Alveolar Ridge Preservation Using a Novel Species-Specific Collagen-Enriched Deproteinized Bovine Bone Mineral: Histological Evaluation of a Prospective Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Histology
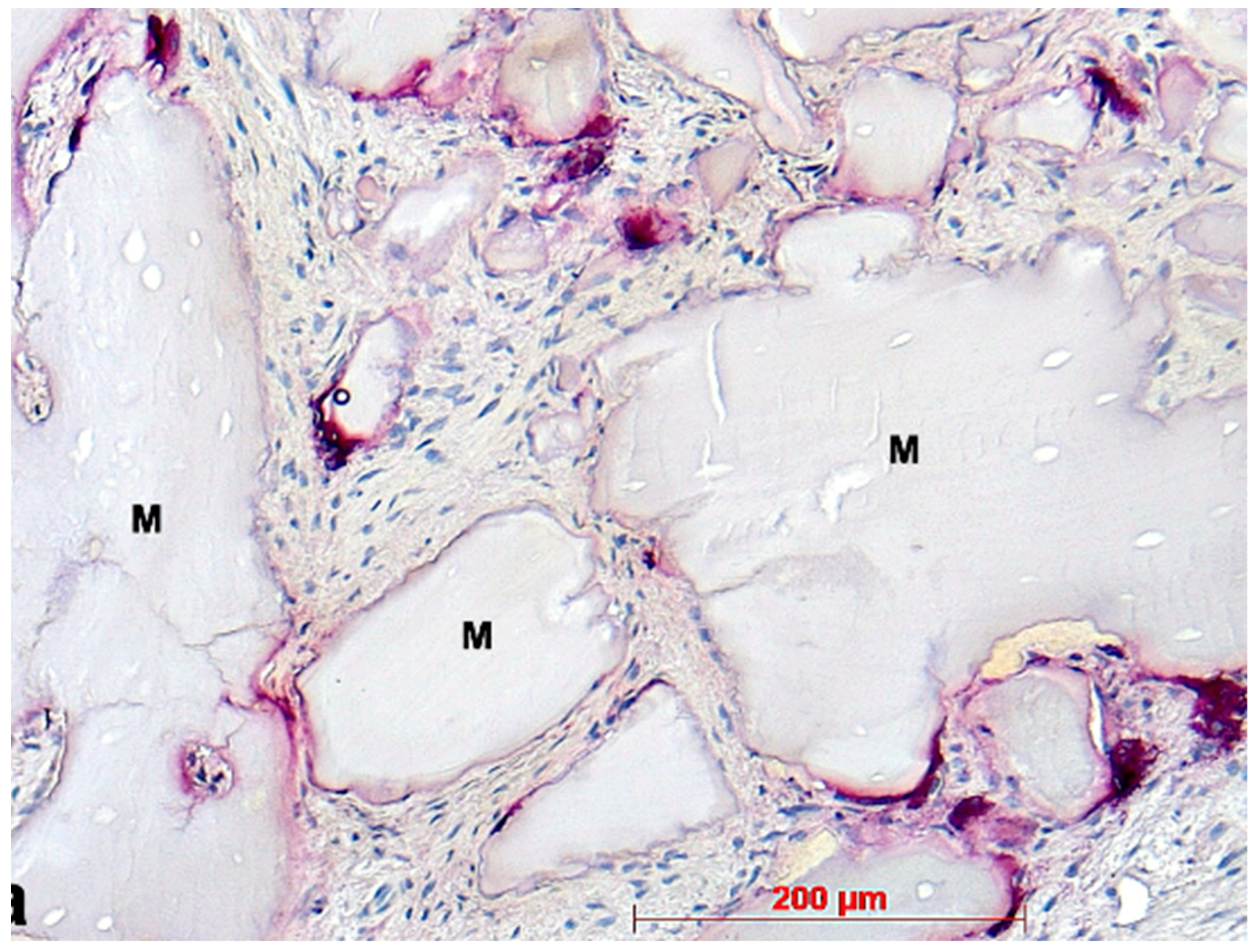
2.2. Immunohistochemistry
2.3. Histological Evaluation
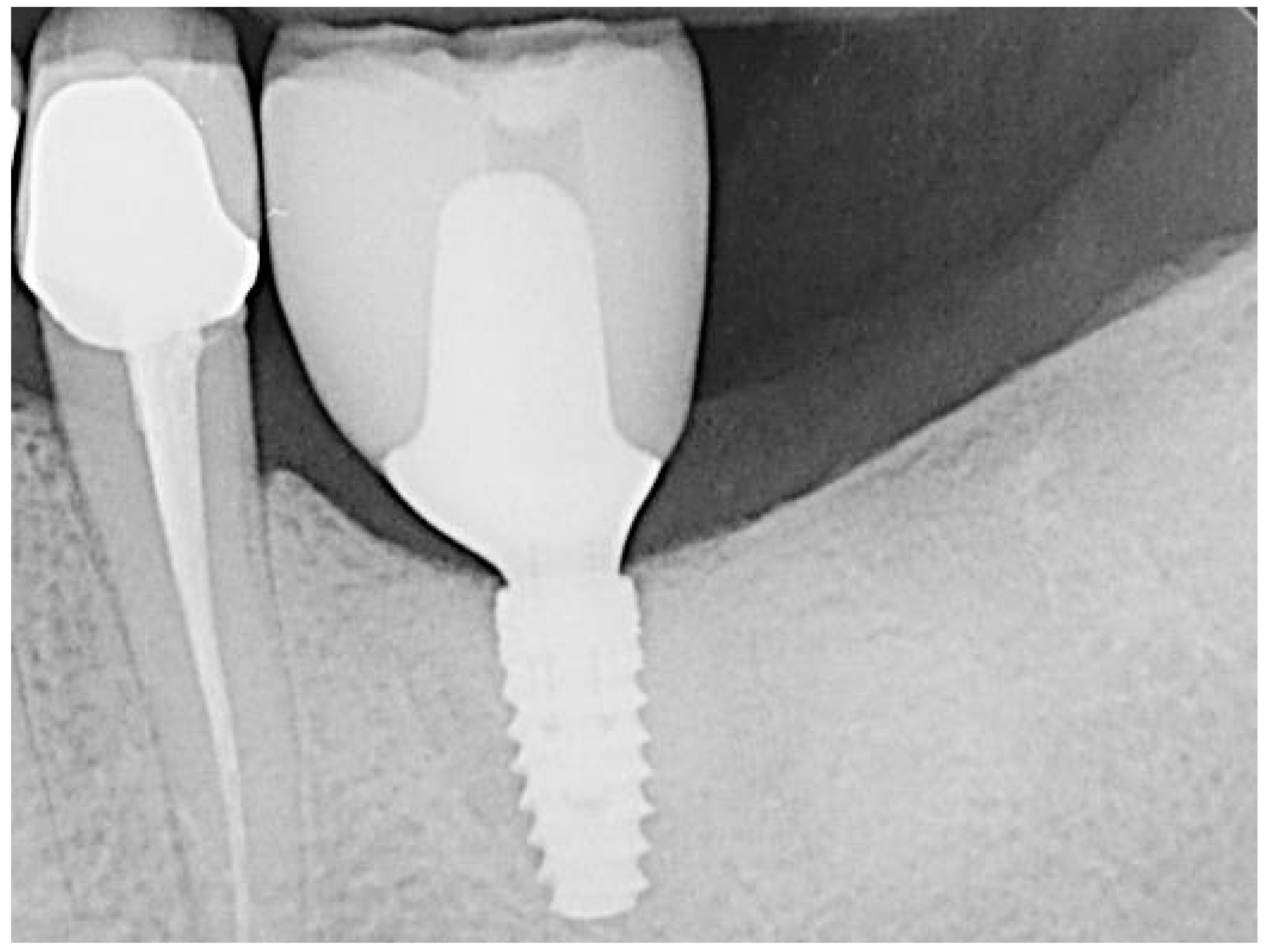
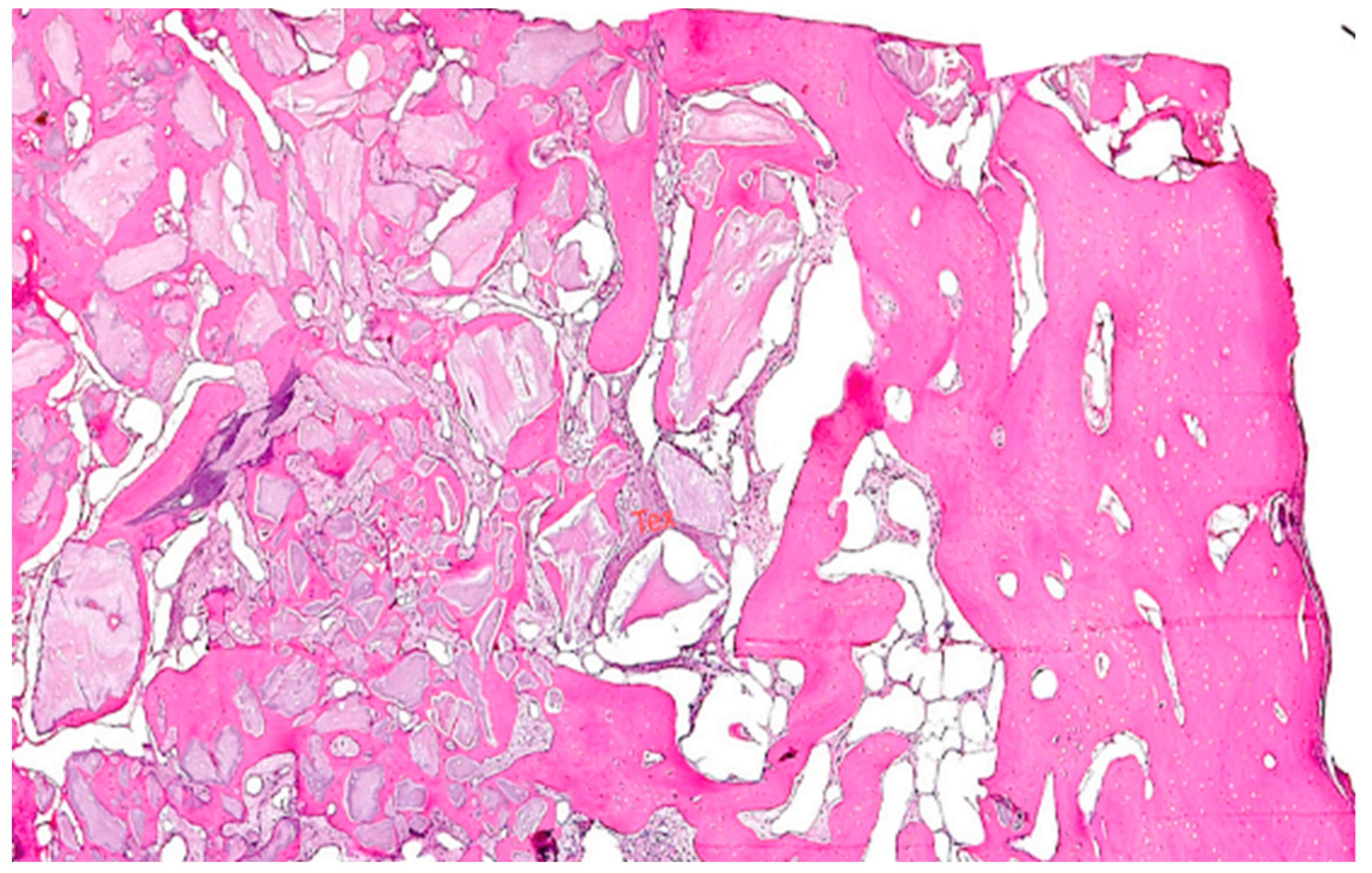
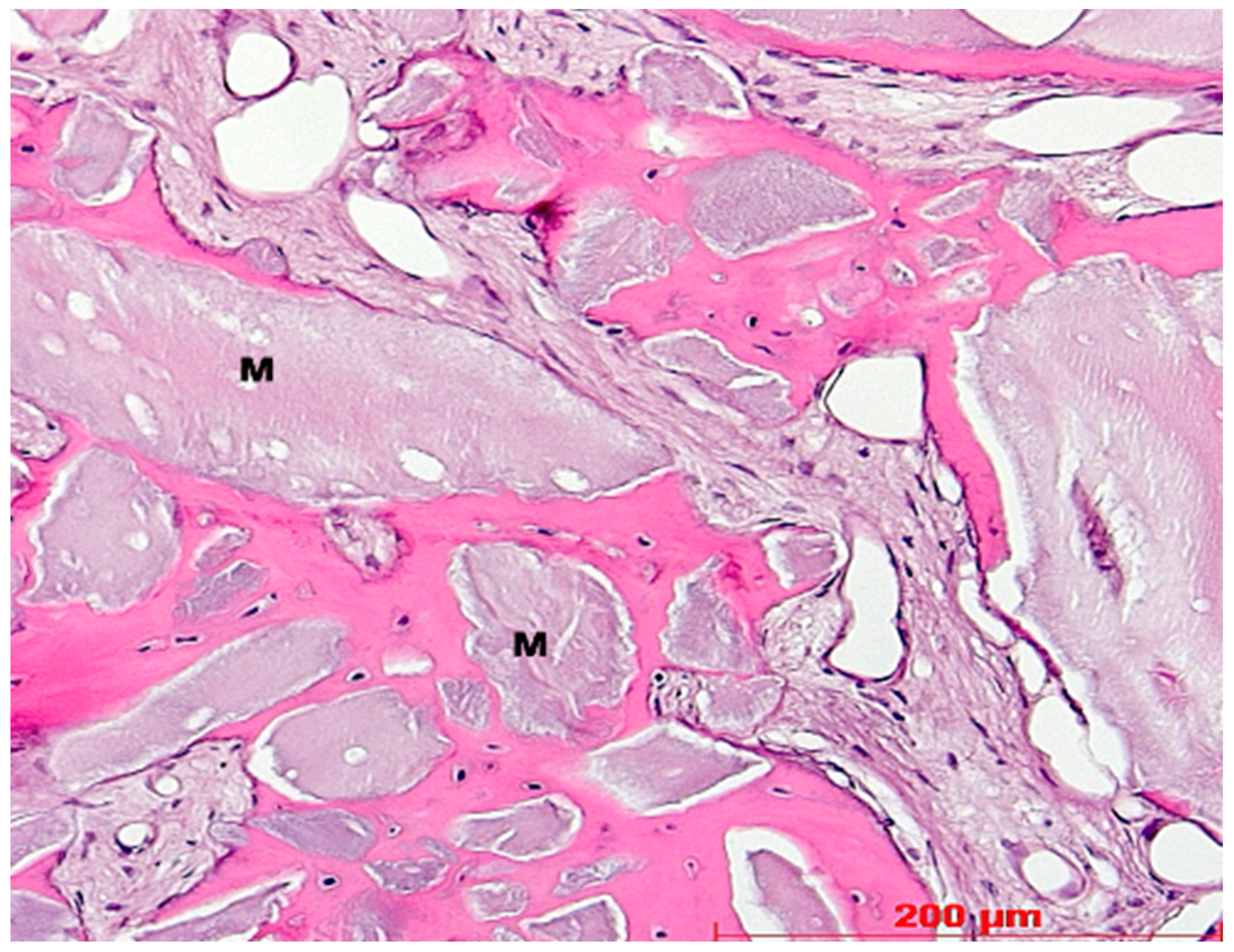
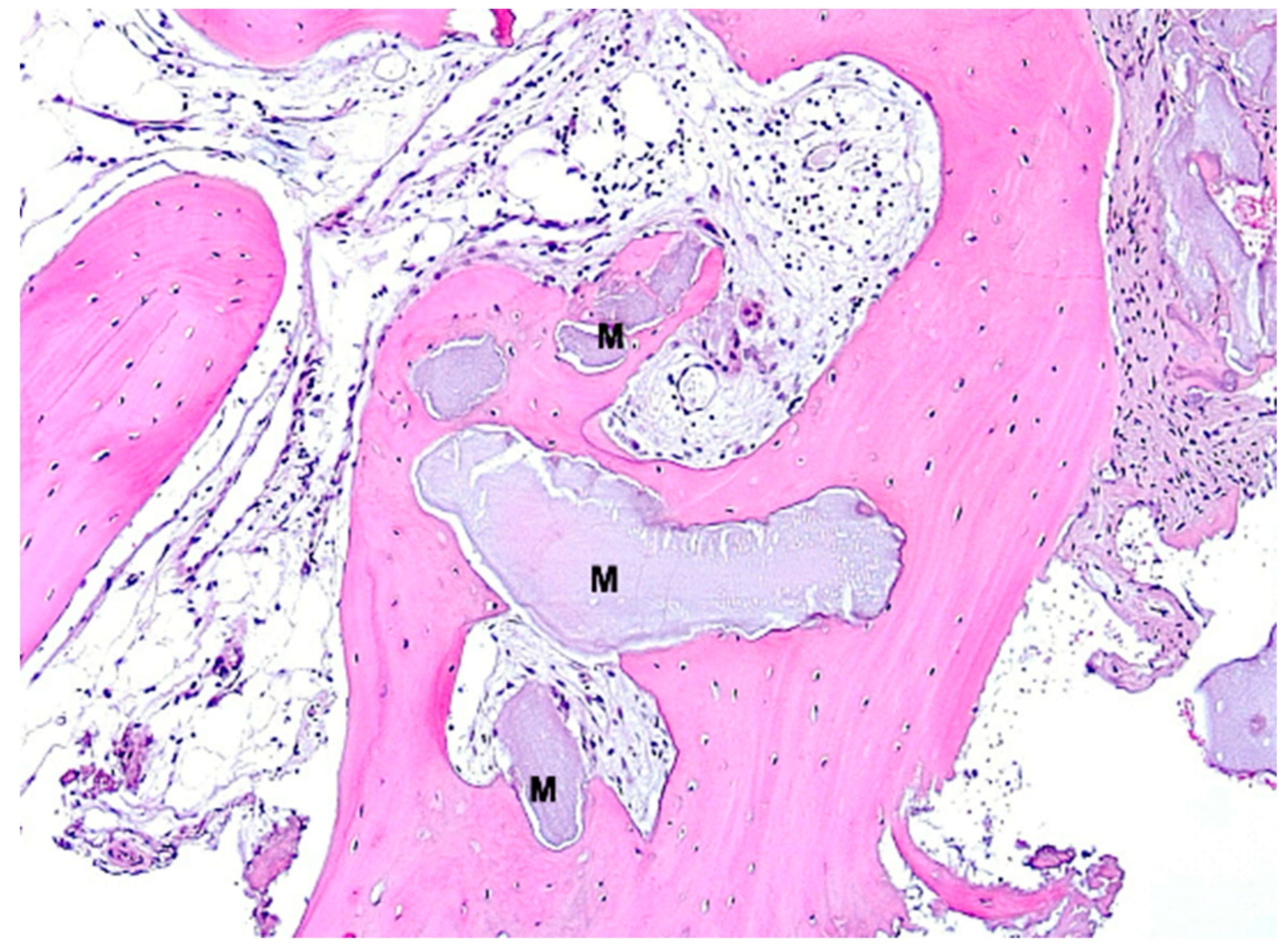
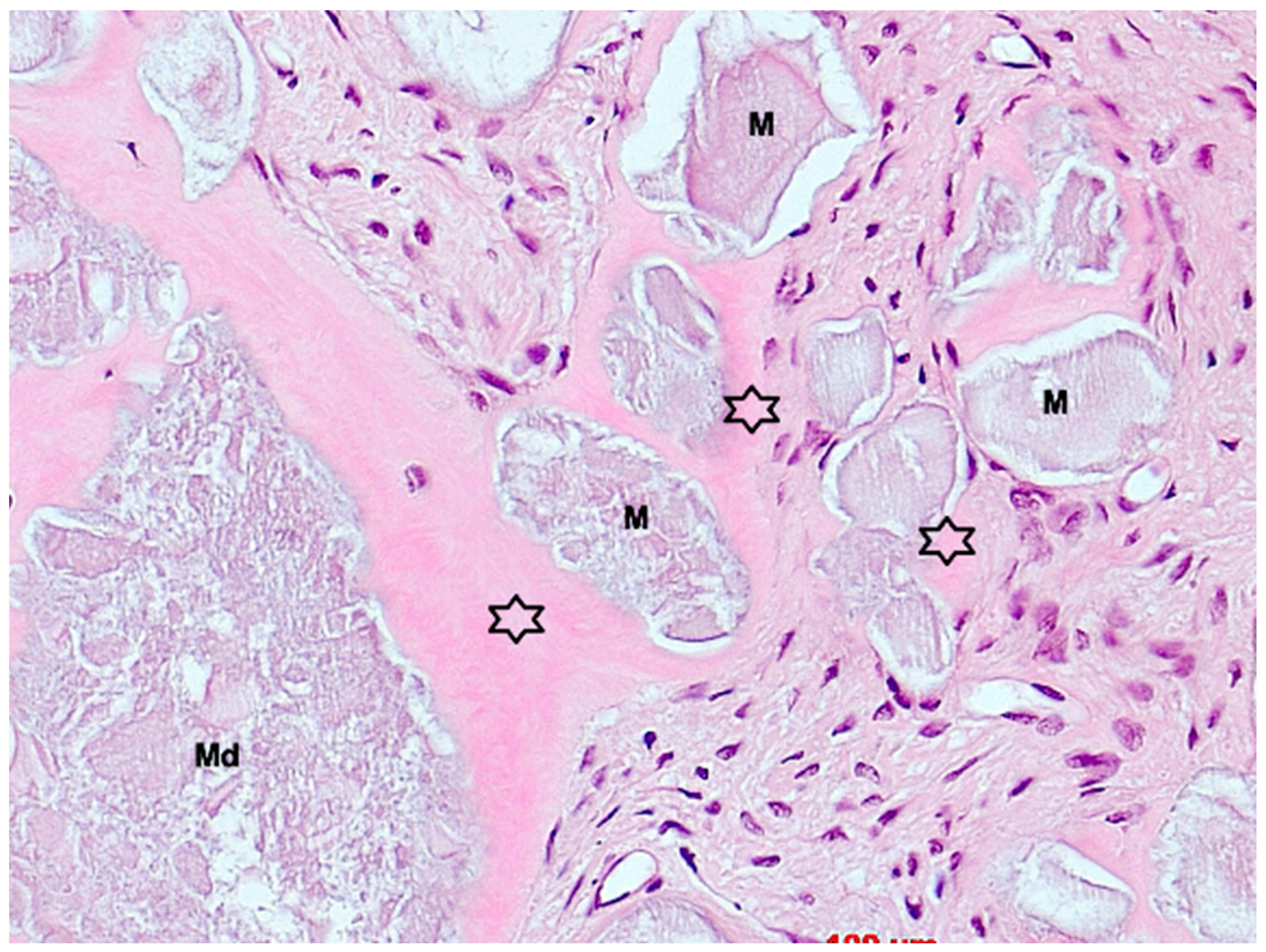
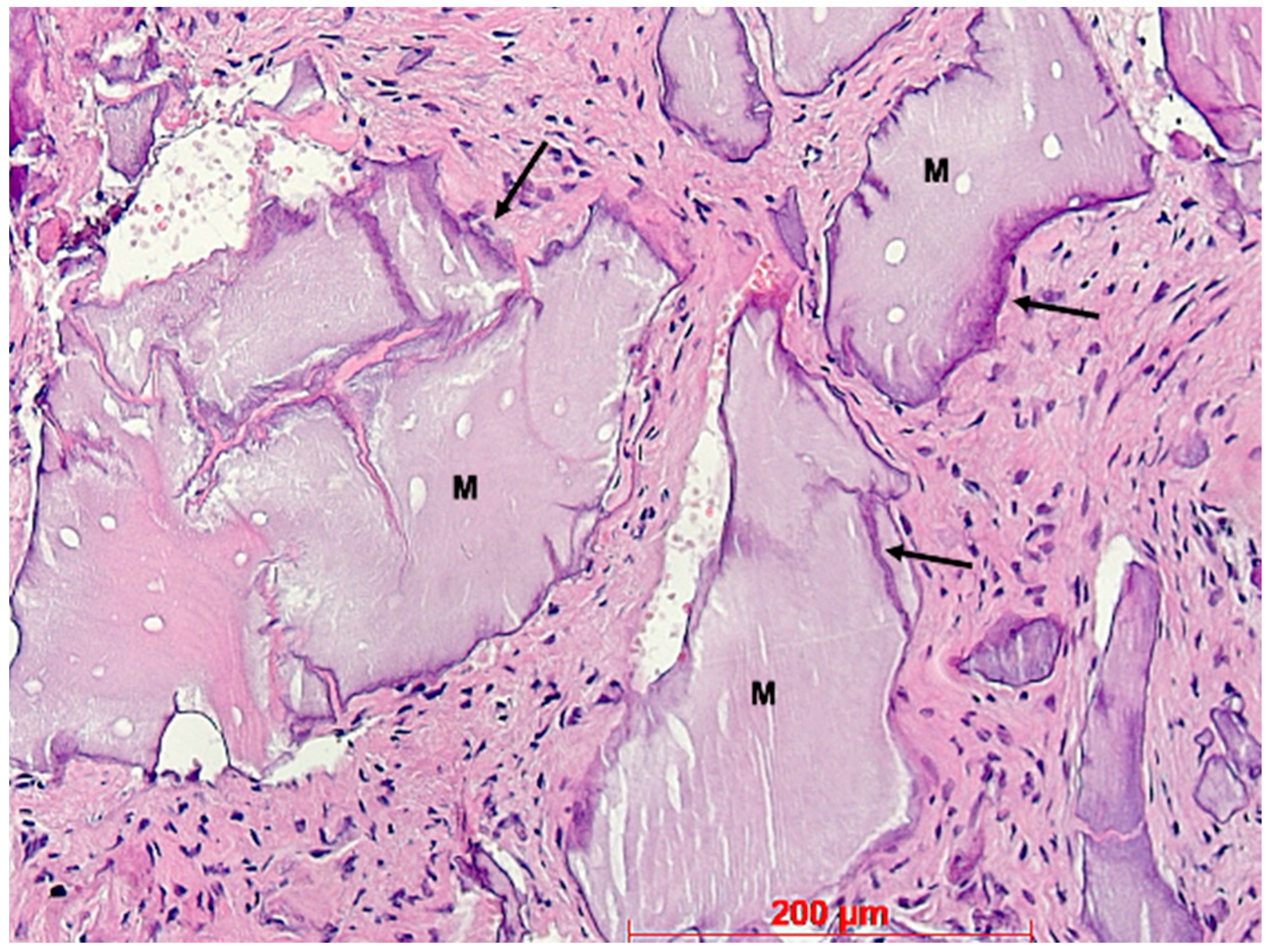
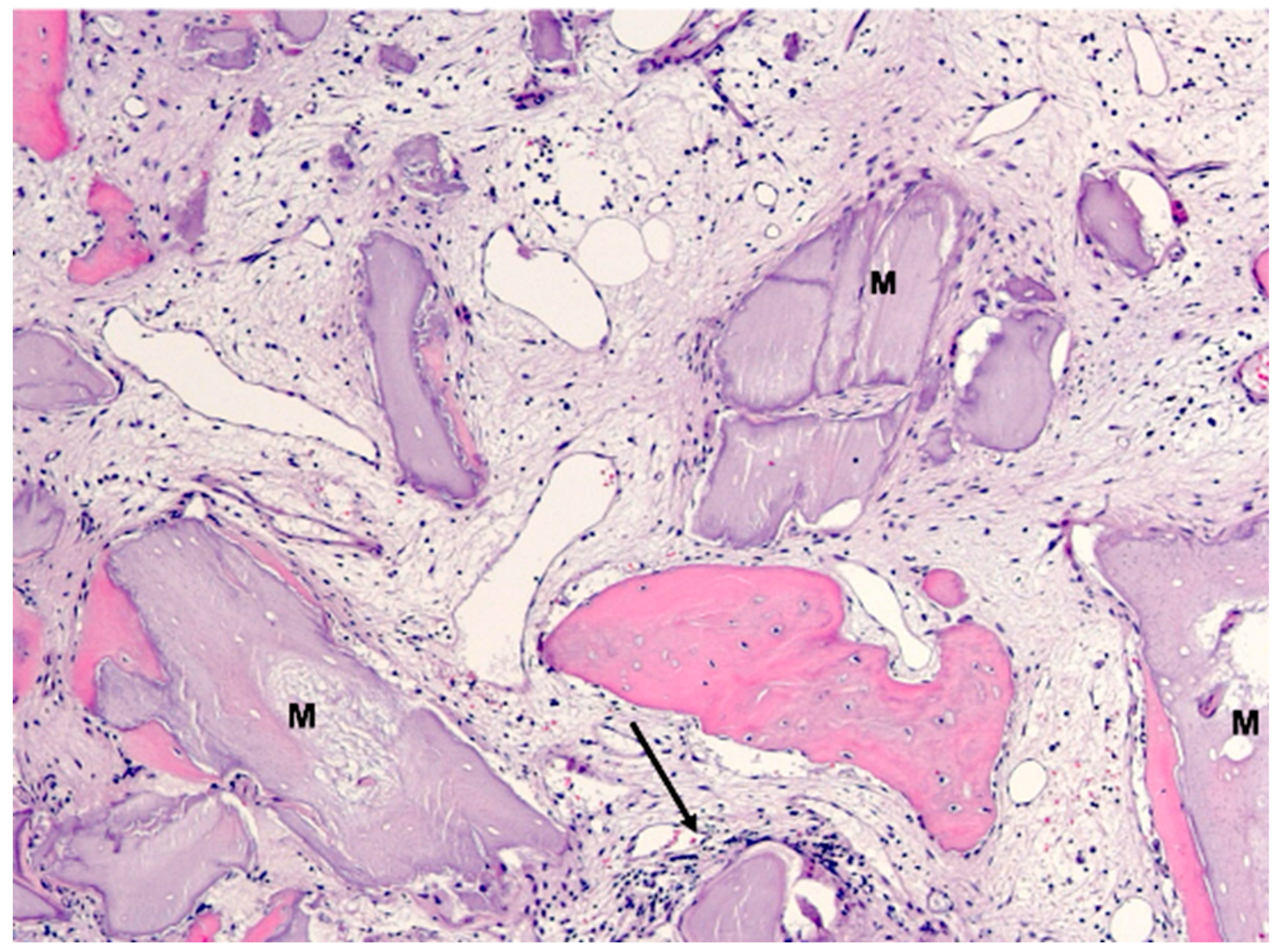
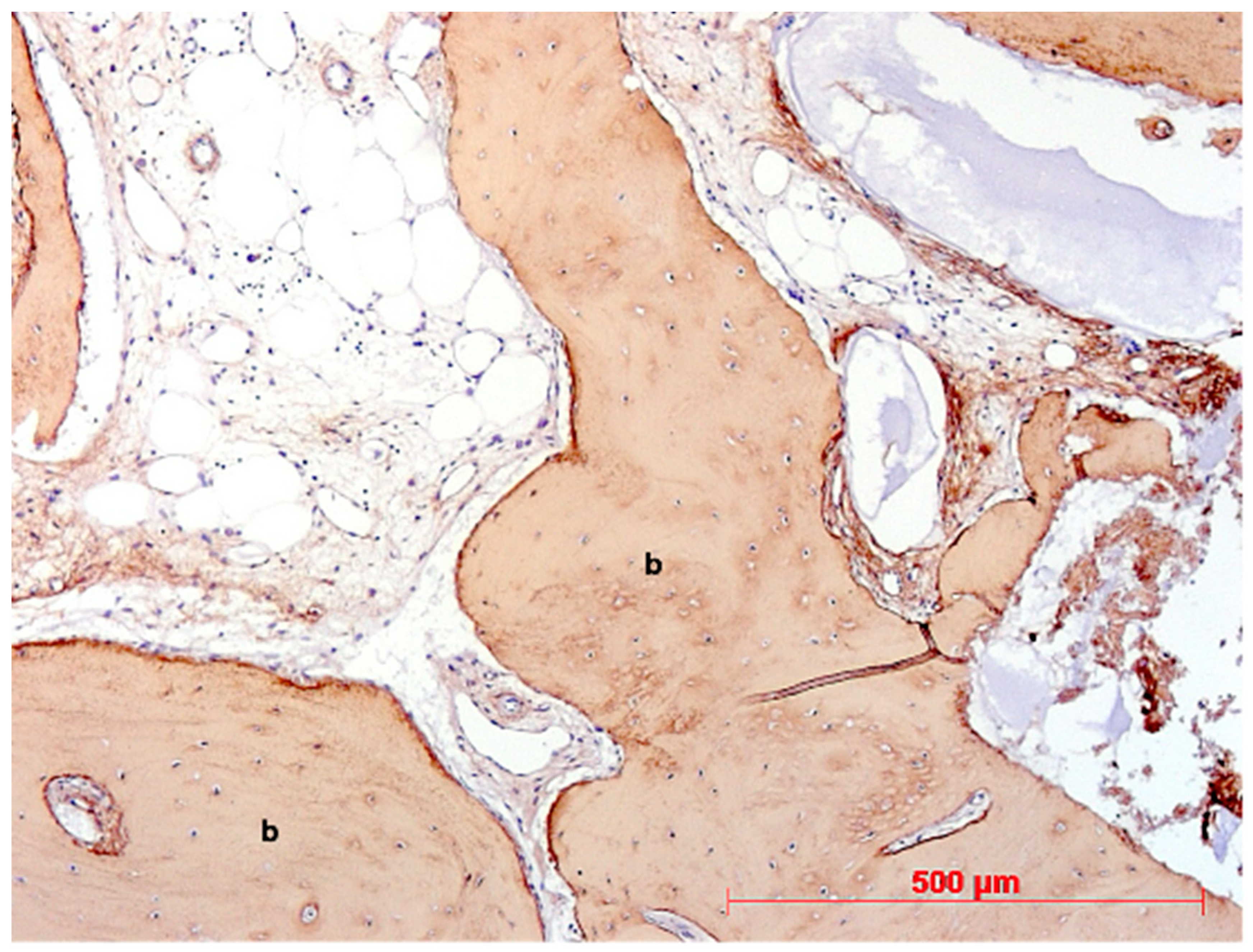
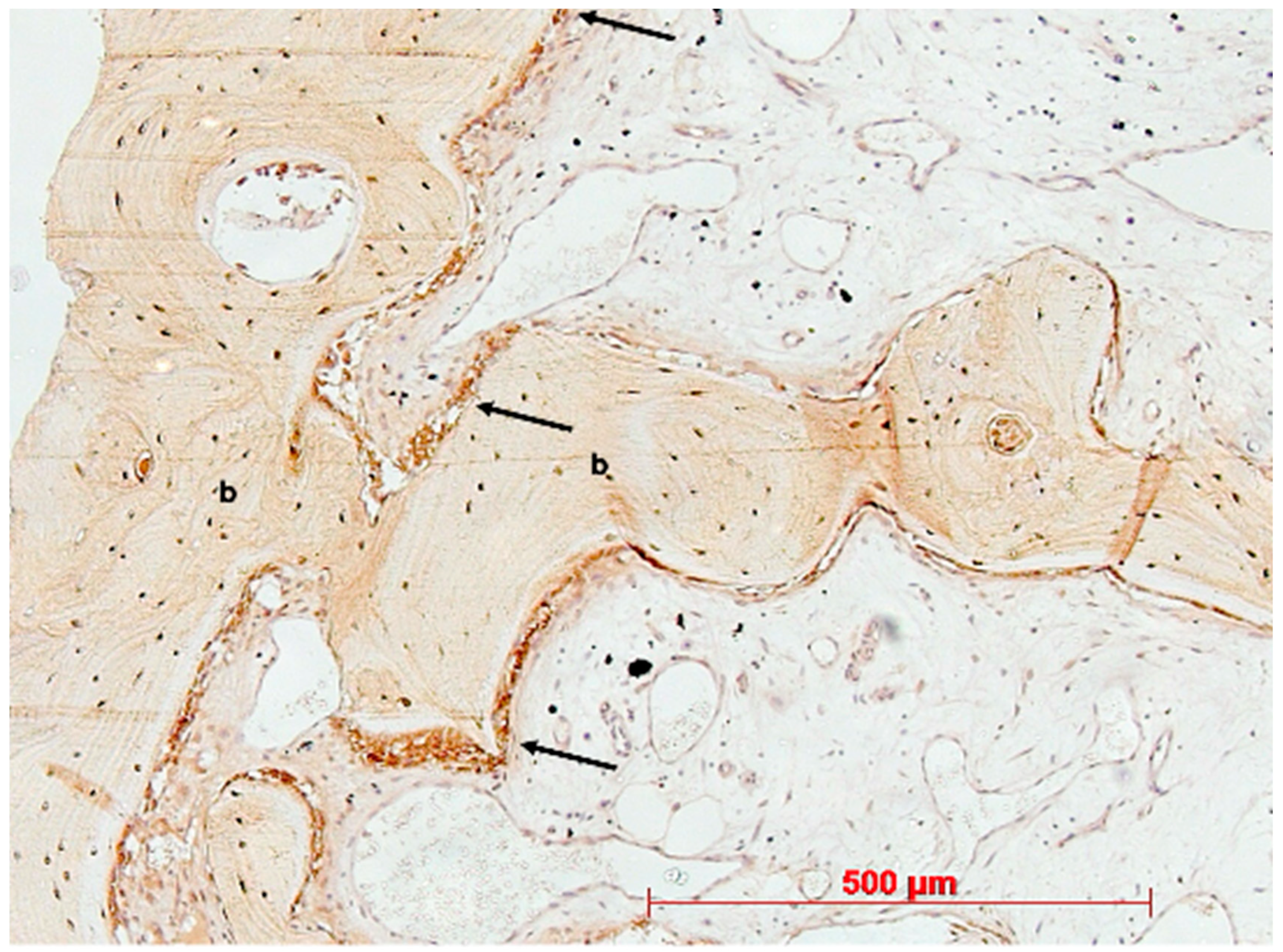
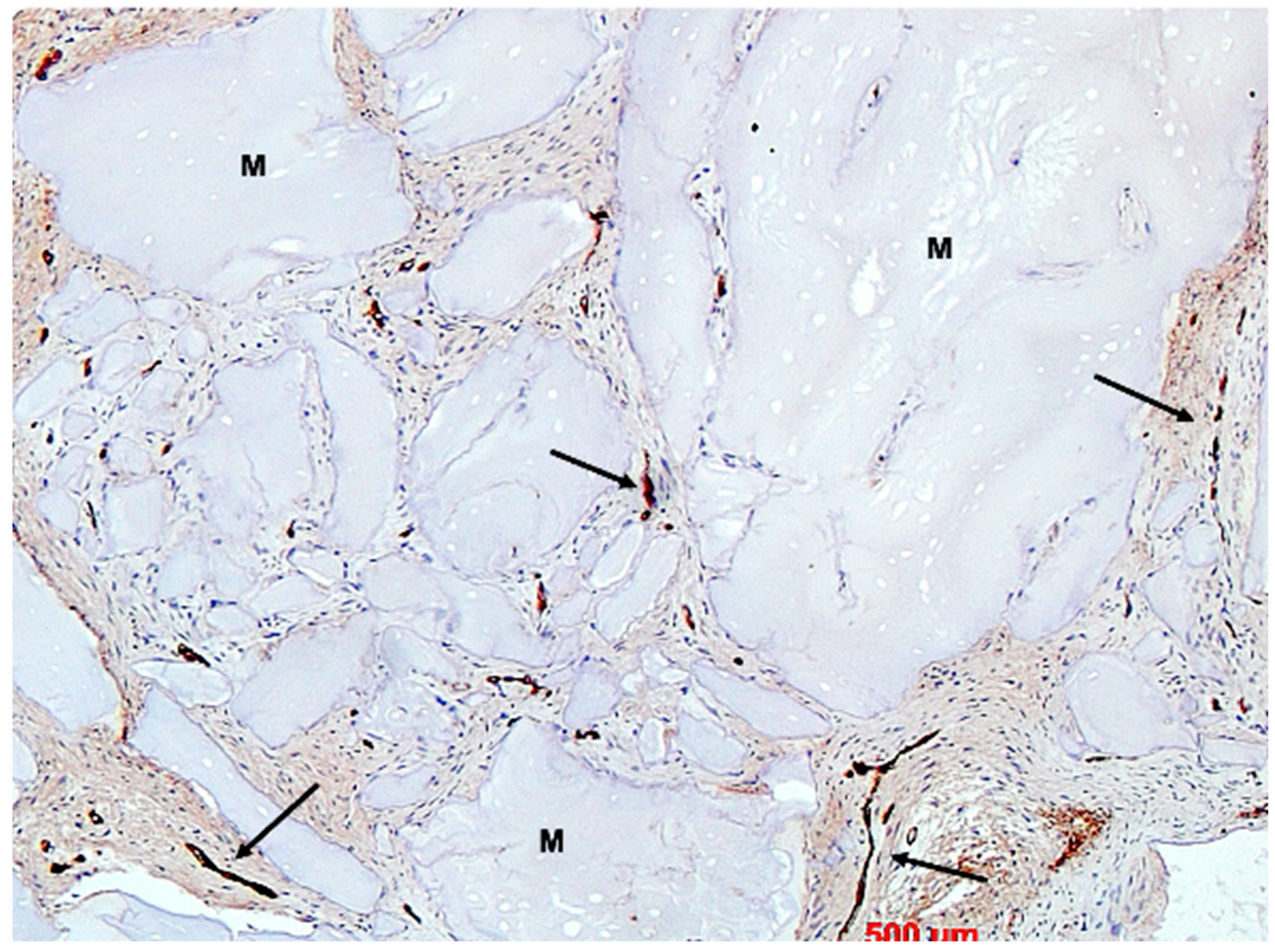
3. Results
4. Discussion
- −
- Surgical technique and sample collection: However, it is considered simple, and the risk is rather low.
- −
- Laboratory processing and histological evaluation: These were conducted by a highly experienced team with decades of experience, and the risk in this case is also considered low.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Oral Health Status Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Åstrøm, A.N.; Mastrovito, B.; Sannevik, J.; Tsakos, G. Role of behavioural and age-related factors in the long-term impact of tooth loss on oral health-related quality of life: A 25-year follow-up of Swedish older adults. Community Dent. Oral Epidemiol. 2022, 51, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Moraschini, V.; Poubel, L.d.C.; Ferreira, V.; Barboza, E.d.S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Tarnow, D. The etiology of hard-and soft-tissue deficiencies at dental implants: A narrative review. J. Clin. Periodontol. 2018, 89 (Suppl. S1), S291–S303. [Google Scholar]
- Agrawal, K.K.; Rao, J.; Anwar, M.; Singh, K.; Himanshu, D. Flapless vs flapped implant insertion in patients with controlled type 2 diabetes subjected to delayed loading: 1-year follow-up results from a randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 403–413. [Google Scholar] [PubMed]
- Vallecillo-Rivas, M.; Reyes-Botella, C.; Vallecillo, C.; Lisbona-González, M.J.; Vallecillo-Capilla, M.; Olmedo-Gaya, M.V. Comparison of Implant Stability between Regenerated and Non-Regenerated Bone. A Prospective Cohort Study. J. Clin. Med. 2021, 10, 3220. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; da Silva, J.C.C.; de Mendonça, A.F.; Lindhe, J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin. Oral Implant. Res. 2015, 26, 407–412. [Google Scholar] [CrossRef]
- Barone, A.; Ricci, M.; Tonelli, P.; Santini, S.; Covani, U. Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin. Oral Implant. Res. 2013, 24, 1231–1237. [Google Scholar] [CrossRef]
- Sculean, A.; Stavropoulos, A.; Bosshardt, D.D. Self-regenerative capacity of intra-oral bone defects. J. Clin. Periodontol. 2019, 46, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar] [PubMed]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Huynh-Ba, G.; Pjetursson, B.E.; Sanz, M.; Cecchinato, D.; Ferrus, J.; Lindhe, J.; Lang, N.P. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin. Oral Implant. Res. 2009, 21, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Shahim, K.; Reyes, M.; Katsaros, C.; Buser, D. Soft Tissue Alterations in Esthetic Postextraction Sites: A 3-Dimensional Analysis. J. Dent. Res. 2015, 94 (Suppl. S9), 187S–193S. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jung, R.E.; Avila-Ortiz, G.; Blanco, J.; Cosyn, J.; Fickl, S.; Figuero, E.; Goldstein, M.; Graziani, F.; Madianos, P.; et al. Management of the extraction socket and timing of implant placement: Consensus report and clinical recommendations of group 3 of the XV European Workshop in Periodontology. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 183–194. [Google Scholar] [CrossRef]
- Wang, R.E.; Lang, N.P. Ridge preservation after tooth extraction. Clin. Oral Implant. Res. 2012, 23 (Suppl. S21), 147–156. [Google Scholar] [CrossRef]
- Landsberg, C.J.; Bichacho, N. A modified surgical/prosthetic approach for optimal single implant supported crown. Part I—The socket seal surgery. Pract. Periodontics Aesthetic Dent. 1994, 6, 11–17; quiz 19. [Google Scholar]
- Fickl, S.; Kauffmann, F.; Stappert, C.; Kauffmann, A.; Schlagenhauf, U. Scar Tissue Formation Following Alveolar Ridge Preservation: A Case Control Study. Int. J. Periodontics Restor. Dent. 2018, 38, E1–E7. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, I.-P.; Mei, C.-C.; Tang, P.-Z.; Hong, H.-H. A Socket seal technique with the use of autologous dental roots for socket seal: A case series. J. Oral Implant. 2023, 49, 473–484. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, A.; Soto-Peñaloza, D.; Gómez, M.; Peñarrocha-Oltra, D.; Alarcón, M.A. Socket seal surgery techniques in the esthetic zone: A systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. Int. J. Implant. Dent. 2021, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Debel, M.; Toma, S.; Vandenberghe, B.; Brecx, M.C.; Lasserre, J.F. Alveolar ridge dimensional changes after two socket sealing techniques. A pilot randomized clinical trial. Clin. Oral Investig. 2021, 25, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Liu, X.; Cai, X.; Sun, L.; Li, T. Could the socket shield technique be better than conventional immediate implantation? A meta-analysis. Clin. Oral Investig. 2022, 26, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.B.; Zuhr, O.; Schupbach, P.; Rebele, S.F.; Emmanouilidis, N.; Fickl, S. The socket-shield technique: A proof-of-principle report. J. Clin. Periodontol. 2010, 37, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Peñarrocha, M.; Uribe, R.; Balaguer, J. Immediate implants after extraction. A review of the current situation. Med. Oral. 2004, 9, 234–242. [Google Scholar] [PubMed]
- Glocker, M.; Attin, T.; Schmidlin, P.R. Ridge Preservation with Modified “Socket-Shield” Technique: A Methodological Case Series. Dent. J. 2014, 2, 11–21. [Google Scholar] [CrossRef]
- Pohl, S.; Binderman, I.; Božić, D.; Shapira, L.; Venkataraman, N. Effectiveness of Autologous Tissue Grafts on Soft Tissue Ingrowth in Patients Following Partial Root Extraction with Socket Shield: A Retrospective Analysis of a Case Series. Int. J. Oral Maxillofac. Implant. 2021, 36, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, H.; Salama, M.; Du Toit, J. Partial Extraction Therapies (PET) Part 1: Maintaining Alveolar Ridge Contour at Pontic and Immediate Implant Sites. Int. J. Periodontics Restor. Dent. 2016, 36, 681–687. [Google Scholar] [CrossRef]
- Neumeyer, S. The Tissue Master Concept (TMC): Innovations for alveolar ridge preservation. Int. J. Esthet. Dent. 2017, 12, 246–257. [Google Scholar]
- Solakoglu, O.; Götz, W.; Heydecke, G.; Schwarzenbach, H. Histological and immunohistochemical comparison of two different allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans. Clin. Implant. Dent. Relat. Res. 2019, 21, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- van Orten, A.; Goetz, W.; Bilhan, H. Tooth-Derived Granules in Combination with Platelet-Rich Fibrin (“Sticky Tooth”) in Socket Preservation: A Histological Evaluation. Dent. J. 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López del Amo, F.; Monje, A. Efficacy of biologics for alveolar ridge preservation/reconstruction and implant site development: An American Academy of Periodontology best evidence systematic review. J. Periodontol. 2022, 93, 1827–1847. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Francetti, L.; Weinstein, R.; Del Fabbro, M. Histomorphometric Results After Postextraction Socket Healing with Different Biomaterials: A Systematic Review of the Literature and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 1001–1017. [Google Scholar] [CrossRef]
- Offner, D.; de Grado, G.F.; Meisels, I.; Pijnenburg, L.; Fioretti, F.; Benkirane-Jessel, N.; Musset, A.-M. Bone Grafts, Bone Substitutes and Regenerative Medicine Acceptance for the Management of Bone Defects Among French Population: Issues about Ethics, Religion or Fear? Cell Med. 2019, 11, 2155179019857661. [Google Scholar] [CrossRef] [PubMed]
- Elian, N.; Cho, S.-C.; Froum, S.; Smith, R.B.; Tarnow, D.P. A simplified socket classification and repair technique. Pract. Proced. Aesthetic Dent. 2007, 19, 99–104. [Google Scholar] [PubMed]
- Jung, R.E.; Philipp, A.; Annen, B.M.; Signorelli, L.; Thoma, D.S.; Hämmerle, C.H.; Schmidlin, P. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 90–98. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 195–223. [Google Scholar] [CrossRef]
- Seyssens, L.; Eghbali, A.; Christiaens, V.; De Bruyckere, T.; Doornewaard, R.; Cosyn, J. A one-year prospective study on alveolar ridge preservation using collagen-enriched deproteinized bovine bone mineral and saddle connective tissue graft: A cone beam computed tomography analysis. Clin. Implant. Dent. Relat. Res. 2019, 21, 853–861. [Google Scholar] [CrossRef]
- Mardas, N.; Trullenque-Eriksson, A.; MacBeth, N.; Petrie, A.; Donos, N. Does ridge preservation following tooth extraction improve implant treatment outcomes: A systematic review: Group 4: Therapeutic concepts & methods. Clin. Oral Implant. Res. 2015, 26 (Suppl. S21), 180–201. [Google Scholar]


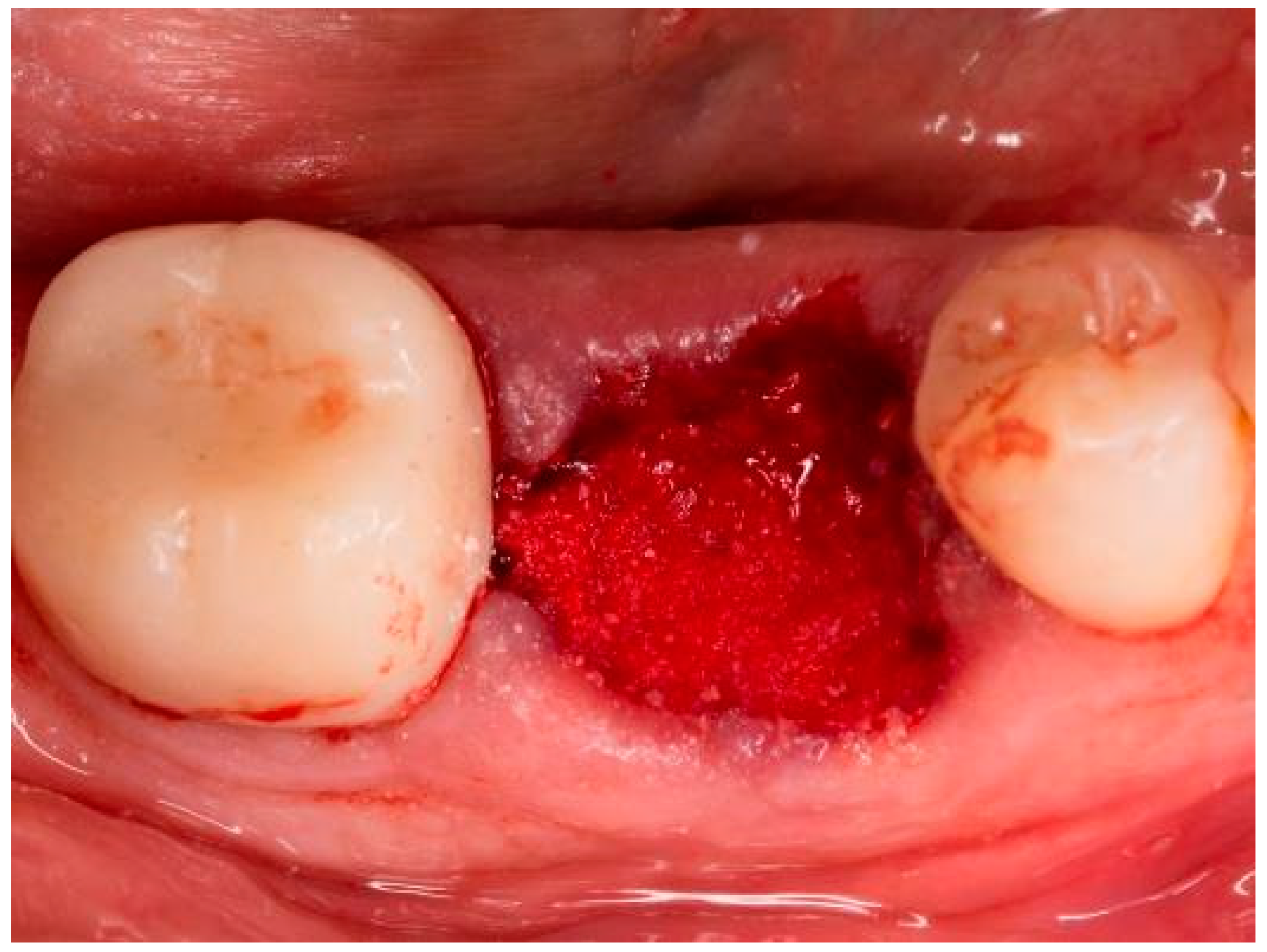
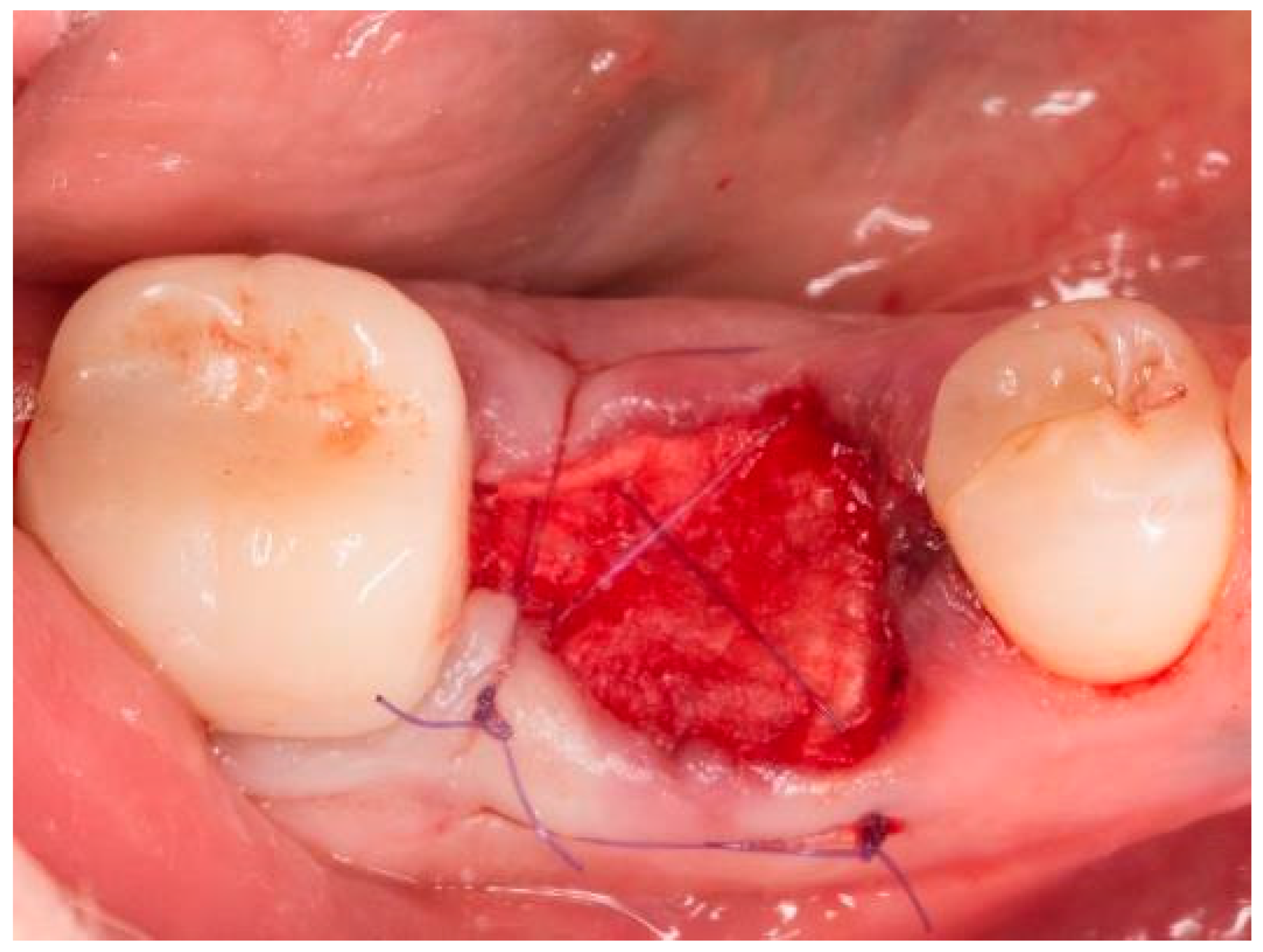

| Case No. | Sex | Age | Regio (FDA): | Reason for Tooth Loss: | Integration Period of the Graft: | Smoking Behavior: | General Medical History: |
|---|---|---|---|---|---|---|---|
| 1 | m | 42 | 25 | fracture | 130 | never | no abnormal medical history |
| 2 | m | 63 | 17 | endodontic | 319 | 10 cig./d | no abnormal medical history |
| 3 | m | 45 | 25 | unrestorable | 133 | never | no abnormal medical history |
| 4 | f | 77 | 36 | fracture | 207 | former smoker | type 2 diabetes, medicated |
| 5 | f | 62 | 46 | endodontic | 190 | never | no abnormal medical history |
| 6 | m | 54 | 37 | endodontic | 253 | former smoker | type 2 diabetes, medicated |
| 7 | m | 55 | 37 | tooth tilting | 292 | never | atopic individual |
| 8 | f | 43 | 26 | endodontic | 187 | former smoker | no abnormal medical history |
| 9 | m | 45 | 25 | unrestorable | 146 | former smoker | atopic individual |
| 10 | f | 44 | 15 | endodontic | 184 | never | no abnormal medical history |
| Antibody | Isotype | Manufacturer | Incubation Protocol |
|---|---|---|---|
| collagen type I | rabbit monoclonal | Abcam (Cambridge, UK) | 1:400, 1 h, rt |
| osteocalcin | mouse monoclonal | Takara (Otsu, Shiga, Japan) | 1:100, 1 h, rt |
| von Willebrand Factor (vWF) | rabbit polyclonal | Linaris (Wertheim, Germany) | 1:200, 1 h, rt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Orten, A.; Goetz, W.; Bilhan, H. Alveolar Ridge Preservation Using a Novel Species-Specific Collagen-Enriched Deproteinized Bovine Bone Mineral: Histological Evaluation of a Prospective Case Series. Bioengineering 2024, 11, 665. https://doi.org/10.3390/bioengineering11070665
van Orten A, Goetz W, Bilhan H. Alveolar Ridge Preservation Using a Novel Species-Specific Collagen-Enriched Deproteinized Bovine Bone Mineral: Histological Evaluation of a Prospective Case Series. Bioengineering. 2024; 11(7):665. https://doi.org/10.3390/bioengineering11070665
Chicago/Turabian Stylevan Orten, Andreas, Werner Goetz, and Hakan Bilhan. 2024. "Alveolar Ridge Preservation Using a Novel Species-Specific Collagen-Enriched Deproteinized Bovine Bone Mineral: Histological Evaluation of a Prospective Case Series" Bioengineering 11, no. 7: 665. https://doi.org/10.3390/bioengineering11070665
APA Stylevan Orten, A., Goetz, W., & Bilhan, H. (2024). Alveolar Ridge Preservation Using a Novel Species-Specific Collagen-Enriched Deproteinized Bovine Bone Mineral: Histological Evaluation of a Prospective Case Series. Bioengineering, 11(7), 665. https://doi.org/10.3390/bioengineering11070665








