Pretreatment Sarcopenia and MRI-Based Radiomics to Predict the Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer
Abstract
1. Introduction
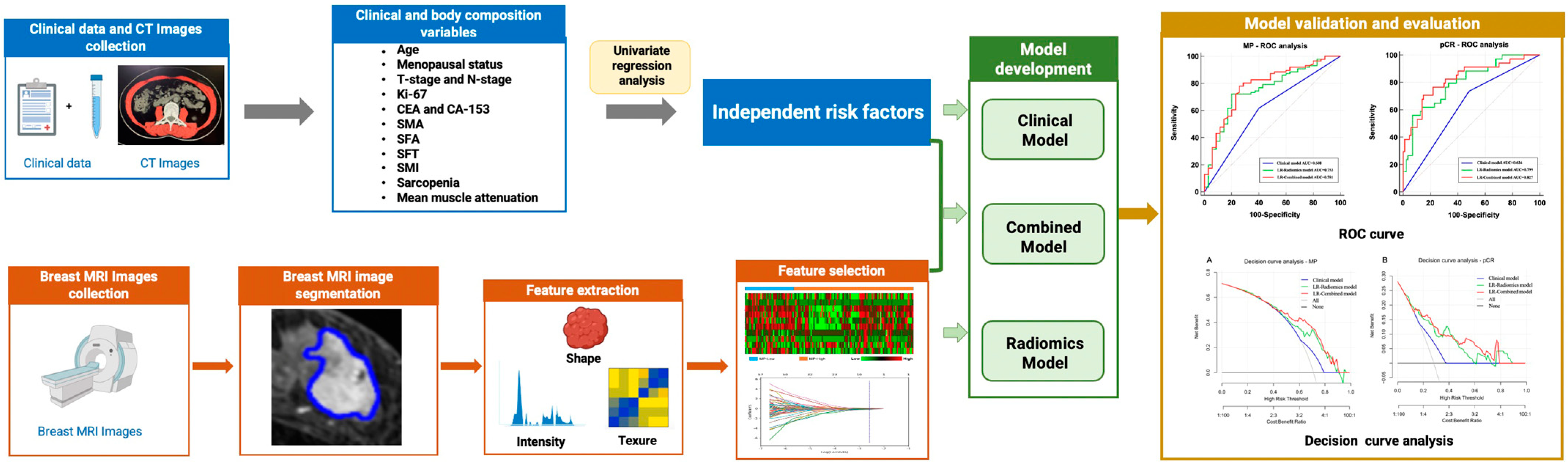
2. Materials and Methods
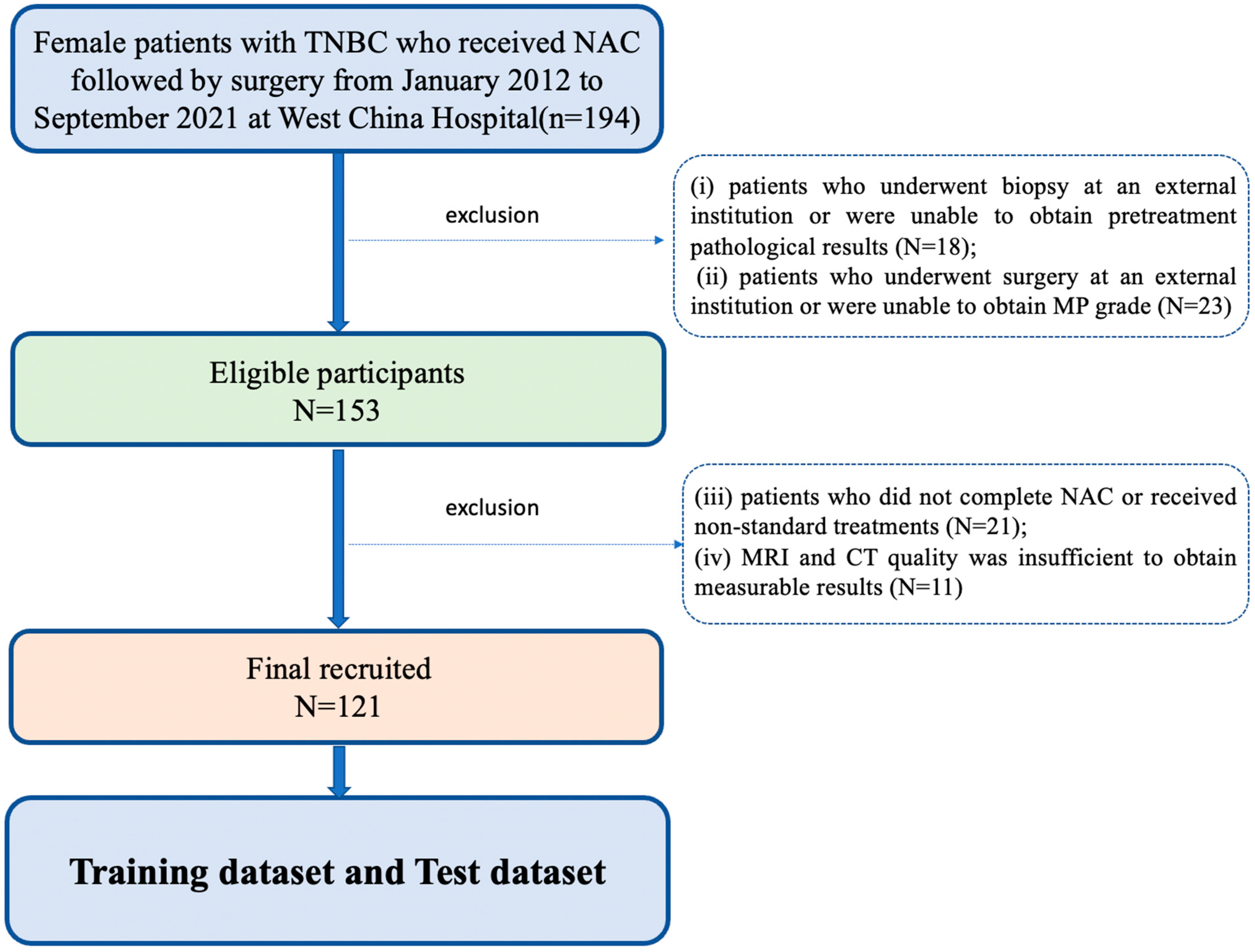
2.1. Patients
2.2. Clinical Feature Collection
2.3. Pathological Examination and Response to Treatment
2.4. Body Composition Quantification and Sarcopenia Assessment
2.5. MRI Protocol and Image Segmentation
2.6. Radiomics Feature Extraction
2.7. Model Development and Evaluation
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Clinical and Body Composition Variables Associated with NAC Efficacy
3.3. Radiomics Feature Selection
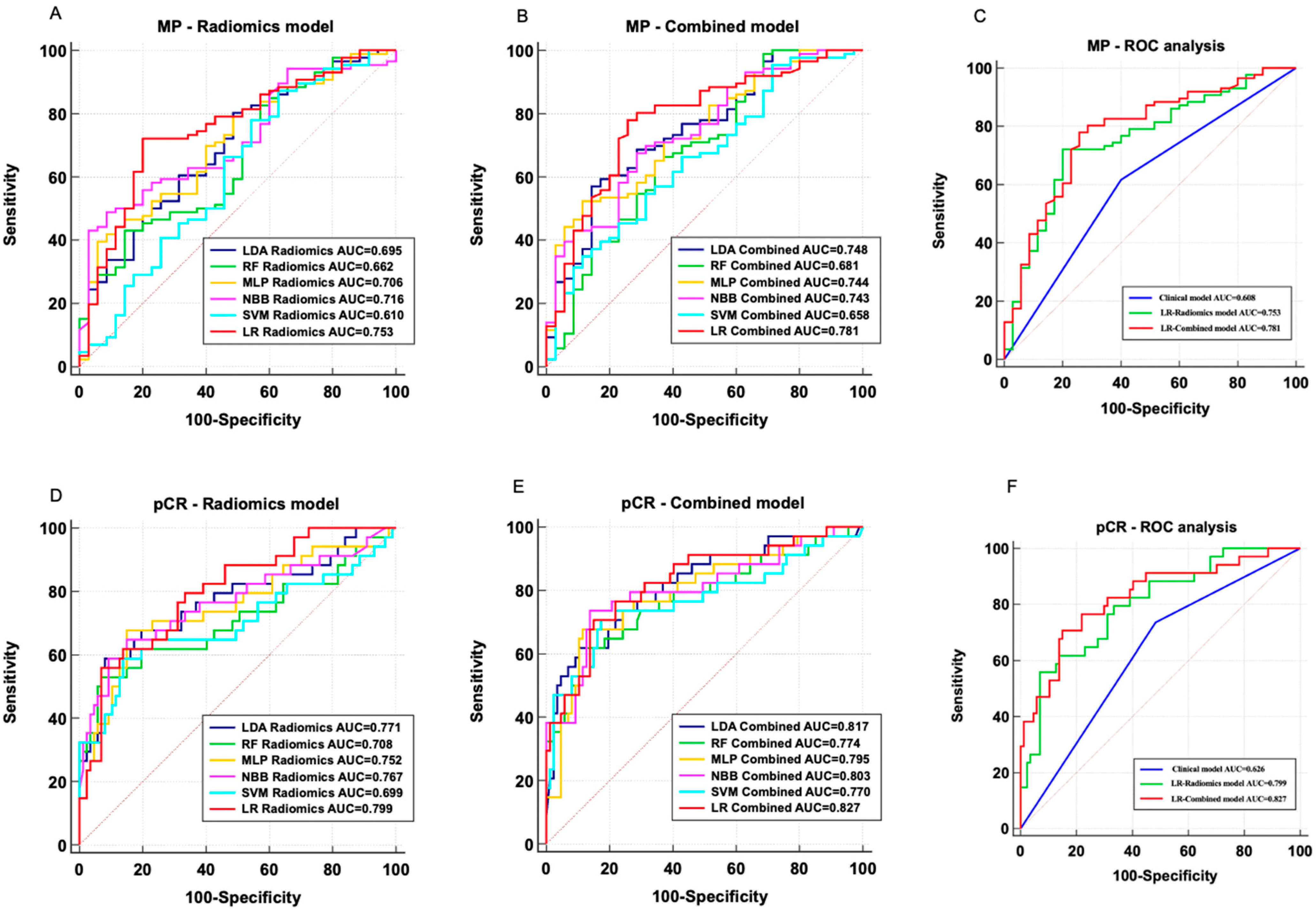
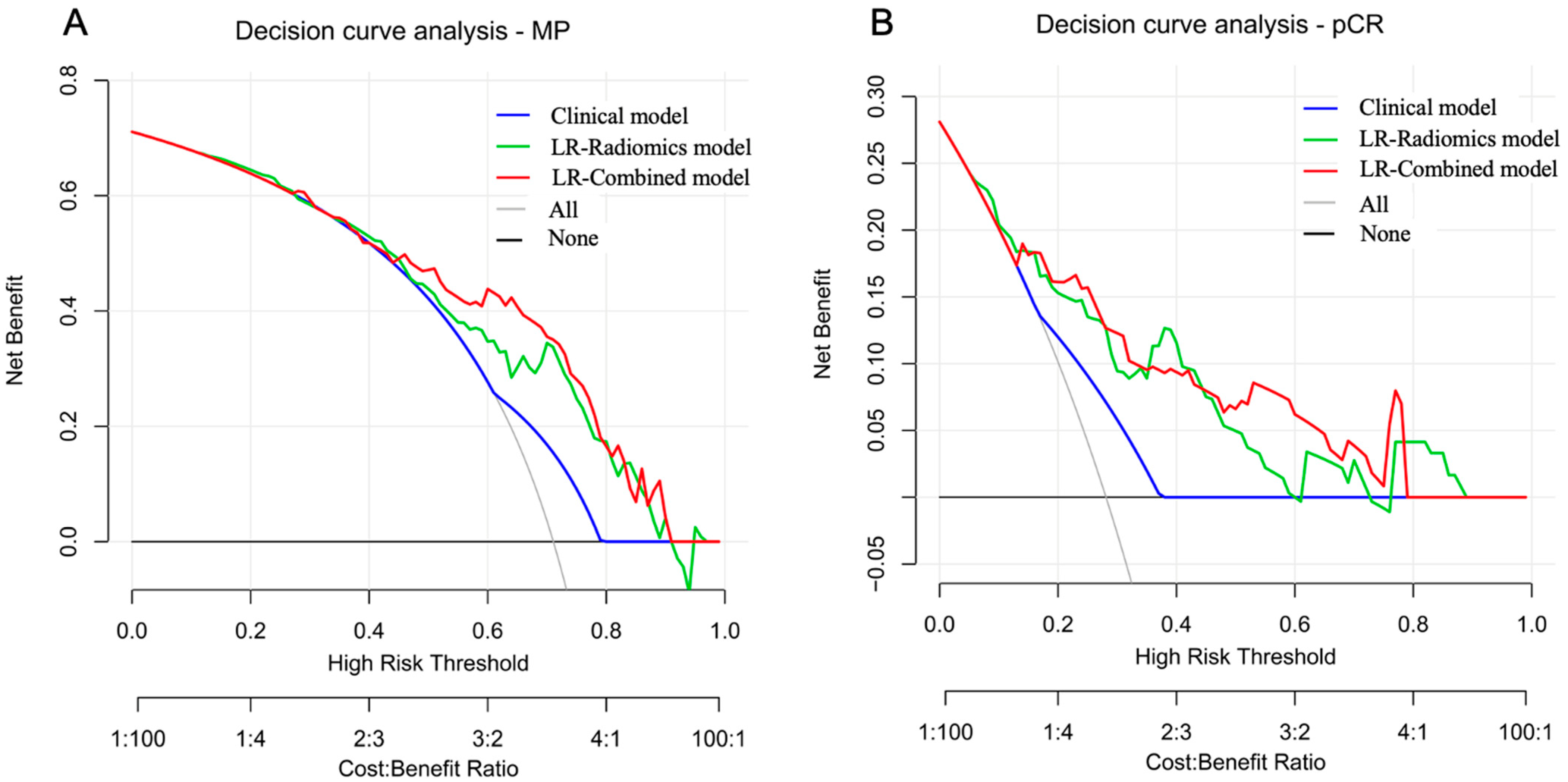
3.4. Performance Evaluation of MP-Low/High Prediction Models
3.5. Performance Evaluation of pCR/Non-pCR Prediction Models
3.6. Important Model Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marra, A.; Curigliano, G. Adjuvant and Neoadjuvant Treatment of Triple-Negative Breast Cancer with Chemotherapy. Cancer J. 2021, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 2012, 23 (Suppl. S6), vi7–vi12. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Chen, P.; Yin, X.; Sun, J.; Li, H.; Ren, G. Efficacy and safety of neoadjuvant chemotherapy regimens for triple-negative breast cancer: A network meta-analysis. Aging 2019, 11, 6286–6311. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. KEYNOTE-522 Investigators. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Zhang, H.; Zhang, S.; Duan, X.; Ye, J.; Xu, L.; Zhao, J.; Cheng, Y.; Liu, Q. Prognostic value of residual cancer burden and Miller-Payne system after neoadjuvant chemotherapy for breast cancer. Gland Surg. 2021, 10, 3211–3221. [Google Scholar] [CrossRef]
- Ma, J.; Deng, Y.; Chen, D.; Li, X.; Yu, Z.; Wang, H.; Zhong, L.; Li, Y.; Wang, C.; Li, X.; et al. Spatial immunophenotypes orchestrate prognosis in triple-negative breast cancer with Miller-Payne grade 4 following neoadjuvant chemotherapy. NPJ Breast Cancer 2023, 9, 57. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Yang, C.; Chen, M.; Shi, Q.; Zhou, C.; Huang, S.; Chen, Y.; Wang, Y.; Li, T. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology 2022, 303, 711–719. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, S.; Park, C.; Doorenbos, A.Z.; Go, J.; Kim, S. Does neoadjuvant chemotherapy regimen affect sarcopenia status in patients with breast cancer? Breast 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, Z.; Ouyang, J.; Tan, Y.; Chen, Y.; Gu, Y.; Mao, L.; Ren, W.; Wang, J.; Lin, L.; et al. Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: A machine learning, multicenter study. EBioMedicine 2021, 69, 103460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Qu, J.; Zhang, R.; Zhou, X.; Li, L.; Sun, K.; Tang, Z.; Jiang, H.; Li, H.; et al. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin. Cancer Res. 2019, 25, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, R.D.; Rowland, J.; McDonald, E.S.; Pantalone, L.; Cohen, E.A.; Gastounioti, A.; Feldman, M.; Schnall, M.; Conant, E.; Kontos, D. Imaging Phenotypes of Breast Cancer Heterogeneity in Preoperative Breast Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI) Scans Predict 10-Year Recurrence. Clin. Cancer Res. 2020, 26, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Breast Imaging; Slanetz, P.J.; Moy, L.; Baron, P.; diFlorio, R.M.; Green, E.D.; Heller, S.L.; Holbrook, A.I.; Lee, S.J.; Lewin, A.A.; et al. ACR Appropriateness Criteria® Monitoring Response to Neoadjuvant Systemic Therapy for Breast Cancer. J. Am. Coll. Radiol. 2017, 14, S462–S475. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, A.; Wengert, G.J.; Helbich, T.H.; Bago-Horvath, Z.; Alaei, S.; Bartsch, R.; Dubsky, P.; Baltzer, P.; Clauser, P.; Kapetas, P.; et al. Impact of Machine Learning with Multiparametric Magnetic Resonance Imaging of the Breast for Early Prediction of Response to Neoadjuvant Chemotherapy and Survival Outcomes in Breast Cancer Patients. Investig. Radiol. 2019, 54, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Zhang, Y.; You, C.; Pei, Y.; Yang, F.; Li, D.; Jiang, Y.-Z.; Shao, Z. Integration of radiogenomic features for early prediction of pathological complete response in patients with triple-negative breast cancer and identification of potential therapeutic targets. J. Transl. Med. 2022, 20, 256. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, T.; Zhang, X.; Li, W.; Zheng, X.; Cheng, M.; Ji, F.; Zhang, L.; Yang, C.; Wu, Z.; et al. Longitudinal MRI-based fusion novel model predicts pathological complete response in breast cancer treated with neoadjuvant chemotherapy: A multicenter, retrospective study. EClinicalMedicine 2023, 58, 101899. [Google Scholar] [CrossRef]
- Hatamikia, S.; George, G.; Schwarzhans, F.; Mahbod, A.; Woitek, R. Breast MRI radiomics and machine learning-based predictions of response to neoadjuvant chemotherapy—How are they affected by variations in tumor delineation? Comput. Struct. Biotechnol. J. 2023, 23, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, A.; Chaudet, P.; Leporq, B.; Heudel, P.E.; Barabas, F.; Tredan, O.; Treilleux, I.; Coulon, A.; Pilleul, F.; Beuf, O. Multicontrast MRI-based radiomics for the prediction of pathological complete response to neoadjuvant chemotherapy in patients with early triple negative breast cancer. MAGMA 2021, 34, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Gan, L.; Liu, Y.; Jiang, Y.; Xin, L.; Liu, Y.; Qin, N.; Cheng, Y.; Liu, Q.; Xu, L.; et al. Radiomics features based on automatic segmented MRI images: Prognostic biomarkers for triple-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Radiol. 2022, 146, 110095. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Wang, H.; Wang, Z.; Qian, Z.; Xiao, Q.; Liu, Y.; Shao, Z.; Zhou, S.; Chai, W.; You, C.; et al. A Combined Nomogram Model to Predict Disease-free Survival in Triple-Negative Breast Cancer Patients with Neoadjuvant Chemotherapy. Front. Genet. 2021, 12, 783513. [Google Scholar] [CrossRef] [PubMed]
- van den Ende, N.S.; Nguyen, A.H.; Jager, A.; Kok, M.; Debets, R.; van Deurzen, C.H.M. Triple-Negative Breast Cancer and Predictive Markers of Response to Neoadjuvant Chemotherapy: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2969. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi Carl, J.; Morris, E.A.; Mendelson, E.B. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Beare, R.; Lowekamp, B.; Yaniv, Z. Image Segmentation, Registration and Characterization in R with SimpleITK. J. Stat. Softw. 2018, 86, 8. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Bates, S.; Hastie, T.; Tibshirani, R. Cross-Validation: What Does It Estimate and How Well Does It Do It? J. Am. Stat. Assoc. 2023, 119, 1434–1445. [Google Scholar] [CrossRef]
- Finazzi, S.; Poole, D.; Luciani, D.; Cogo, P.E.; Bertolini, G. Calibration belt for quality-of-care assessment based on dichotomous outcomes. PLoS ONE 2011, 6, e16110. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Iwase, T.; Wang, X.; Shrimanker, T.V.; Kolonin, M.G.; Ueno, N.T. Body composition and breast cancer risk and treatment: Mechanisms and impact. Breast Cancer Res. Treat. 2021, 186, 273–283. [Google Scholar] [CrossRef]
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; McCullough, A.J.; Muc, S.; Schneyer, A.; Bennett, C.D.; Dodig, M.; Kalhan, S.C. Sarcopenia associated with porto-systemic shunting is reversed by follistatin. J. Hepatol. 2011, 54, 915–921. [Google Scholar] [CrossRef]
- Au, P.C.; Li, H.L.; Lee, G.K.; Li, G.H.; Chan, M.; Cheung, B.M.; Wong, I.C.; Lee, V.H.; Mok, J.; Yip, B.H.; et al. Sarcopenia and mortality in cancer: A meta-analysis. Osteoporos Sarcopenia 2021, 7 (Suppl. S1), S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, K.; Murakami, S.; Sato, A.; Ogawa, S.; Shinagawa, H.; Kamohara, Y. Integrated Evaluation of Inflammatory, Nutritional, and Sarcopenia Markers to Predict Survival in Metastatic Breast Cancer Patients. In Vivo 2023, 37, 811–817. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, S.; Park, C.; Doorenbos, A.; Go, J.; Kim, S. Hematologic toxicities, sarcopenia, and body composition change in breast cancer patients undergoing neoadjuvant chemotherapy. Support. Care Cancer 2023, 31, 419. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shu, Z.; Li, Y.; Chen, B.; Tang, L.; Mo, W.; Shao, G.; Shao, F. Machine Learning-Based Radiomics Nomogram Using Magnetic Resonance Images for Prediction of Neoadjuvant Chemotherapy Efficacy in Breast Cancer Patients. Front. Oncol. 2020, 10, 1410. [Google Scholar] [CrossRef]
- Miao, S.; Jia, H.; Cheng, K.; Hu, X.; Li, J.; Huang, W.; Wang, R. Deep learning radiomics under multimodality explore association between muscle/fat and metastasis and survival in breast cancer patients. Brief. Bioinform. 2022, 23, bbac432. [Google Scholar] [CrossRef]
- Qi, H.; An, Y.; Hu, X.; Miao, S.; Li, J. Explainable Machine Learning Explores Association between Sarcopenia and Breast Cancer Distant Metastasis. IEEE Access 2023, 11, 65725–65738. [Google Scholar] [CrossRef]
| Clinical Variables | MP-Low (n = 35) | MP-High (n = 86) | p-Value | Non-pCR (n = 87) | pCR (n = 34) | p-Value |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 50.4 ± 11.1 | 48.1 ± 11.0 | 0.295 | 49.1 ± 11.0 | 47.8 ± 11.0 | 0.587 |
| Weight (kg, mean ± SD) | 58.5 ± 4.38 | 61 ± 5.87 | 0.308 | 59.7 ± 4.07 | 60.55 ± 6.89 | 0.726 |
| BMI (kg/m2, mean ± SD) | 24.8 ± 2.62 | 24.97 ± 3.06 | 0.897 | 25.43 ± 2.48 | 24.27 ± 3.25 | 0.314 |
| Menopausal status (no/yes) | 17/18 | 41/45 | 0.929 | 43/44 | 15/19 | 0.601 |
| T staging (T1/T2/T3/T4) | 1/13/10/11 | 6/42/18/20 | 0.431 | 3/38/20/26 | 4/17/8/5 | 0.149 |
| N staging (N0/N1/N2/N3) | 4/15/11/5 | 6/38/22/20 | 0.596 | 8/39/23/17 | 2/14/10/8 | 0.881 |
| Ki67 (mean ± SD) | 0.52 ± 0.21 | 0.47 ± 0.23 | 0.226 | 0.48 ± 0.23 | 0.48 ± 0.20 | 0.972 |
| CEA (mean ± SD) | 3.88 ± 11.02 | 3.61 ± 7.87 | 0.883 | 4.13 ± 10.38 | 2.56 ± 2.10 | 0.387 |
| CA153 (mean ± SD) | 21.83 ± 13.76 | 20.40 ± 12.70 | 0.589 | 21.95 ± 13.37 | 17.91 ± 11.63 | 0.128 |
| Chemotherapy regimens (three-drug/two-drug) | 8/27 | 25/61 | 0.488 | 27/60 | 6/28 | 0.139 |
| Chemotherapy cycles (<6/≥6) | 12/23 | 35/51 | 0.514 | 37/50 | 10/24 | 0.185 |
| SMA (cm2, mean ± SD) | 96.51 ± 11.64 | 100.04 ± 12.85 | 0.165 | 97.66 ± 13.41 | 102.50 ± 9.46 | 0.059 |
| SMI (cm2/m2, mean ± SD) | 39.84 ± 5.13 | 40.78 ± 5.12 | 0.367 | 40.31 ± 5.59 | 41.01 ± 3.69 | 0.507 |
| SFA (cm2, mean ± SD) | 171.38 ± 51.93 | 159.47 ± 57.62 | 0.295 | 164.33 ± 56.05 | 159.31 ± 56.75 | 0.662 |
| SFT (cm, mean ± SD) | 2.47 ± 0.56 | 2.43 ± 0.76 | 0.790 | 2.42 ± 0.64 | 2.52 ± 0.85 | 0.492 |
| Mean muscle attenuation (HU, mean ± SD) | 30.84 ± 8.55 | 30.94 ± 9.89 | 0.956 | 30.65 ± 9.67 | 31.58 ± 9.09 | 0.632 |
| Sarcopenia (no/yes) | 14/21 | 53/33 | 0.031 | 42/45 | 25/9 | 0.012 |
| Clinical Variables | Univariate Regression—MP | Univariate Regression—pCR | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | p-Value | |
| Age | 0.9809 | 0.9462–1.0168 | 0.2929 | 1.0268 | 0.9901–1.0649 | 0.1539 |
| Weight | 0.7483 | 0.3685–1.1481 | 0.2323 | 1.1522 | 0.5497–1.7547 | 0.7355 |
| BMI | 0.9796 | 0.7129–1.2223 | 0.2577 | 1.0881 | 0.4823–1.6939 | 0.1567 |
| CA153 | 0.9919 | 0.9634–1.0213 | 0.5863 | 0.9780 | 0.9444–1.0128 | 0.2125 |
| CEA | 0.9968 | 0.9553–1.0401 | 0.8816 | 0.9648 | 0.8779–1.0603 | 0.4566 |
| Ki67 | 0.3347 | 0.0568–1.9723 | 0.2265 | 0.8196 | 0.1406–4.7792 | 0.8250 |
| Menopausal status | 1.0366 | 0.4721–2.2759 | 0.9286 | 1.5714 | 0.7078–3.4888 | 0.2667 |
| N staging | 1.2276 | 0.7892–1.9094 | 0.3630 | 1.0968 | 0.7108–1.6926 | 0.6763 |
| Chemotherapy regimens | 0.7230 | 0.2893–1.8069 | 0.4876 | 2.2119 | 0.8219–5.9527 | 0.1160 |
| Chemotherapy cycles | 0.7602 | 0.3349–1.7260 | 0.5123 | 0.9340 | 0.4180–2.0867 | 0.8677 |
| T staging | 0.7551 | 0.4961–1.1493 | 0.1899 | 0.6597 | 0.4277–1.0174 | 0.0598 |
| Mean muscle attenuation | 1.0012 | 0.9608–1.0433 | 0.9556 | 1.0105 | 0.9684–1.0544 | 0.6294 |
| Sarcopenia | 0.4151 | 0.1858–0.9274 | 0.0321 | 0.3360 | 0.1407–0.8022 | 0.0140 |
| SFA | 0.9963 | 0.9893–1.0032 | 0.2937 | 0.9984 | 0.9913–1.0055 | 0.6595 |
| SFT | 0.9269 | 0.5335–1.6104 | 0.7876 | 1.2158 | 0.6989–2.1150 | 0.4891 |
| SMA | 1.0230 | 0.9906–1.0564 | 0.1657 | 1.0316 | 0.9985–1.0658 | 0.0616 |
| SMI | 1.0369 | 0.9588–1.1214 | 0.3644 | 1.0265 | 0.9506–1.1086 | 0.5041 |
| Model | AUC | Threshold | 95% CI | p | SEN | SPE | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Clinical | 0.608 | N/A | 0.515–0.696 | reference | 61.6% | 60% | 79.1% | 38.9% |
| LDA Radiomics | 0.695 | 0.8246 | 0.605–0.775 | 0.0902 | 80.2% | 51.4% | 80.2% | 51.4% |
| RF Radiomics | 0.662 | 0.8146 | 0.571–0.746 | 0.0308 | 43.0% | 85.7% | 88.1% | 38.0% |
| MLP Radiomics | 0.706 | 0.8019 | 0.616–0.785 | 0.1828 | 39.5% | 94.3% | 94.4% | 38.8% |
| NBB Radiomics | 0.716 | 0.9193 | 0.627–0.794 | 0.3556 | 48.8% | 91.4% | 93.3% | 42.1% |
| SVM Radiomics | 0.610 | 0.6636 | 0.517–0.697 | 0.0091 | 87.2% | 37.1% | 77.3% | 54.2% |
| LR Radiomics | 0.753 | 0.5604 | 0.667–0.827 | reference | 72.1% | 80.0% | 89.9% | 53.8% |
| LDA Combined | 0.748 | 0.9569 | 0.660–0.822 | 0.3723 | 57.0% | 85.7% | 90.7% | 44.8% |
| RF Combined | 0.681 | 0.7261 | 0.590–0.763 | 0.0340 | 65.1% | 65.7% | 82.4% | 43.4% |
| MLP Combined | 0.744 | 0.7562 | 0.657–0.819 | 0.3167 | 52.3% | 88.6% | 91.8% | 43.1% |
| NBB Combined | 0.743 | 0.5121 | 0.656–0.818 | 0.4226 | 67.4% | 71.4% | 85.3% | 47.2% |
| SVM Combined | 0.658 | 0.4808 | 0.566–0.742 | 0.0005 | 95.4% | 28.6% | 76.6% | 71.4% |
| LR Combined | 0.781 | 0.4998 | 0.697–0.851 | reference | 77.9% | 74.3% | 88.2% | 57.8% |
| Model | AUC | Threshold | 95% CI | p | SEN | SPE | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Clinical | 0.626 | N/A | 0.534–0.713 | reference | 73.5% | 51.7% | 37.3% | 83.3% |
| LDA Radiomics | 0.771 | 0.2477 | 0.686–0.842 | 0.3984 | 58.8% | 92.0% | 74.1% | 85.1% |
| RF Radiomics | 0.708 | 0.4474 | 0.619–0.787 | 0.0333 | 52.9% | 93.1% | 75.0% | 83.5% |
| MLP Radiomics | 0.752 | 0.3331 | 0.665–0.826 | 0.2692 | 67.7% | 85.1% | 63.9% | 87.1% |
| NBB Radiomics | 0.767 | 0.4885 | 0.682–0.839 | 0.4112 | 64.7% | 85.1% | 62.9% | 86.0% |
| SVM Radiomics | 0.699 | 0.3596 | 0.609–0.779 | 0.0711 | 58.8% | 86.2% | 62.5% | 84.3% |
| LR Radiomics | 0.799 | 0.5044 | 0.716–0.866 | reference | 55.9% | 93.1% | 76.0% | 84.4% |
| LDA Combined | 0.817 | 0.6769 | 0.737–0.882 | 0.7568 | 61.8% | 89.7% | 70.0% | 85.7% |
| RF Combined | 0.774 | 0.3549 | 0.689–0.845 | 0.1062 | 61.8% | 87.4% | 65.6% | 85.4% |
| MLP Combined | 0.795 | 0.2064 | 0.712–0.863 | 0.3861 | 67.7% | 88.5% | 69.7% | 87.5% |
| NBB Combined | 0.803 | 0.3108 | 0.721–0.870 | 0.3216 | 73.5% | 86.2% | 67.6% | 89.3% |
| SVM Combined | 0.770 | 0.3704 | 0.684–0.841 | 0.1721 | 70.6% | 82.8% | 61.5% | 87.8% |
| LR Combined | 0.827 | 0.5001 | 0.747–0.889 | reference | 70.6% | 85.1% | 64.9% | 88.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Meng, W.; Li, Q.; Zheng, Y.; Yin, H.; Liu, Y.; Zhao, S.; Ma, J. Pretreatment Sarcopenia and MRI-Based Radiomics to Predict the Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Bioengineering 2024, 11, 663. https://doi.org/10.3390/bioengineering11070663
Guo J, Meng W, Li Q, Zheng Y, Yin H, Liu Y, Zhao S, Ma J. Pretreatment Sarcopenia and MRI-Based Radiomics to Predict the Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Bioengineering. 2024; 11(7):663. https://doi.org/10.3390/bioengineering11070663
Chicago/Turabian StyleGuo, Jiamin, Wenjun Meng, Qian Li, Yichen Zheng, Hongkun Yin, Ying Liu, Shuang Zhao, and Ji Ma. 2024. "Pretreatment Sarcopenia and MRI-Based Radiomics to Predict the Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer" Bioengineering 11, no. 7: 663. https://doi.org/10.3390/bioengineering11070663
APA StyleGuo, J., Meng, W., Li, Q., Zheng, Y., Yin, H., Liu, Y., Zhao, S., & Ma, J. (2024). Pretreatment Sarcopenia and MRI-Based Radiomics to Predict the Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Bioengineering, 11(7), 663. https://doi.org/10.3390/bioengineering11070663







