Modified Clover Technique Using Automated Suture Placement and Securing Technology in a Passive Beating Heart Model
Abstract
1. Introduction
2. Material and Methods
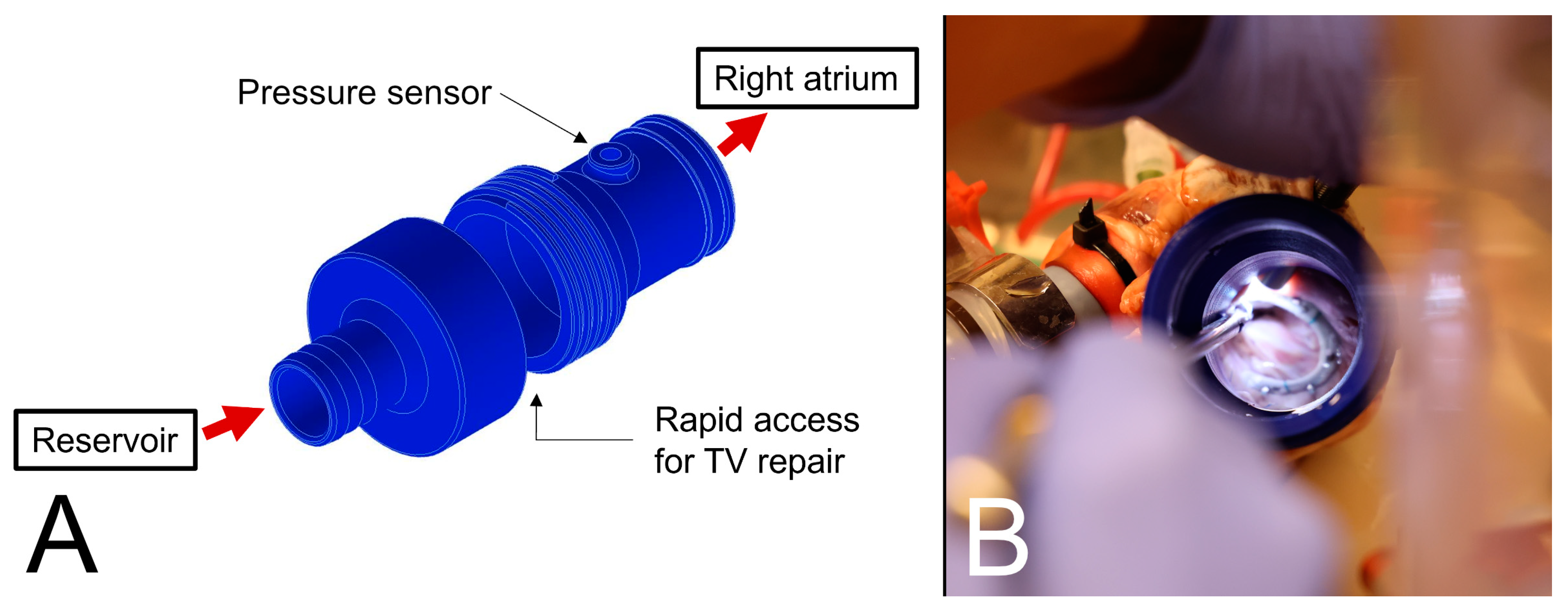
2.1. Passive Beating Heart Model
2.2. Surgical Repair Techniques
2.3. Statistical Analysis
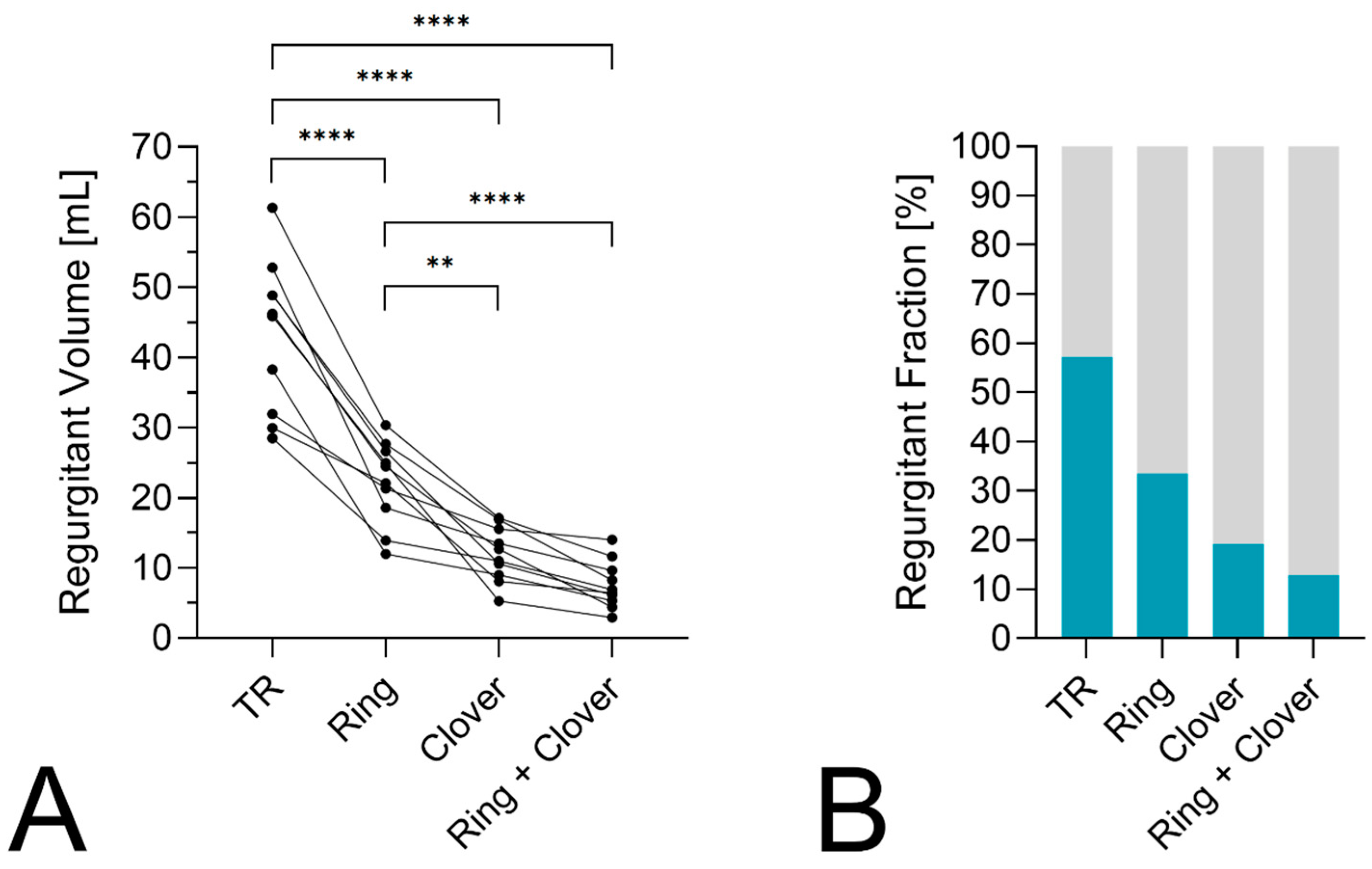
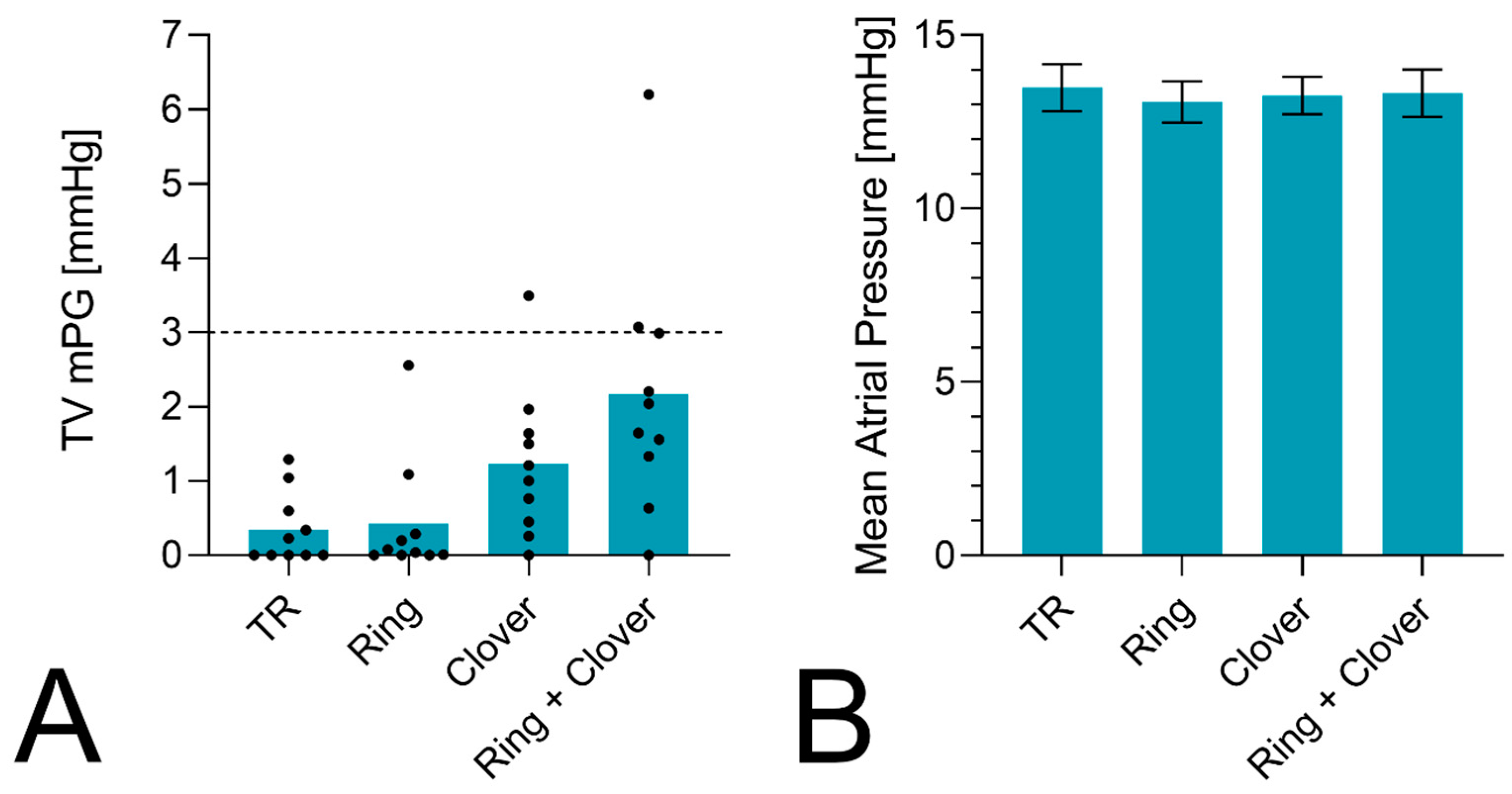
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIED | Cardiovascular implantable electronic device |
| mPG | Mean pressure gradient |
| PAC | Pulmonary artery connector |
| RAC | Right atrium connector |
| RF | Regurgitant fraction |
| TVr | Tricuspid valve repair |
| TR | Tricuspid regurgitation |
References
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, D.; Bursi, F.; Fanti, D.; Dotto, A.; Ciceri, L.; Springhetti, P.; Bergamini, C.; Tafciu, E.; Maffeis, C.; Scarsini, R.; et al. Outpatient tricuspid regurgitation in the community: Clinical context and outcome. Int. J. Cardiol. 2024, 396, 131443. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T. Tricuspid Regurgitation. N. Engl. J. Med. 2023, 388, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.; Burri, H.; Praz, F.; Soliman, O.; Badano, L.; Barreiro, M.; Cavalcante, J.L.; de Potter, T.; Doenst, T.; Friedrichs, K.; et al. Tricuspid valve disease and cardiac implantable electronic devices. Eur. Heart J. 2024, 45, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; Delgado, V.; Leon, M.B.; Enriquez-Sarano, M.; Topilsky, Y.; Bax, J.J. Morphologic Types of Tricuspid Regurgitation: Characteristics and Prognostic Implications. JACC Cardiovasc. Imaging 2019, 12, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kassis, N.; Layoun, H.; Goyal, A.; Dong, T.; Saad, A.M.; Puri, R.; Griffin, B.P.; Heresi, G.A.; Tonelli, A.R.; Kapadia, S.R.; et al. Mechanistic Insights into Tricuspid Regurgitation Secondary to Pulmonary Arterial Hypertension. Am. J. Cardiol. 2022, 175, 97–105. [Google Scholar] [CrossRef]
- Shiran, A.; Sagie, A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J. Am. Coll. Cardiol. 2009, 53, 401–408. [Google Scholar] [CrossRef]
- Muraru, D.; Badano, L.P.; Hahn, R.T.; Lang, R.M.; Delgado, V.; Wunderlich, N.C.; Donal, E.; Taramasso, M.; Duncan, A.; Lurz, P.; et al. Atrial secondary tricuspid regurgitation: Pathophysiology, definition, diagnosis, and treatment. Eur. Heart J. 2024, 45, 895–911. [Google Scholar] [CrossRef]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Di Mauro, M.; Saitto, G.; Lio, A.; Berretta, P.; Taramasso, M.; Scrofani, R.; Della Corte, A.; Sponga, S.; Greco, E.; et al. Outcome of patients undergoing isolated tricuspid repair or replacement surgery. Eur. J. Cardiothorac. Surg. 2022, 62, ezac230. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Di Mauro, M.; Saitto, G.; Lio, A.; Berretta, P.; Taramasso, M.; Scrofani, R.; Della Corte, A.; Sponga, S.; Greco, E.; et al. Beating Versus Arrested Heart Isolated Tricuspid Valve Surgery: Long-term Outcomes. Ann. Thorac. Surg. 2022, 113, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Musumeci, F.; Ranocchi, F.; Andreas, M. Prediction of mortality in isolated tricuspid surgery. J. Card. Surg. 2022, 37, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Saitto, G.; Lio, A.; Di Mauro, M.; Berretta, P.; Taramasso, M.; Scrofani, R.; Della Corte, A.; Sponga, S.; Greco, E.; et al. Observed versus predicted mortality after isolated tricuspid valve surgery. J. Card. Surg. 2022, 37, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, M.; Sitges, M.; Luis Pomar, J.; Romiti, S.; Domenech-Ximenos, B.; D’Abramo, M.; Wretschko, E.; Miraldi, F.; Greco, E. Functional Tricuspid Regurgitation: Behind the Scenes of a Long-Time Neglected Disease. Front. Cardiovasc. Med. 2022, 9, 836441. [Google Scholar] [CrossRef]
- Alfieri, O.; De Bonis, M.; Lapenna, E.; Agricola, E.; Quarti, A.; Maisano, F. The “clover technique” as a novel approach for correction of post-traumatic tricuspid regurgitation. J. Thorac. Cardiovasc. Surg. 2003, 126, 75–79. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, M.; Lapenna, E.; Di Sanzo, S.; Del Forno, B.; Pappalardo, F.; Castiglioni, A.; Vicentini, L.; Pozzoli, A.; Giambuzzi, I.; Latib, A.; et al. Long-term results (up to 14 years) of the clover technique for the treatment of complex tricuspid valve regurgitation. Eur. J. Cardiothorac. Surg. 2017, 52, 125–130. [Google Scholar] [CrossRef]
- Abdelbar, A.; Kenawy, A.; Zacharias, J. Minimally invasive tricuspid valve surgery. J. Thorac. Dis. 2021, 13, 1982–1992. [Google Scholar] [CrossRef]
- Chen, J.; Ma, W.; Ming, Y.; Wang, W.; Liu, S.; Yang, Y.; Lin, Y.; Wei, L.; Wang, C. Minimally Invasive Valve Replacement for Late Tricuspid Regurgitation After Left-Sided Valve Surgery. Ann. Thorac. Surg. 2021, 111, e381–e383. [Google Scholar] [CrossRef] [PubMed]
- Strobel, R.J.; Hawkins, R.B.; Mehaffey, J.H.; Rotar, E.P.; Yount, K.W.; Teman, N.R.; Ailawadi, G. Minimally Invasive Approaches Are Safe for Concomitant Mitral and Tricuspid Valve Surgery. Innovations 2022, 17, 416–423. [Google Scholar] [CrossRef]
- Salurso, E.; Perico, F.; Pappalardo, F.; Gard, M.; Antoniotti, M.; Passanante, E.; Zanotti, D.; De Bonis, M.; Alfieri, O.; Vismara, R. A Novel Transcatheter Device for the Edge-to-Edge Treatment of Tricuspid Regurgitation: A Preliminary Evaluation. Ann. Biomed. Eng. 2024, 52, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.N.; Tay, E.L.W.; Kabinejadian, F.; Ong, C.W.; Ismail, M.; Leo, H.L. Ventricular vortex loss analysis due to various tricuspid valve repair techniques: An ex vivo study. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1312–H1327. [Google Scholar] [CrossRef]
- Amedi, A.; Onohara, D.; Xu, D.; Suresh, K.S.; Padala, M. Hemodynamic outcomes after undersizing ring annuloplasty and focal suture annuloplasty for surgical repair of functional tricuspid regurgitation. J. Thorac. Cardiovasc. Surg. 2022, 164, 76–87.e71. [Google Scholar] [CrossRef] [PubMed]
- Algarni, K.D.; Alfonso, J.; Pragliola, C.; Kheirallah, H.; Adam, A.I.; Arafat, A.A. Long-term Outcomes of Tricuspid Valve Repair: The Influence of the Annuloplasty Prosthesis. Ann. Thorac. Surg. 2021, 112, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Deloche, A.; Dauptain, J.; Soyer, R.; Blondeau, P.; Piwnica, A.; Dubost, C.; McGoon, D.C. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J. Thorac. Cardiovasc. Surg. 1971, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hisata, Y.; Hazama, S.; Izumi, K.; Eishi, K. ‘Clover technique’ for tricuspid regurgitation due to pacemaker lead. J. Heart Valve Dis. 2011, 20, 464–465. [Google Scholar] [PubMed]
- Pozzi, M.; Pavlakovic, I.; Dondas, A.; Sebbag, L.; Boissonnat, P.; Obadia, J.F. The clover technique in endomyocardial biopsy-induced tricuspid regurgitation. Int. J. Cardiol. 2016, 203, 731–732. [Google Scholar] [CrossRef]
- Hausleiter, J.; Braun, D.; Massberg, S.; Nabauer, M. Percutaneous edge-to-edge tricuspid repair applying the ‘clover’ technique. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1261. [Google Scholar] [CrossRef]
- Lapenna, E.; Gramegna, F.; Del Forno, B.; Scarale, M.G.; Nonis, A.; Carino, D.; Ancona, F.; Faggi, A.; Schiavi, D.; Alfieri, O.; et al. Long-term results of clover and edge-to-edge leaflet repair for complex tricuspid regurgitation. Ann. Thorac. Surg. 2024, 13, S0003-4975(24)00364-3. [Google Scholar] [CrossRef]
- Gammie, J.S.; Chu, M.W.A.; Falk, V.; Overbey, J.R.; Moskowitz, A.J.; Gillinov, M.; Mack, M.J.; Voisine, P.; Krane, M.; Yerokun, B.; et al. Concomitant Tricuspid Repair in Patients with Degenerative Mitral Regurgitation. N. Engl. J. Med. 2022, 386, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23; quiz 101–102. [Google Scholar] [CrossRef]
- Pitsis, A.; Marvaki, A.; Lolakos, K.; Andreas, M. Totally Endoscopic Redo Tricuspid Repair With a Modified Clover Triple Edge-to-Edge Technique. Innovations 2024, 15569845241253279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ke, Y.; Xie, X.; Guo, H.; Zeng, Q.; Huang, H. Outcomes of Totally Endoscopic Beating-Heart Tricuspid Repair in Redo Cardiac Surgery. Heart Lung Circ. 2020, 29, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Abdelbar, A.; Niranjan, G.; Tynnson, C.; Saravanan, P.; Knowles, A.; Laskawski, G.; Zacharias, J. Endoscopic Tricuspid Valve Surgery is a Safe and Effective Option. Innovations 2020, 15, 66–73. [Google Scholar] [CrossRef]
- Pitsis, A. Totally Endoscopic Triple-Valve Surgery With Transcatheter Valve in Mitral Annular Calcification, Aortic Valve Replacement, and Tricuspid Repair. Innovations 2024, 19, 118–119. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laengle, S.; Suria, A.; Poschner, T.; Tasdelen, S.; Pitsis, A.; Kocher, A.; Andreas, M. Modified Clover Technique Using Automated Suture Placement and Securing Technology in a Passive Beating Heart Model. Bioengineering 2024, 11, 666. https://doi.org/10.3390/bioengineering11070666
Laengle S, Suria A, Poschner T, Tasdelen S, Pitsis A, Kocher A, Andreas M. Modified Clover Technique Using Automated Suture Placement and Securing Technology in a Passive Beating Heart Model. Bioengineering. 2024; 11(7):666. https://doi.org/10.3390/bioengineering11070666
Chicago/Turabian StyleLaengle, Severin, Aldo Suria, Thomas Poschner, Sahra Tasdelen, Antonios Pitsis, Alfred Kocher, and Martin Andreas. 2024. "Modified Clover Technique Using Automated Suture Placement and Securing Technology in a Passive Beating Heart Model" Bioengineering 11, no. 7: 666. https://doi.org/10.3390/bioengineering11070666
APA StyleLaengle, S., Suria, A., Poschner, T., Tasdelen, S., Pitsis, A., Kocher, A., & Andreas, M. (2024). Modified Clover Technique Using Automated Suture Placement and Securing Technology in a Passive Beating Heart Model. Bioengineering, 11(7), 666. https://doi.org/10.3390/bioengineering11070666











