Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation
Abstract
1. Introduction
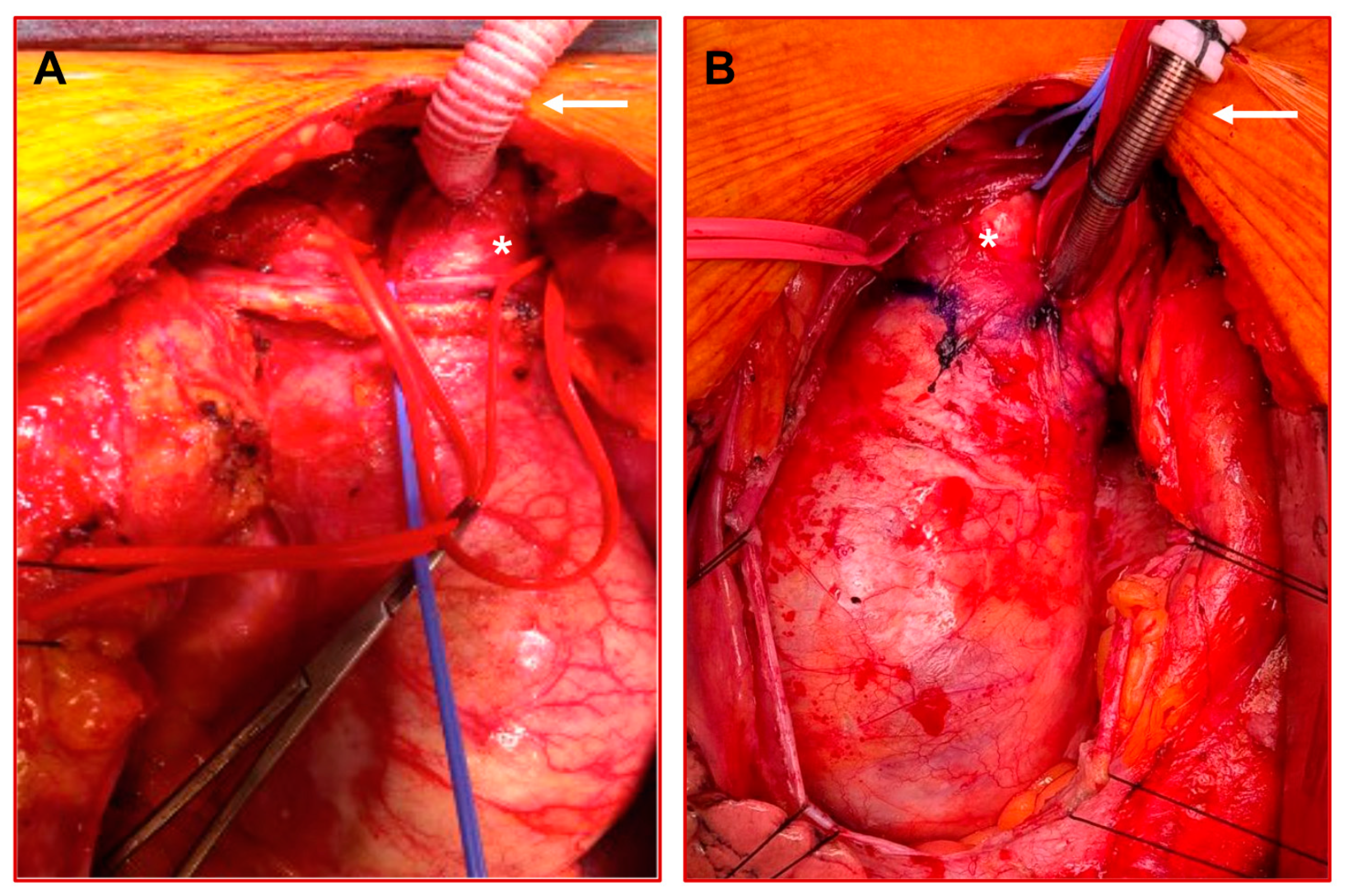
2. Cerebral Perfusion Modes
3. Temperature Management
4. Pharmacological Strategies of Neuroprotection
4.1. Corticosteroids
4.2. Magnesium
4.3. Mannitol
4.4. Lidocaine
4.5. Barbiturates
4.6. Propofol
4.7. Inhaled Anesthetics
4.8. Ketamine
4.9. Nitric Oxide
5. Neuromonitoring
5.1. NIRS
5.2. EEG and SSEPs
5.3. Alpha-Stat vs. pH-Stat
6. Future Perspectives
6.1. Cerebral Monitoring
6.2. Biomarkers
6.3. Enhanced Recovery after Surgery (ERAS)
6.4. Visualization and Augmented Reality
6.5. AI and Machine Learning
6.6. Endovascular and Hybrid Approaches
7. The Vienna Approach
8. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urbanski, P.P.; Luehr, M.; di Bartolomeo, R.; Diegeler, A.; de Paulis, R.; Esposito, G.; Bonser, R.S.; Etz, C.D.; Kallenbach, K.; Rylski, B.; et al. Multicentre analysis of current strategies and outcomes in open aortic arch surgery: Heterogeneity is still an issue. Eur. J. Cardiothorac. Surg. 2016, 50, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Zierer, A.; Detho, F.; Dzemali, O.; Aybek, T.; Moritz, A.; Bakhtiary, F. Antegrade cerebral perfusion with mild hypothermia for aortic arch replacement: Single-center experience in 245 consecutive patients. Ann. Thorac. Surg. 2011, 91, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Angleitner, P.; Stelzmueller, M.E.; Mahr, S.; Kaider, A.; Laufer, G.; Ehrlich, M. Bilateral or unilateral antegrade cerebral perfusion during surgery for acute type A dissection. J. Thorac. Cardiovasc. Surg. 2020, 159, 2159–2167.e2. [Google Scholar] [CrossRef] [PubMed]
- Griepp, R.B.; Stinson, E.B.; Hollingsworth, J.F.; Buehler, D. Prosthetic replacement of the aortic arch. J. Thorac. Cardiovasc. Surg. 1975, 70, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Miki, S.; Kusuhara, K.; Okita, Y.; Tahata, T.; Yamanaka, K. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J. Cardiovasc. Surg. 1990, 31, 553–558. [Google Scholar]
- Mills, N.L.; Ochsner, J.L. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. J. Thorac. Cardiovasc. Surg. 1980, 80, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.P.; Fang, W.C.; Grabenwöger, M.; Kocher, A.; Ankersmit, J.; Laufer, G.; Grubhofer, G.; Havel, M.; Wolner, E. Impact of retrograde cerebral perfusion on aortic arch aneurysm repair. J. Thorac. Cardiovasc. Surg. 1999, 118, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Bavaria, J.E.; Pochettino, A. Retrograde cerebral perfusion (RCP) in aortic arch surgery: Efficacy and possible mechanisms of brain protection. Semin. Thorac. Cardiovasc. Surg. 1997, 9, 222–232. [Google Scholar] [PubMed]
- Ehrlich, M.; Hagl, C.; McCullough, J.N.; Zhang, N.; Shiang, H.; Bodian, C.A.; Griepp, R.B. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J. Thorac. Cardiovasc. Surg. 2001, 122, 331–338. [Google Scholar] [CrossRef]
- Bachet, J.; Guilmet, D.; Goudot, B.; Termignon, J.L.; Teodori, G.; Dreyfus, G.; Brodaty, D.; Dubois, C.; Delentdecker, P.; Cabrol, C. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J. Thorac. Cardiovasc. Surg. 1991, 102, 85–93, discussion-4. [Google Scholar] [CrossRef]
- Kazui, T.; Inoue, N.; Komatsu, S. Surgical treatment of aneurysms of the transverse aortic arch. J. Cardiovasc. Surg. 1989, 30, 402–406. [Google Scholar]
- Tian, D.H.; Wan, B.; Bannon, P.G.; Misfeld, M.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; Elefteriades, J.A.; Bavaria, J.E.; Coselli, J.S.; et al. A meta-analysis of deep hypothermic circulatory arrest alone versus with adjunctive selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2013, 2, 261–270. [Google Scholar] [PubMed]
- Yan, T.D.; Bannon, P.G.; Bavaria, J.; Coselli, J.S.; Elefteriades, J.A.; Griepp, R.B.; Hughes, G.C.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; et al. Consensus on hypothermia in aortic arch surgery. Ann. Cardiothorac. Surg. 2013, 2, 163–168. [Google Scholar] [PubMed]
- Urbanski, P.P.; Lenos, A.; Bougioukakis, P.; Neophytou, I.; Zacher, M.; Diegeler, A. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: A change of paradigm? Eur. J. Cardiothorac. Surg. 2012, 41, 185–191. [Google Scholar] [CrossRef]
- Carrel, T.; Schmiady, M.; Ouda, A.; Vogt, P.R. Universus bilateral antegrade cerebral perfusion during repair of acute aortic dissection: Still a discussed matter! JTCVS Tech. 2023, 17, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zierer, A.; Risteski, P.; El-Sayed Ahmad, A.; Moritz, A.; Diegeler, A.; Urbanski, P.P. The impact of unilateral versus bilateral antegrade cerebral perfusion on surgical outcomes after aortic arch replacement: A propensity-matched analysis. J. Thorac. Cardiovasc. Surg. 2014, 147, 1212–1217, discussion 7–8. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Piperata, A.; Watanabe, M.; Pernot, M.; Metras, A.; Kalscheuer, G.; Avesani, M.; Barandon, L.; Peltan, J.; Lorenzoni, G.; Jorgji, V.; et al. Unilateral versus bilateral cerebral perfusion during aortic surgery for acute type A aortic dissection: A multicentre study. Eur. J. Cardiothorac. Surg. 2022, 61, 828–835. [Google Scholar] [CrossRef]
- Kruger, T.; Weigang, E.; Hoffmann, I.; Blettner, M.; Aebert, H.; Investigators, G. Cerebral protection during surgery for acute aortic dissection type A: Results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011, 124, 434–443. [Google Scholar] [CrossRef]
- O’Hara, D.; McLarty, A.; Sun, E.; Itagaki, S.; Tannous, H.; Chu, D.; Egorova, N.; Chikwe, J. Type-A Aortic Dissection and Cerebral Perfusion: The Society of Thoracic Surgeons Database Analysis. Ann. Thorac. Surg. 2020, 110, 1461–1467. [Google Scholar] [CrossRef]
- Okita, Y.; Miyata, H.; Motomura, N.; Takamoto, S.; Japan Cardiovascular Surgery Database Organization. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: Analysis based on the Japan Adult Cardiovascular Surgery Database. J. Thorac. Cardiovasc. Surg. 2015, 149 (Suppl. S2), S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Dimagli, A.; Cooper, G.; Uppal, R.; Mariscalco, G.; Krasopoulos, G.; Goodwin, A.; Trivedi, U.; Kendall, S.; Sinha, S.; et al. Neuroprotective strategies in acute aortic dissection: An analysis of the UK National Adult Cardiac Surgical Audit. Eur. J. Cardiothorac. Surg. 2021, 60, 1437–1444. [Google Scholar] [CrossRef]
- Norton, E.L.; Wu, X.; Kim, K.M.; Patel, H.J.; Deeb, G.M.; Yang, B. Unilateral is comparable to bilateral antegrade cerebral perfusion in acute type A aortic dissection repair. J. Thorac. Cardiovasc. Surg. 2020, 160, 617–625.e5. [Google Scholar] [CrossRef] [PubMed]
- Zierer, A.; El-Sayed Ahmad, A.; Papadopoulos, N.; Moritz, A.; Diegeler, A.; Urbanski, P.P. Selective antegrade cerebral perfusion and mild (28 degrees C-30 degrees C) systemic hypothermic circulatory arrest for aortic arch replacement: Results from 1002 patients. J. Thorac. Cardiovasc. Surg. 2012, 144, 1042–1049. [Google Scholar] [CrossRef]
- Preventza, O.; Coselli, J.S.; Akvan, S.; Kashyap, S.A.; Garcia, A.; Simpson, K.H.; Price, M.D.; Mayor, J.; de la Cruz, K.I.; Cornwell, L.D.; et al. The impact of temperature in aortic arch surgery patients receiving antegrade cerebral perfusion for >30 minutes: How relevant is it really? J. Thorac. Cardiovasc. Surg. 2017, 153, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.H.; Weller, J.; Hasmat, S.; Preventza, O.; Forrest, P.; Kiat, H.; Yan, T.D. Temperature Selection in Antegrade Cerebral Perfusion for Aortic Arch Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2019, 108, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Abjigitova, D.; Veen, K.M.; van Tussenbroek, G.; Mokhles, M.M.; Bekkers, J.A.; Takkenberg, J.J.M.; Bogers, A.J. Cerebral protection in aortic arch surgery: Systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac128. [Google Scholar] [CrossRef]
- Hughes, G.C.; Chen, E.P.; Browndyke, J.N.; Szeto, W.Y.; DiMaio, J.M.; Brinkman, W.T.; Gaca, J.G.; Blumenthal, J.A.; Karhausen, J.A.; Bisanar, T.; et al. Cognitive Effects of Body Temperature During Hypothermic Circulatory Arrest Trial (GOT ICE): A Randomized Clinical Trial Comparing Outcomes after Aortic Arch Surgery. Circulation 2024, 149, 658–668. [Google Scholar] [CrossRef]
- Trivedi, D.; Navid, F.; Balzer, J.R.; Joshi, R.; Lacomis, J.M.; Jovin, T.G.; Althouse, A.D.; Gleason, T.G. Aggressive Aortic Arch and Carotid Replacement Strategy for Type A Aortic Dissection Improves Neurologic Outcomes. Ann. Thorac. Surg. 2016, 101, 896–903, Discussion-5. [Google Scholar] [CrossRef]
- Manetta, F.; Mullan, C.W.; Catalano, M.A. Neuroprotective Strategies in Repair and Replacement of the Aortic Arch. Int. J. Angiol. 2018, 27, 98–109. [Google Scholar]
- Sandercock, P.A.; Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 2011, CD000064. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996, 1, 1–19. [Google Scholar] [CrossRef]
- Pearce, A.; Lockwood, C.; van den Heuvel, C.; Pearce, J. The use of therapeutic magnesium for neuroprotection during global cerebral ischemia associated with cardiac arrest and cardiac surgery in adults: A systematic review. JBI Database System Rev. Implement. Rep. 2017, 15, 86–118. [Google Scholar] [CrossRef]
- Mathew, J.P.; White, W.D.; Schinderle, D.B.; Podgoreanu, M.V.; Berger, M.; Milano, C.A.; Laskowitz, D.T.; Stafford-Smith, M.; Blumenthal, J.A.; Newman, M.F.; et al. Intraoperative magnesium administration does not improve neurocognitive function after cardiac surgery. Stroke 2013, 44, 3407–3413. [Google Scholar] [CrossRef]
- Bhudia, S.K.; Cosgrove, D.M.; Naugle, R.I.; Rajeswaran, J.; Lam, B.-K.; Walton, E.; Petrich, J.; Palumbo, R.; Marc Gillinov, A.; Apperson-Hansen, C.; et al. Magnesium as a neuroprotectant in cardiac surgery: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2006, 131, 853–861. [Google Scholar] [CrossRef]
- Kruger, T.; Hoffmann, I.; Blettner, M.; Borger, M.A.; Schlensak, C.; Weigang, E. Intraoperative neuroprotective drugs without beneficial effects? Results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur. J. Cardiothorac. Surg. 2013, 44, 939–946. [Google Scholar] [CrossRef]
- Hung, K.C.; Ho, C.N.; Liu, W.C.; Yew, M.; Chang, Y.J.; Lin, Y.T.; Hung, I.Y.; Chen, J.Y.; Huang, P.W.; Sun, C.K. Prophylactic effect of intravenous lidocaine against cognitive deficit after cardiac surgery: A PRISMA-compliant meta-analysis and trial sequential analysis. Medicine 2022, 101, e30476. [Google Scholar] [CrossRef]
- Jovin, D.G.; Katlaps, K.G.; Ellis, B.K.; Dharmaraj, B. Neuroprotection against stroke and encephalopathy after cardiac surgery. Interv. Med. Appl. Sci. 2019, 11, 27–37. [Google Scholar] [CrossRef]
- Li, X.; Yao, L.; Liang, Q.; Qu, H.; Cai, H. Propofol Protects Hippocampal Neurons from Hypoxia-Reoxygenation Injury by Decreasing Calcineurin-Induced Calcium Overload and Activating YAP Signaling. Oxidative Med. Cell. Longev. 2018, 2018, 1725191. [Google Scholar] [CrossRef]
- Kumagai, H.; Isaka, M.; Sugawara, Y.; Okada, K.; Imai, K.; Orihashi, K.; Sueda, T. Intra-aortic injection of propofol prevents spinal cord injury during aortic surgery. Eur. J. Cardiothorac. Surg. 2006, 29, 714–719. [Google Scholar] [CrossRef]
- Roach, G.F.; Newman, M.F.; Murkin, J.M.; Martzke, J.; Ruskin, A.; Li, J.; Guo, A.; Wisniewski, A.B.; Mangano, D.T. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology 1999, 90, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Bickler, P.E.; Zhan, X.; Fahlman, C.S. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: Role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology 2005, 103, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Duan, G.; Wu, Z.; Zuo, Z.; Li, H. Comparison of the cerebroprotective effect of inhalation anaesthesia and total intravenous anaesthesia in patients undergoing cardiac surgery with cardiopulmonary bypass: A systematic review and meta-analysis. BMJ Open 2017, 7, e014629. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Byrne, A.J.; Hudetz, A.G.; Pagel, P.S.; Warltier, D.C. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol. Scand. 2009, 53, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Avidan, M.S.; Maybrier, H.R.; Abdallah, A.B.; Jacobsohn, E.; Vlisides, P.E.; Pryor, K.O.; Veselis, R.A.; Grocott, H.P.; Emmert, D.A.; Rogers, E.M.; et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomised clinical trial. Lancet 2017, 390, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Linardi, D.; Mani, R.; Murari, A.; Dolci, S.; Mannino, L.; Decimo, I.; Tessari, M.; Martinazzi, S.; Gottin, L.; Luciani, G.B.; et al. Nitric Oxide in Selective Cerebral Perfusion Could Enhance Neuroprotection During Aortic Arch Surgery. Front. Cardiovasc. Med. 2021, 8, 772065. [Google Scholar] [CrossRef] [PubMed]
- Fedorow, C.; Grocott, H.P. Cerebral monitoring to optimize outcomes after cardiac surgery. Curr. Opin. Anaesthesiol. 2010, 23, 89–94. [Google Scholar] [CrossRef]
- Montisci, A.; Maj, G.; Cavozza, C.; Audo, A.; Benussi, S.; Rosati, F.; Cattaneo, S.; di Bacco, L.; Pappalardo, F. Cerebral Perfusion and Neuromonitoring during Complex Aortic Arch Surgery: A Narrative Review. J. Clin. Med. 2023, 12, 3470. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardiothorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Zheng, F.; Sheinberg, R.; Yee, M.S.; Ono, M.; Zheng, Y.; Hogue, C.W. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: A systematic review. Anesth. Analg. 2013, 116, 663–676. [Google Scholar] [CrossRef]
- Friess, J.-O.; Beeler, M.; Yildiz, M.; Guensch, D.P.; Levis, A.; Gerber, D.; Wollborn, J.; Jenni, H.; Huber, M.; Schönhoff, F. Determination of selective antegrade perfusion flow rate in aortic arch surgery to restore baseline cerebral near-infrared spectroscopy values: A single-centre observational study. Eur. J. Cardiothorac. Surg. 2023, 63, ezad047. [Google Scholar] [CrossRef] [PubMed]
- Falasa, M.P.; Arnaoutakis, G.J.; Janelle, G.M.; Beaver, T.M. Neuromonitoring and neuroprotection advances for aortic arch surgery. JTCVS Tech. 2021, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Brown, J.A.; Serna-gallegos, D.; Thirumala, P.D.; Balzer, J.R.; Paras, S.; Fleseriu, C.; Crammond, D.J.; Anetakis, K.M.; Kilic, A.; et al. Intraoperative neurophysiologic monitoring during aortic arch surgery. J. Thorac. Cardiovasc. Surg. 2023, 165, 1971–1981.e2. [Google Scholar] [CrossRef]
- Ghincea, C.V.; Anderson, D.A.; Ikeno, Y.; Roda, G.F.; Eldeiry, M.; Bronsert, M.R.; Aunkst, K.; Fullerton, D.A.; Reece, T.B.; Aftab, M. Utility of neuromonitoring in hypothermic circulatory arrest cases for early detection of stroke: Listening through the noise. J. Thorac. Cardiovasc. Surg. 2021, 162, 1035–1045.e5. [Google Scholar] [CrossRef]
- Abdul Aziz, K.A.; Meduoye, A. Is pH-stat or alpha-stat the best technique to follow in patients undergoing deep hypothermic circulatory arrest? Interact. Cardiovasc. Thorac. Surg. 2010, 10, 271–282. [Google Scholar] [CrossRef]
- Jonas, R.A. Technique of circulatory arrest makes a difference. J. Thorac. Cardiovasc. Surg. 2018, 156, 40–41. [Google Scholar] [CrossRef]
- Du Plessis, A.J.; Jonas, R.A.; Wypij, D.; Hickey, P.R.; Riviello, J.; Wessel, D.L.; Roth, S.J.; Burrows, F.A.; Walter, G.; Farrell, D.M.; et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J. Thorac. Cardiovasc. Surg. 1997, 114, 991–1000, discussion 1000–1001. [Google Scholar] [CrossRef]
- Lohbusch, B.; Olson, K.; Magowan, B.; Cherichella, R.; Wolverton, J.; Dell’Aiera, L.; Likosky, D.S.; Fitzgerald, D. Adult Clinical Perfusion Practice Survey: 2020 results. J. Extra Corpor. Technol. 2023, 55, 3–22. [Google Scholar] [CrossRef]
- Ghazy, T.; Darwisch, A.; Schmidt, T.; Nguyen, P.; Elmihy, S.; Fajfrova, Z.; Matschke, K.; Kappert, U. The Transcranial Doppler Sonography for Optimal Monitoring and Optimization of Cerebral Perfusion in Aortic Arch Surgery: A Case Series. Heart Surg. Forum. 2017, 20, E085–E088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, M.U. Robotic Transcranial Doppler in aortic surgery: A New Standard of Care. In Proceedings of the SCTS Annual Meeting 2024, Newport, UK, 17–19 March 2024. Wales2024. 03/18/2024. [Google Scholar]
- Si, Y.; Duan, W.; Xie, J.; Duan, C.; Liu, S.; Wang, Q.; Zhao, X.; Wu, D.; Wang, Y.; Wang, L.; et al. Biomarkers for prediction of neurological complications after acute Stanford type A aortic dissection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0281352. [Google Scholar] [CrossRef]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Abjigitova, D.; Sadeghi, A.H.; Peek, J.J.; Bekkers, J.A.; Bogers, A.; Mahtab, E.A.F. Virtual Reality in the Preoperative Planning of Adult Aortic Surgery: A Feasibility Study. J. Cardiovasc. Dev. Dis. 2022, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Condino, S.; Piazza, R.; Carbone, M.; Bath, J.; Troisi, N.; Ferrari, M.; Berchiolli, R. Bioengineering, augmented reality, and robotic surgery in vascular surgery: A literature review. Front. Surg. 2022, 9, 966118. [Google Scholar] [CrossRef] [PubMed]
- Nedadur, R.; Bhatt, N.; Chung, J.; Chu, M.W.A.; Ouzounian, M.; Wang, B. Machine learning and decision making in aortic arch repair. J. Thorac. Cardiovasc. Surg. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Basha, A.M.; Moore, R.D.; Rommens, K.L.; Herget, E.J.; McClure, R.S. A Systematic Review of Total Endovascular Aortic Arch Repair: A Promising Technology. Can. J. Cardiol. 2023, 39, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Smorenburg, S.P.M.; Montesano, M.; Hoogteijling, T.J.; Truijers, M.; Symersky, P.; Jansen, E.K.; Zandbergen, H.R.; Wisselink, W.; van Schaik, T.G.; Yeung, K.K. Anatomic Suitability for Branched Thoracic Endovascular Repair in Patients with Aortic Arch Pathological Features. J. Am. Heart Assoc. 2020, 9, e016695. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Hauck, S.R.; Dachs, T.M.; Haider, L.; Stelzmüller, M.E.; Ehrlich, M.; Loewe, C.; Funovics, M.A. Endovascular repair in type A aortic dissection: Anatomical candidacy for currently manufactured stent grafts and conceptual valve-carrying devices for an Endo-Bentall procedure. Eur. J. Cardiothorac. Surg. 2023, 63, ezad085. [Google Scholar] [CrossRef]
- Khullar, V.; Schaff, H.V.; Dearani, J.A.; Daly, R.C.; Greason, K.L.; Joyce, L.D.; Pochettino, A. Open Surgical Repair Remains the Gold Standard for Treating Aortic Arch Pathology. Ann. Thorac. Surg. 2017, 103, 1413–1420. [Google Scholar] [CrossRef]

| Author, Year | Study Type | Patient Collective | Cerebral Perfusion Technique | CA Time (Minutes) | Mean Temperature (°C) | Neurological Deficit (PND or TND Rates) | Conclusions |
|---|---|---|---|---|---|---|---|
| Piperata et al., 2022 [18] | Multi-center, retrospective | ATAAD n = 646 PSM analysis: n = 378 | uACP: 39% bACP: 61% PSM analysis uACP n = 189 bACP n = 189 | uACP: 34 (28–42) bACP: 37 (28–50) | uACP: 28 °C (28–28 °C) bACP: 27.5 °C (25–28 °C) | PND uACP: 4% bACP: 14% (p < 0.001) TND uACP: 11% bACP: 12% (p = 0.061) | - uACP and bACP are both valid brain protection strategies - PNDs are significantly less frequent in uACP - All combined-complications are significantly less frequent in uACP |
| Benedetto et al., 2021 [22] | National Adult Cardiac Surgical Audit, prospective | ATAAD n = 1929 | uACP: 6.1% bACP: 39.4% RCP: 11.5% DHCA: 43% | 37.2 +/− 28.5 | n/a | uACP: 9.4% bACP: 14.6% RCP: 13.1% DHCA: 14.2% (p: n/a) | - uACP and bACP are superior to DHCA alone regarding death and CVA - uACP may be superior to bACP in short CA times |
| Angleitner et al., 2020 [3] | Single- center, retrospective | ATAAD n = 184 | uACP: 51% bACP: 49% | 34 (26–49) | n/a | PND uACP: 19.4% bACP: 18.7% (p = 0.753) TND uACP: 9.7% bACP: 7.7% (p = 0.226) | - bACP vs. uACP have similar neurological outcomes - bACP may be superior to uACP in perfusion durations > 50 minutes |
| Norton et al., 2020 [23] | Single-center, retrospective | ATAAD n = 307 | uACP: 45.6% bACP: 54.4% | uACP: 29 (23–38) bACP: 45 (38–55) | bACP: 17 °C (16–18 °C) uACP: 20 °C (18–24 °C) | uACP: 6% bACP: 9% (p = 0.4) | - uACP and bACP are equally effective - uACP recommended for simplicity and less manipulation of arch branch vessels |
| O´Hara et al., 2020 [20] | STS Adult Cardiac Surgical Database, retrospective | ATAAD n = 6387 | ACP: 46.2% RCP: 22.6% DHCA: 31.2% | ACP: 25 (26–48) RCP: 33 (25–45) DHCA: 26 (20–34) | ACP: 22 °C (18.4–25) RCP:17.6 °C (19–21.9) DHCA: 18.8 °C (17.7–21.4) | ACP: 12.5% RCP: 11.2% DHCA: 13% (p = 0.06) | - Cerebral perfusion techniques such as ACP and RCP are associated with reduced death and stroke risk |
| Okita et al., 2015 [21] | Japan Adult Cardiovascular Surgery Database, retrospective | Arch repair excl. ATAAD All n = 8169 PSM analysis n = 2282 | DHCA/RCP: n = 1141 (14%) ACP: n = 7038 (86%) PSM analysis: DHCA/RCP: n = 1141 ACP: n = 1141 | n/a | ACP: 24.2 °C +/− 3.2 °C DHCA/RCP: 21.2 °C +/− 3.7 °C | uACP: 6.7% bACP: 8.6% (p = 0.83) | - HCA/RCP and ACP have comparable outcomes (death, stroke, reoperation) - Longer ICU stays in HCA/RCP group - ACP might be preferred for complicated aortic arch procedures |
| Zierer et al., 2012 [24] | Multi-center, prospective | Aortic Arch replacement incl. ATAAD (35%) n = 1002 | uACP: 33% bACP: 67% | 36 +/− 19 | 28–30 °C | PND uACP: 2% bACP: 4% (p = 0.6) | - uACP offers at least equal brain protection as bACP |
| Kruger et al., 2011 [19] | German Registry for Acute Aortic Dissection Type A (GERAADA) | ATAAD n = 1558 | DHCA: 22.8% RCP: 2.2% uACP: 40.3% bACP: 29.1% | DHCA: 22.7 +/− 14.3 RCP: n/a uACP: 32.2 +/− 17.9 bACP: 37.4 +/− 23.6 | n/a | PND HCA: 14.9% uACP: 12.6% bACP: 14.1% (p: n/a) | - Similar results with times < 30 min - During Longer periods ACP is advisable - uACP and bACP are equivalent |
| Agent | Author, Year | Conclusions |
|---|---|---|
| Corticosteroids | Manetta et al., 2018 [30], Sandercock & Soane 2011 [31], Sapolsky 1996 [32] |
|
| Magnesium | Pearce et al., 2017 [33], Mathew et al., 2013 [34], Bhudia et al., 2006 [35] |
|
| Mannitol | Kruger et al., 2013 [36] |
|
| Lidocaine | Hung et al., 2022 [37], Jovin et al., 2019 [38] |
|
| Barbiturates | Jovin et al., 2019 [38] |
|
| Propofol | Li et al., 2018 [39], Kumagai et al., 2006 [40], Roach et al., 1999 [41] |
|
| Inhaled anesthetics | Bickler et al., 2005 [42], Chen et al., 2017 [43] |
|
| Ketamine | Hudetz et al., 2009 [44], Avidan et al. 2017 [45] |
|
| Nitric Oxide | Linardi et al., 2021 [46] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, P.; Winter, M.; Mahr, S.; Stelzmueller, M.-E.; Zimpfer, D.; Ehrlich, M. Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation. Bioengineering 2024, 11, 775. https://doi.org/10.3390/bioengineering11080775
Werner P, Winter M, Mahr S, Stelzmueller M-E, Zimpfer D, Ehrlich M. Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation. Bioengineering. 2024; 11(8):775. https://doi.org/10.3390/bioengineering11080775
Chicago/Turabian StyleWerner, Paul, Martin Winter, Stephané Mahr, Marie-Elisabeth Stelzmueller, Daniel Zimpfer, and Marek Ehrlich. 2024. "Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation" Bioengineering 11, no. 8: 775. https://doi.org/10.3390/bioengineering11080775
APA StyleWerner, P., Winter, M., Mahr, S., Stelzmueller, M.-E., Zimpfer, D., & Ehrlich, M. (2024). Cerebral Protection Strategies in Aortic Arch Surgery—Past Developments, Current Evidence, and Future Innovation. Bioengineering, 11(8), 775. https://doi.org/10.3390/bioengineering11080775









