Leverage Effective Deep Learning Searching Method for Forensic Age Estimation
Abstract
1. Introduction
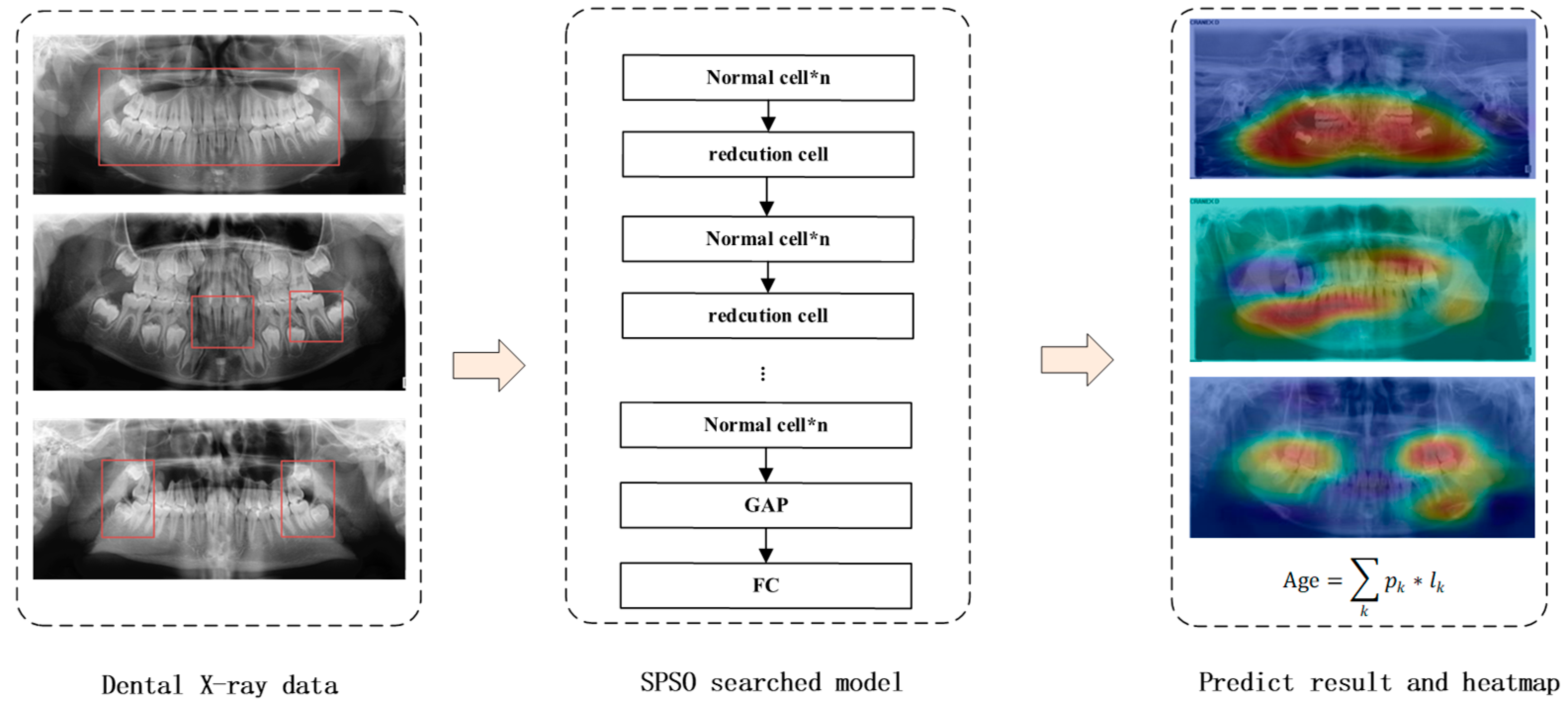
2. Methods
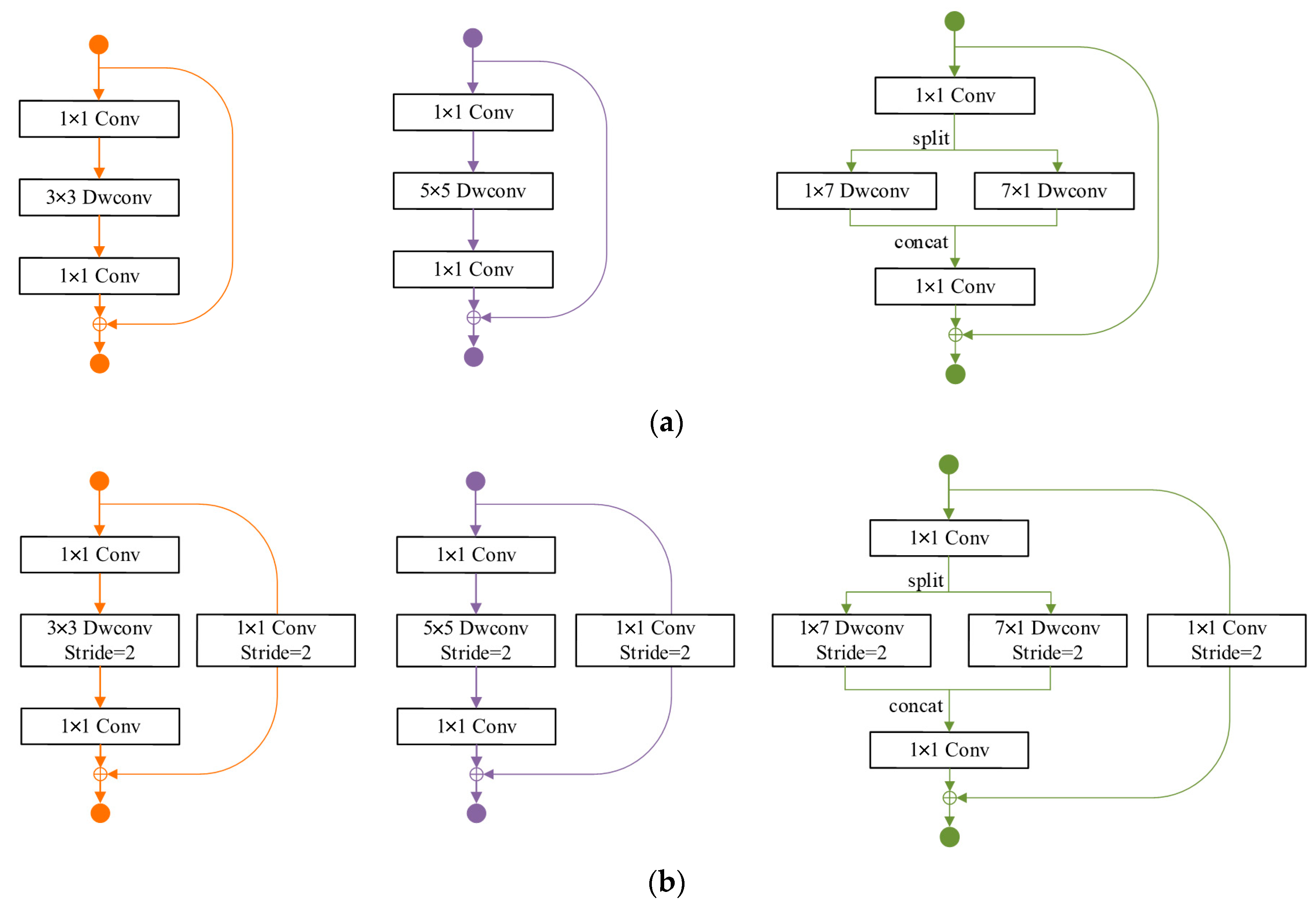
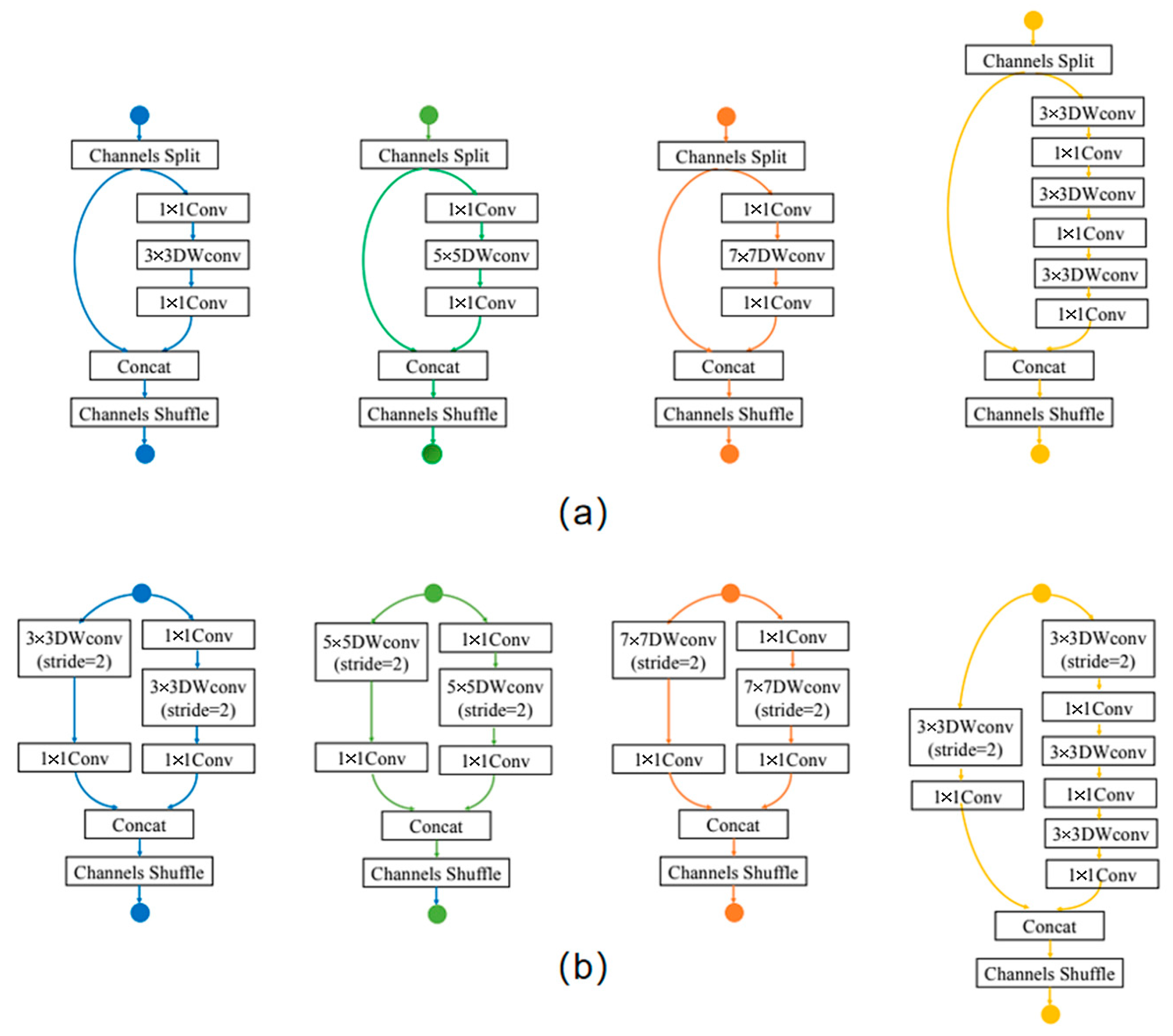
2.1. Exploration of Neural Network Elements for OPG
2.2. Model Search Based on Proposed Rules for OPGs
- (1)
- The depth of the parallel asymmetric convolution block is equivalent to that of the original MobileNetV2 block, ensuring seamless adherence to Rule 3.
- (2)
- Let and denote the number of input channels and output channels, respectively, in a MobileNetV2 block. Here, represents the expand ratios and represents the kernel size. The resulting output feature map has dimensions (for convenience; we assume that the number of output channels of all layers in the map are h × w, which does not affect our analysis and conclusion);then, the FLOPs of the block is:
3. Experiments
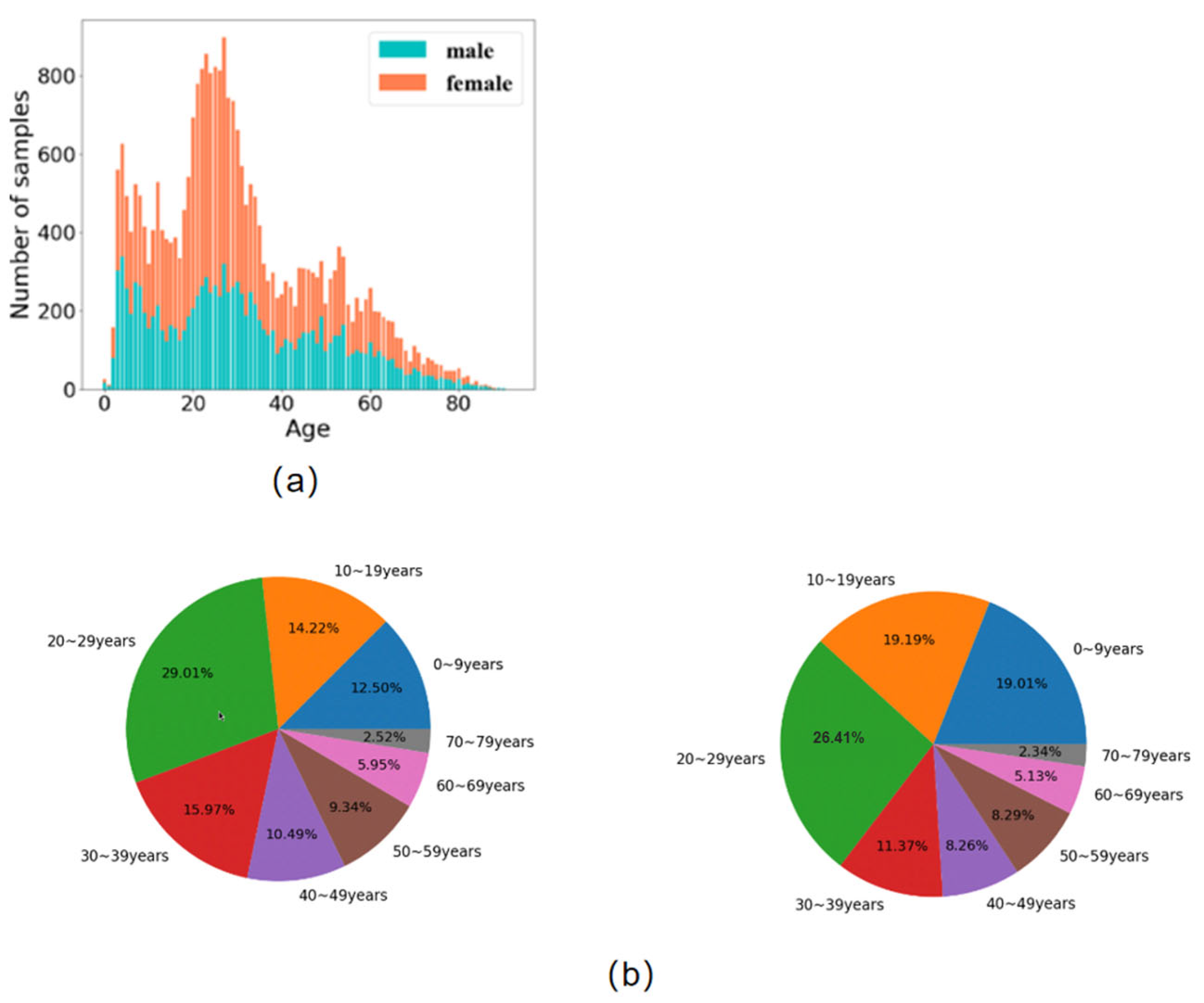
3.1. Dataset of X-ray
3.2. Experimental Settings
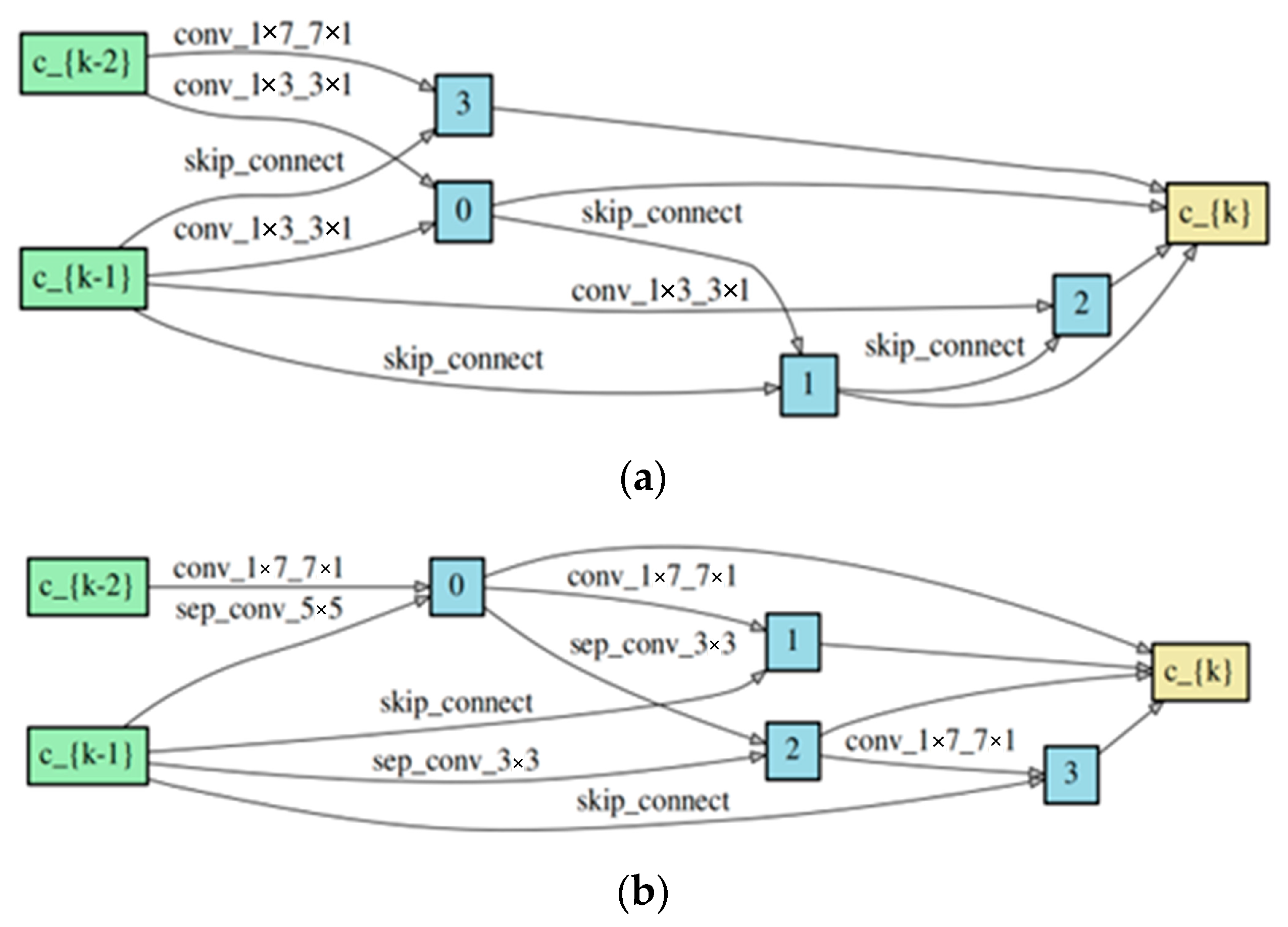
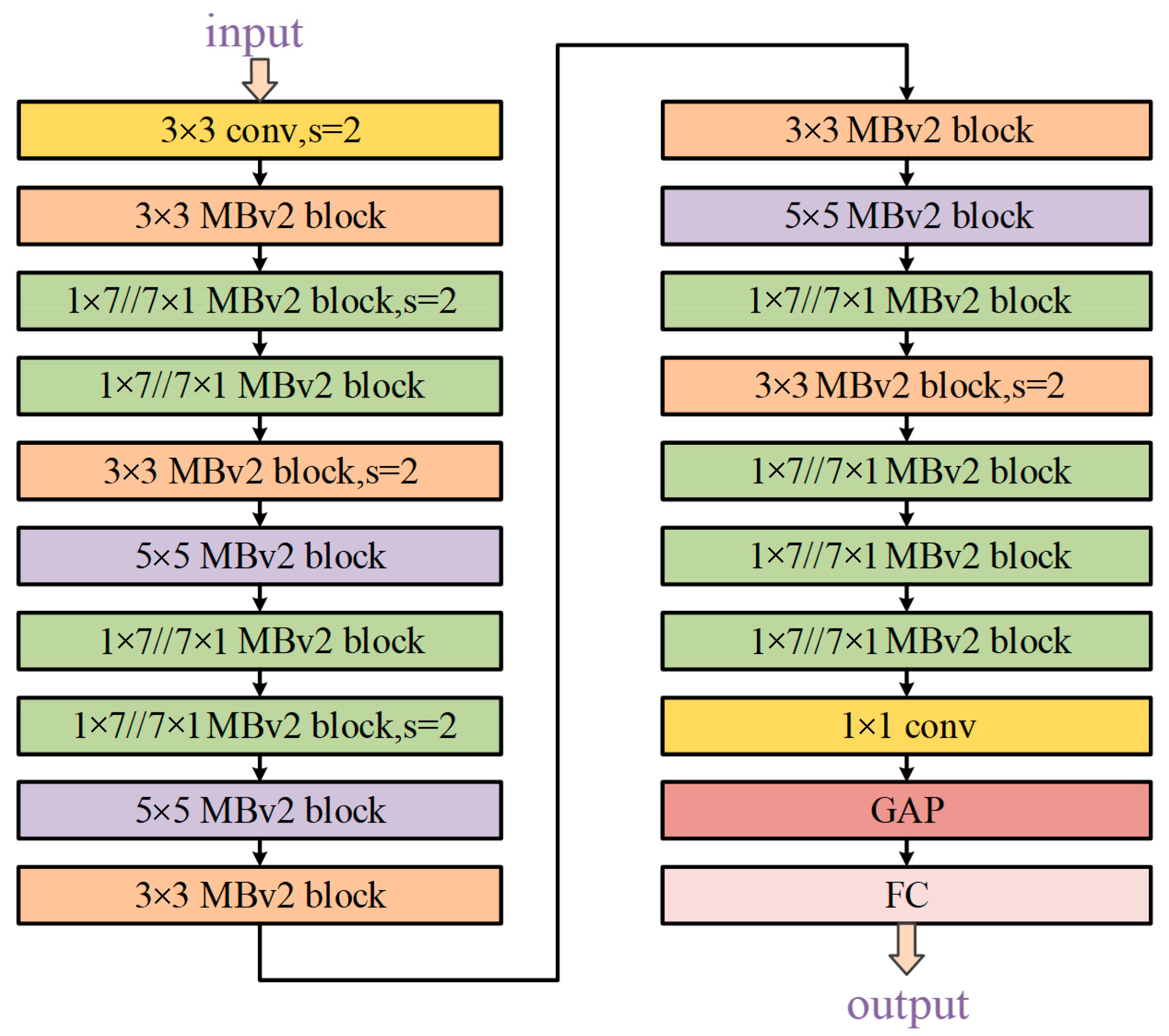
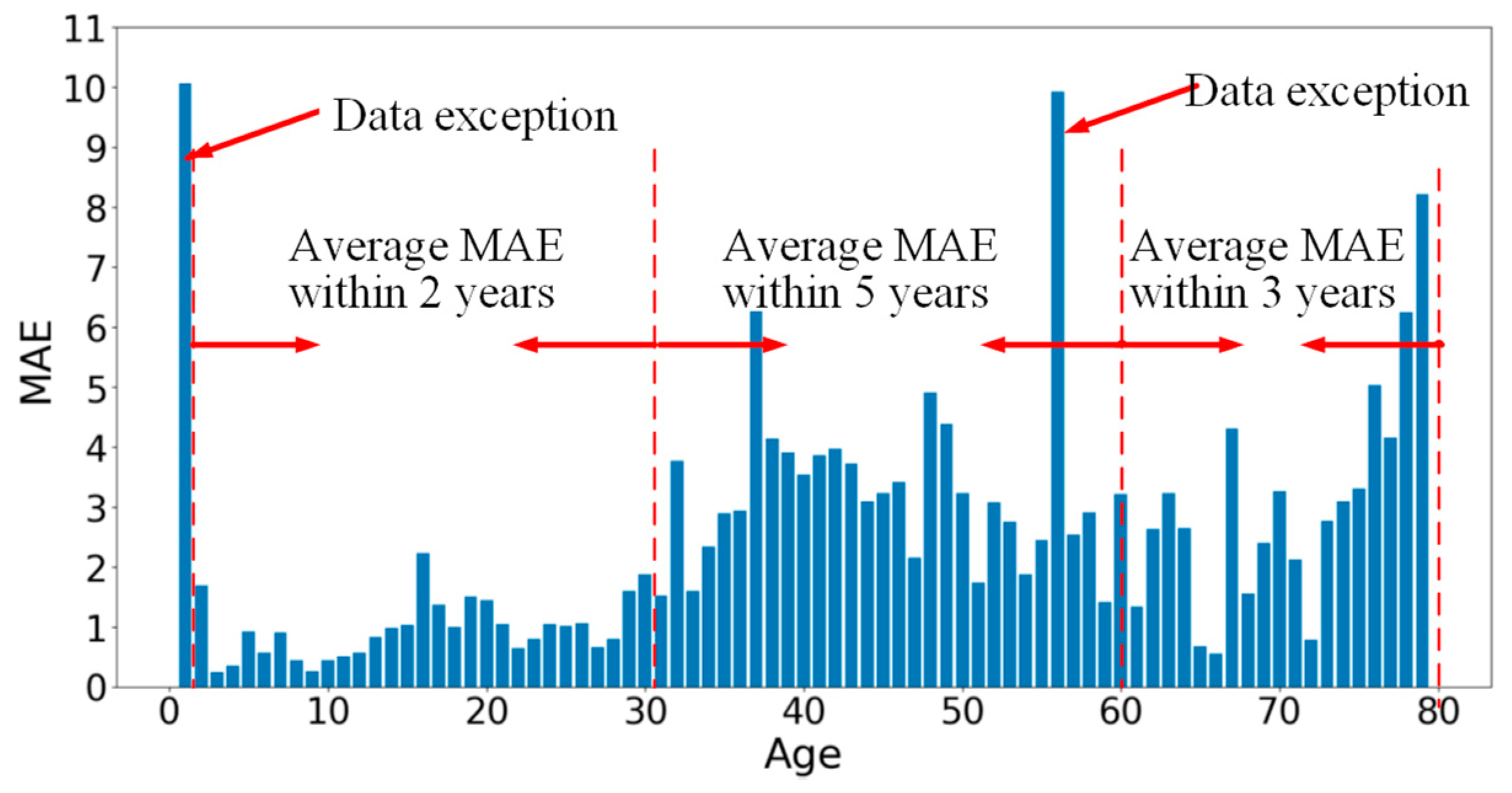
3.3. Experimental Results for DNN Architecture Searching
3.4. Analysis for Our Rules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinchliffe, J. Forensic Odontology, Part 2. Major Disasters. Br. Dent. J. 2011, 210, 269–274. [Google Scholar] [CrossRef]
- Schuller-Götzburg, P.; Suchanek, J. Forensic Odontologists Successfully Identify Tsunami Victims in Phuket, Thailand. Forensic Sci. Int. 2007, 171, 204–207. [Google Scholar] [CrossRef]
- Wedel, V.; Found, G.; Nusse, G. A 37-Year-Old Cold Case Identification Using Novel and Collaborative Methods. J. Forensic Identif. 2015, 65, 733–749. [Google Scholar]
- Sweet, D. Sweet Forensic Dental Identification. Forensic Sci. Int. 2010, 201, 3–4. [Google Scholar] [CrossRef]
- Saxena, S.; Sharma, P.; Gupta, N. Experimental Studies of Forensic Odontology to Aid in the Identification Process. J. Forensic Dent. Sci. 2010, 2, 69–76. [Google Scholar] [CrossRef]
- Krishan, K.; Kanchan, T.; Garg, A.K. Dental Evidence in Forensic Identification—An Overview, Methodology and Present Status. Open Dent. J. 2015, 9, 250–256. [Google Scholar] [CrossRef]
- Gonsior, M.; Ramsthaler, F.; Gehl, A.; Verhoff, M.A. Morphology as a Cause for Different Classification of the Ossification Stage of the Medial Clavicular Epiphysis by Ultrasound, Computed Tomography, and Macroscopy. Int. J. Legal. Med. 2013, 127, 1013–1021. [Google Scholar] [CrossRef]
- Savall, F.; Hérin, F.; Peyron, P.A.; Rougé, D.; Baccino, E.; Saint-Martin, P.; Telmon, N. Age Estimation at Death Using Pubic Bone Analysis of a Virtual Reference Sample. Int. J. Legal. Med. 2018, 132, 609–615. [Google Scholar] [CrossRef]
- Dedouit, F.; Auriol, J.; Rousseau, H.; Rougé, D.; Crubézy, E.; Telmon, N. Age Assessment by Magnetic Resonance Imaging of the Knee: A Preliminary Study. Forensic Sci. Int. 2012, 217, 232.e1–232.e7. [Google Scholar] [CrossRef]
- Vieth, V.; Schulz, R.; Heindel, W.; Pfeiffer, H.; Buerke, B.; Schmeling, A.; Ottow, C. Forensic Age Assessment by 3.0T MRI of the Knee: Proposal of a New MRI Classification of Ossification Stages. Eur. Radiol. 2018, 28, 3255–3262. [Google Scholar] [CrossRef]
- Hackman, L.; Davies, C.M.; Black, S. Age Estimation Using Foot Radiographs from a Modern Scottish Population. J. Forensic Sci. 2013, 58 (Suppl. 1), S146–S150. [Google Scholar] [CrossRef]
- Whitaker, J.M.; Rousseau, L.; Williams, T.; Rowan, R.A.; Hartwig, W.C. Scoring System for Estimating Age in the Foot Skeleton. Am. J. Phys. Anthropol. 2002, 118, 385–392. [Google Scholar] [CrossRef]
- Cameriere, R.; De Luca, S.; Biagi, R.; Cingolani, M.; Farronato, G.; Ferrante, L. Accuracy of Three Age Estimation Methods in Children by Measurements of Developing Teeth and Carpals and Epiphyses of the Ulna and Radius. J. Forensic Sci. 2012, 57, 1263–1270. [Google Scholar] [CrossRef]
- Murray, R.O. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method). Proc. R Soc. Med. 1976, 69, 542. [Google Scholar]
- Bayer, L.M. Radiographic atlas of skeletal development of the hand and wrist. Calif. Med. 1959, 91, 53. [Google Scholar]
- Manigandan, T.; Sumathy, C.; Elumalai, M.; Sathasivasubramanian, S.; Kannan, A. Forensic Radiology in Dentistry. J. Pharm. Bioallied Sci. 2015, 7, S260–S264. [Google Scholar] [CrossRef]
- Mincer, H.H.; Harris, E.F.; Berryman, H.E. The A.B.F.O. Study of Third Molar Development and Its Use as an Estimator of Chronological Age. J. Forensic Sci. 1993, 38, 379–390. [Google Scholar] [CrossRef]
- Moorrees, C.F.; Fanning, E.A.; Hunt, E.E., Jr. Age variation of formation stages for ten permanent teeth. J. Dent. Res. 1963, 42, 1490–1502. [Google Scholar] [CrossRef]
- Manjrekar, S.; Deshpande, S.; Katge, F.; Jain, R.; Ghorpade, T. Age Estimation in Children by the Measurement of Open Apices in Teeth: A Study in the Western Indian Population. Int. J. Dent. 2022, 2022, 9513501. [Google Scholar] [CrossRef]
- Mohamed, E.G.; Redondo, R.P.D.; Koura, A.; EL-Mofty, M.S.; Kayed, M. Dental Age Estimation Using Deep Learning: A Comparative Survey. Computation 2023, 11, 18. [Google Scholar] [CrossRef]
- Büyükçakır, B.; Bertels, J.; Claes, P.; Vandermeulen, D.; de Tobel, J.; Thevissen, P.W. OPG-Based Dental Age Estimation Using a Data-Technical Exploration of Deep Learning Techniques. J. Forensic Sci. 2024, 69, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning. arXiv 2016, arXiv:1602.07261. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.-C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar]
- Ma, N.; Zhang, X.; Zheng, H.-T.; Sun, J. ShuffleNet V2: Practical Guidelines for Efficient CNN Architecture Design. In Computer Vision—ECCV 2018; Ferrari, V., Hebert, M., Sminchisescu, C., Weiss, Y., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2018; Volume 11218, pp. 122–138. ISBN 978-3-030-01263-2. [Google Scholar]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. arXiv 2020, arXiv:1905.11946. [Google Scholar]
- Xie, S.; Girshick, R.; Dollár, P.; Tu, Z.; He, K. Aggregated Residual Transformations for Deep Neural Networks. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 5987–5995. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely Connected Convolutional Networks. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 2261–2269. [Google Scholar]
- Geng, X.; Zhou, Z.-H.; Zhang, Y.; Li, G.; Dai, H. Learning from Facial Aging Patterns for Automatic Age Estimation. In Proceedings of the 14th ACM International Conference on Multimedia, Santa Barbara, CA, USA, 23–27 October 2006; Association for Computing Machinery: New York, NY, USA, 2006; pp. 307–316. [Google Scholar]
- Xu, Y.; Xie, L.; Zhang, X.; Chen, X.; Qi, G.-J.; Tian, Q.; Xiong, H. PC-DARTS: Partial Channel Connections for Memory-Efficient Architecture Search. arXiv 2020, arXiv:1907.05737. [Google Scholar]
- Liu, H.; Simonyan, K.; Yang, Y. DARTS: Differentiable Architecture Search. arXiv 2019, arXiv:1806.09055. [Google Scholar]
- Guo, Z.; Zhang, X.; Mu, H.; Heng, W.; Liu, Z.; Wei, Y.; Sun, J. Single Path One-Shot Neural Architecture Search with Uniform Sampling. arXiv 2020, arXiv:1904.00420. [Google Scholar]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-Level Arrhythmia Detection and Classification in Ambulatory Electrocardiograms Using a Deep Neural Network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Hou, W.; Liu, L.; Gao, J.; Zhu, A.; Pan, K.; Sun, H.; Zheng, N. Exploring Effective DNN Models for Forensic Age Estimation Based on Panoramic Radiograph Images. In Proceedings of the 2021 International Joint Conference on Neural Networks (IJCNN), Shenzhen, China, 18–22 July 2021; pp. 1–8. [Google Scholar]


| Model | FLOPs/s × 10−9 | Params/s × 10−6 | MAE/Year |
|---|---|---|---|
| Human experts | - | - | >2 |
| ResNeXt50 | 12.59 | 23.14 | 1.74 |
| Inception-v4 | 20.46 | 41.27 | 1.70 |
| MobileNetV2 (1.0×) | 0.96 | 2.33 | 1.98 |
| EfficientNet-B0 | 1.22 | 4.11 | 1.94 |
| AGENet-Small | 3.54 | 7.53 | 1.70 |
| AGENet-Large | 7.49 | 16.89 | 1.64 |
| HSCNet | 3.99 | 7.78 | 1.64 |
| AGE-SPOS (0.5×) | 0.29 | 0.72 | 2.40 |
| AGE-SPOS (1.0×) | 0.95 | 2.17 | 1.80 |
| AGE-SPOS (1.5×) | 1.97 | 4.44 | 1.77 |
| DNN Architecture | MAE/Year | |
|---|---|---|
| Without SE Module | With SE Module | |
| AGE-SPOS (0.5×) | 2.40 | 2.61 (+0.21) |
| AGE-SPOS (1.0×) | 1.80 | 1.80 (−0.00) |
| AGE-SPOS (1.5×) | 1.77 | 1.75 (−0.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-Y.; Yan, C.-X.; Min, Q.-M.; Zhang, Y.-X.; Jing, W.-F.; Hou, W.-X.; Pan, K.-Y. Leverage Effective Deep Learning Searching Method for Forensic Age Estimation. Bioengineering 2024, 11, 674. https://doi.org/10.3390/bioengineering11070674
Zhang Z-Y, Yan C-X, Min Q-M, Zhang Y-X, Jing W-F, Hou W-X, Pan K-Y. Leverage Effective Deep Learning Searching Method for Forensic Age Estimation. Bioengineering. 2024; 11(7):674. https://doi.org/10.3390/bioengineering11070674
Chicago/Turabian StyleZhang, Zhi-Yong, Chun-Xia Yan, Qiao-Mei Min, Yu-Xiang Zhang, Wen-Fan Jing, Wen-Xuan Hou, and Ke-Yang Pan. 2024. "Leverage Effective Deep Learning Searching Method for Forensic Age Estimation" Bioengineering 11, no. 7: 674. https://doi.org/10.3390/bioengineering11070674
APA StyleZhang, Z.-Y., Yan, C.-X., Min, Q.-M., Zhang, Y.-X., Jing, W.-F., Hou, W.-X., & Pan, K.-Y. (2024). Leverage Effective Deep Learning Searching Method for Forensic Age Estimation. Bioengineering, 11(7), 674. https://doi.org/10.3390/bioengineering11070674








