize Brain Flexibility Associated with Multi-Dimensional Symptoms in Major Depressive Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109866. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Liao, X.; Chen, X.; Zhao, T.; Xu, Y.; Xia, M.; Zhang, J.; Xia, Y.; Sun, X.; Wei, Y.; et al. Progressive Stabilization of Brain Network Dynamics during Childhood and Adolescence. Cereb. Cortex 2022, 32, 1024–1039. [Google Scholar] [CrossRef]
- Yin, W.; Li, T.; Mucha, P.J.; Cohen, J.R.; Zhu, H.; Zhu, Z.; Lin, W. Altered Neural Flexibility in Children with Attention-Deficit/Hyperactivity Disorder. Mol. Psychiatry 2022, 27, 4673–4679. [Google Scholar] [CrossRef]
- Betzel, R.F.; Satterthwaite, T.D.; Gold, J.I.; Bassett, D.S. Positive Affect, Surprise, and Fatigue Are Correlates of Network Flexibility. Sci. Rep. 2017, 7, 520. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Shu, Z.; Han, J.; Yu, N. A Dynamic Brain Network Decomposition Method Discovers Effective Brain Hemodynamic Sub-Networks for Parkinson’s Disease. J. Neural Eng. 2024, 21, 026047. [Google Scholar] [CrossRef]
- Broeders, T.A.A.; Linsen, F.; Louter, T.S.; Nawijn, L.; Penninx, B.W.J.H.; van Tol, M.J.; van der Wee, N.J.A.; Veltman, D.J.; van der Werf, Y.D.; Schoonheim, M.M.; et al. Dynamic Reconfigurations of Brain Networks in Depressive and Anxiety Disorders: The Influence of Antidepressants. Psychiatry Res. 2024, 334, 115774. [Google Scholar] [CrossRef]
- Harlalka, V.; Bapi, R.S.; Vinod, P.K.; Roy, D. Atypical Flexibility in Dynamic Functional Connectivity Quantifies the Severity in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019, 13, 6. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Z.; Cao, H.; Yang, J.; Wu, Z.; Long, Y. Childhood Trauma Is Linked to Decreased Temporal Stability of Functional Brain Networks in Young Adults. J. Affect. Disord. 2021, 290, 23–30. [Google Scholar] [CrossRef]
- Long, Y.; Liu, X.; Liu, Z. Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives. Brain Sci. 2023, 13, 429. [Google Scholar] [CrossRef]
- Cao, H.; McEwen, S.C.; Forsyth, J.K.; Gee, D.G.; Bearden, C.E.; Addington, J.; Goodyear, B.; Cadenhead, K.S.; Mirzakhanian, H.; Cornblatt, B.A.; et al. Toward Leveraging Human Connectomic Data in Large Consortia: Generalizability of Fmri-Based Brain Graphs across Sites, Sessions, and Paradigms. Cereb. Cortex 2019, 29, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Sao, A.K.; Minhas, A.S. Analyzing the Effect of Resolution of Network Nodes on the Resting State Functional Connectivity Maps of Schizophrenic Human Brains. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 6695–6698. [Google Scholar]
- Di Martino, A.; Yan, C.-G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M.; et al. The Autism Brain Imaging Data Exchange: Towards a Large-Scale Evaluation of the Intrinsic Brain Architecture in Autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar] [CrossRef]
- Cameron, C.; Yassine, B.; Carlton, C.; Francois, C.; Alan, E.; András, J.; Budhachandra, K.; John, L.; Qingyang, L.; Michael, M.; et al. The Neuro Bureau Preprocessing Initiative: Open Sharing of Preprocessed Neuroimaging Data and Derivatives. Front. Neuroinform 2013, 7, 5. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; Dilavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule-Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Tang, S.; Wu, Z.; Cao, H.; Chen, X.; Wu, G.; Tan, W.; Liu, D.; Yang, J.; Long, Y.; Liu, Z. Age-Related Decrease in Default-Mode Network Functional Connectivity Is Accelerated in Patients With Major Depressive Disorder. Front. Aging Neurosci. 2022, 13, 809853. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, H.; Yan, C.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.; Bo, Q.; Chen, G.; Chen, N.; et al. Altered Resting-State Dynamic Functional Brain Networks in Major Depressive Disorder: Findings from the REST-Meta-MDD Consortium. Neuroimage Clin. 2020, 26, 102163. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.J.; Bo, Q.J.; Cao, J.; Chen, G.M.; Chen, N.X.; Chen, W.; et al. Reduced Default Mode Network Functional Connectivity in Patients with Recurrent Major Depressive Disorder. Proc. Natl. Acad. Sci. USA 2019, 116, 9078–9083. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, X.; Chen, Z.B.; Li, L.; Li, X.Y.; Castellanos, F.X.; Bai, T.J.; Bo, Q.J.; Cao, J.; Chang, Z.K.; et al. Disrupted Intrinsic Functional Brain Topology in Patients with Major Depressive Disorder. Mol. Psychiatry 2021, 26, 7363–7371. [Google Scholar] [CrossRef]
- Aquino, K.M.; Fulcher, B.D.; Parkes, L.; Sabaroedin, K.; Fornito, A. Identifying and Removing Widespread Signal Deflections from FMRI Data: Rethinking the Global Signal Regression Problem. Neuroimage 2020, 212, 116614. [Google Scholar] [CrossRef]
- Wanger, T.J.; Janes, A.C.; Frederick, B.B. Spatial Variation of Changes in Test–Retest Reliability of Functional Connectivity after Global Signal Regression: The Effect of Considering Hemodynamic Delay. Hum. Brain Mapp. 2023, 44, 668–678. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Nardos, B.; Cohen, A.L.; Fair, D.A.; Power, J.D.; Church, J.A.; Nelson, S.M.; Wig, G.S.; Vogel, A.C.; Lessov-Schlaggar, C.N.; et al. Prediction of Individual Brain Maturity Using FMRI. Science 2010, 329, 1358–1361. [Google Scholar] [CrossRef]
- Tan, W.; Ouyang, X.; Huang, D.; Wu, Z.; Liu, Z.; He, Z.; Long, Y. Disrupted Intrinsic Functional Brain Network in Patients with Late-Life Depression: Evidence from a Multi-Site Dataset. J. Affect. Disord. 2023, 323, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Z.; Liu, Z.; Liu, D.; Huang, D.; Long, Y. Acute Effect of Betel Quid Chewing on Brain Network Dynamics: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2021, 12, 701420. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Wan, D.; Zhao, R.; Canario, E.; Meng, C.; Biswal, B.B. The Complexity of Spontaneous Brain Activity Changes in Schizophrenia, Bipolar Disorder, and ADHD Was Examined Using Different Variations of Entropy. Hum. Brain Mapp. 2023, 44, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, Z.; Wang, J.; Wang, L.; Shen, X.; Bai, L.; Li, Z.; Dong, M.; Liu, C.; Yi, G.; et al. Evolution of Brain Network Dynamics in Early Parkinson’s Disease with Mild Cognitive Impairment. Cogn. Neurodyn 2023, 17, 681–694. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Ye, H.; Jiang, W.; Zhang, Y.; Kong, Y.; Yuan, Y. Abnormal Changes of Dynamic Topological Characteristics in Patients with Major Depressive Disorder. J. Affect. Disord. 2024, 345, 349–357. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, C.; He, L. Abnormal Dynamic Functional Network Connectivity in Patients with Early-Onset Bipolar Disorder. Front. Psychiatry 2023, 14, 1169488. [Google Scholar] [CrossRef]
- Sun, Y.; Collinson, S.L.; Suckling, J.; Sim, K. Dynamic Reorganization of Functional Connectivity Reveals Abnormal Temporal Efficiency in Schizophrenia. Schizophr. Bull. 2019, 45, 659–669. [Google Scholar] [CrossRef]
- Long, Y.; Chen, C.; Deng, M.; Huang, X.; Tan, W.; Zhang, L.; Fan, Z.; Liu, Z. Psychological Resilience Negatively Correlates with Resting-State Brain Network Flexibility in Young Healthy Adults: A Dynamic Functional Magnetic Resonance Imaging Study. Ann. Transl. Med. 2019, 7, 809. [Google Scholar] [CrossRef]
- Mucha, P.J.; Richardson, T.; Macon, K.; Porter, M.A.; Onnela, J.P. Community Structure in Time-Dependent, Multiscale, and Multiplex Networks. Science 2010, 328, 876–878. [Google Scholar] [CrossRef]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic Reconfiguration of Frontal Brain Networks during Executive Cognition in Humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef] [PubMed]
- Jeub, L.G.S.; Bazzi, M.; Jutla, I.S.; Mucha, P.J. A Generalized Louvain Method for Community Detection Implemented in MATLAB. Available online: https://github.com/GenLouvain/GenLouvain (accessed on 1 April 2024).
- Bassett, D.S.; Wymbs, N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic Reconfiguration of Human Brain Networks during Learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef]
- Paban, V.; Modolo, J.; Mheich, A.; Hassan, M. Psychological Resilience Correlates with EEG Source-Space Brain Network Flexibility. Netw. Neurosci. 2019, 3, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Mash, L.E.; Linke, A.C.; Olson, L.A.; Fishman, I.; Liu, T.T.; Müller, R. Transient States of Network Connectivity Are Atypical in Autism: A Dynamic Functional Connectivity Study. Hum. Brain Mapp. 2019, 40, 2377–2389. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Z.; Xia, M.; Liu, J.; Shou, X.; Cui, Z.; Liao, X.; He, Y. Alterations in Connectome Dynamics in Autism Spectrum Disorder: A Harmonized Mega- and Meta-Analysis Study Using the Autism Brain Imaging Data Exchange Dataset. Biol. Psychiatry 2022, 91, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Rees, G. Brain Network Dynamics in High-Functioning Individuals with Autism. Nat. Commun. 2017, 8, 16048. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Wise, T.; Marwood, L.; Perkins, A.M.; Herane-Vives, A.; Joules, R.; Lythgoe, D.J.; Luh, W.-M.; Williams, S.C.R.; Young, A.H.; Cleare, A.J.; et al. Instability of Default Mode Network Connectivity in Major Depression: A Two-Sample Confirmation Study. Transl. Psychiatry 2017, 7, e1105. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, S.; Lu, B.; Liao, J.; Yang, Z.; Li, H.; Pei, H.; Li, J.; Iturria-Medina, Y.; Yao, D.; et al. The Role of the Primary Sensorimotor System in Generalized Epilepsy: Evidence from the Cerebello–Cerebral Functional Integration. Hum. Brain Mapp. 2024, 45, e26551. [Google Scholar] [CrossRef]
- Javaheripour, N.; Li, M.; Chand, T.; Krug, A.; Kircher, T.; Dannlowski, U.; Nenadić, I.; Hamilton, J.P.; Sacchet, M.D.; Gotlib, I.H.; et al. Altered Resting-State Functional Connectome in Major Depressive Disorder: A Mega-Analysis from the PsyMRI Consortium. Transl. Psychiatry 2021, 11, 511. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Barnes, J.L.; Wheelwright, S.J.; Baron-Cohen, S. Self-Referential Cognition and Empathy in Autism. PLoS ONE 2007, 2, e883. [Google Scholar] [CrossRef]
- Burrows, C.A.; Usher, L.V.; Mundy, P.C.; Henderson, H.A. The Salience of the Self: Self-referential Processing and Internalizing Problems in Children and Adolescents with Autism Spectrum Disorder. Autism Res. 2017, 10, 949–960. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Lynch, C.J.; Schaer, M.; Menon, V. The Default Mode Network in Autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.L.H.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263. [Google Scholar] [CrossRef]
- Mosconi, M.W.; Sweeney, J.A. Sensorimotor Dysfunctions as Primary Features of Autism Spectrum Disorders. Sci. China Life Sci. 2015, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hannant, P.; Cassidy, S.; Tavassoli, T.; Mann, F. Sensorimotor Difficulties Are Associated with the Severity of Autism Spectrum Conditions. Front. Integr. Neurosci. 2016, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Coll, S.-M.; Foster, N.E.V.; Meilleur, A.; Brambati, S.M.; Hyde, K.L. Sensorimotor Skills in Autism Spectrum Disorder: A Meta-Analysis. Res. Autism Spectr. Disord. 2020, 76, 101570. [Google Scholar] [CrossRef]
- Beffara, B.; Hadj-Bouziane, F.; Hamed, S.B.; Boehler, C.N.; Chelazzi, L.; Santandrea, E.; Macaluso, E. Separate and Overlapping Mechanisms of Statistical Regularities and Salience Processing in the Occipital Cortex and Dorsal Attention Network. Hum. Brain Mapp. 2023, 44, 6439–6458. [Google Scholar] [CrossRef]
- Jung, M.; Tu, Y.; Lang, C.A.; Ortiz, A.; Park, J.; Jorgenson, K.; Kong, X.-J.; Kong, J. Decreased Structural Connectivity and Resting-State Brain Activity in the Lateral Occipital Cortex Is Associated with Social Communication Deficits in Boys with Autism Spectrum Disorder. Neuroimage 2019, 190, 205–212. [Google Scholar] [CrossRef]
- Matsuoka, K.; Makinodan, M.; Kitamura, S.; Takahashi, M.; Yoshikawa, H.; Yasuno, F.; Ishida, R.; Kishimoto, N.; Yasuda, Y.; Hashimoto, R.; et al. Increased Dendritic Orientation Dispersion in the Left Occipital Gyrus Is Associated with Atypical Visual Processing in Adults with Autism Spectrum Disorder. Cereb. Cortex 2020, 30, 5617–5625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).