Anoikis-Related Long Non-Coding RNA Signatures to Predict Prognosis and Immune Infiltration of Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. The Capture and Pre-Processing of Patients’ Data
2.2. Screening of Ar-lncRNAs
2.3. Creation and Validation of Risk Signature
2.4. Establishment of an Anoikis-Related Nomogram
2.5. Analysis of Gene Set Enrichment
2.6. Analysis of the Immunity Signature
2.7. Investigation of the Model in Clinical Therapy
2.8. Consensus Clustering
2.9. Statistical Analysis
3. Results
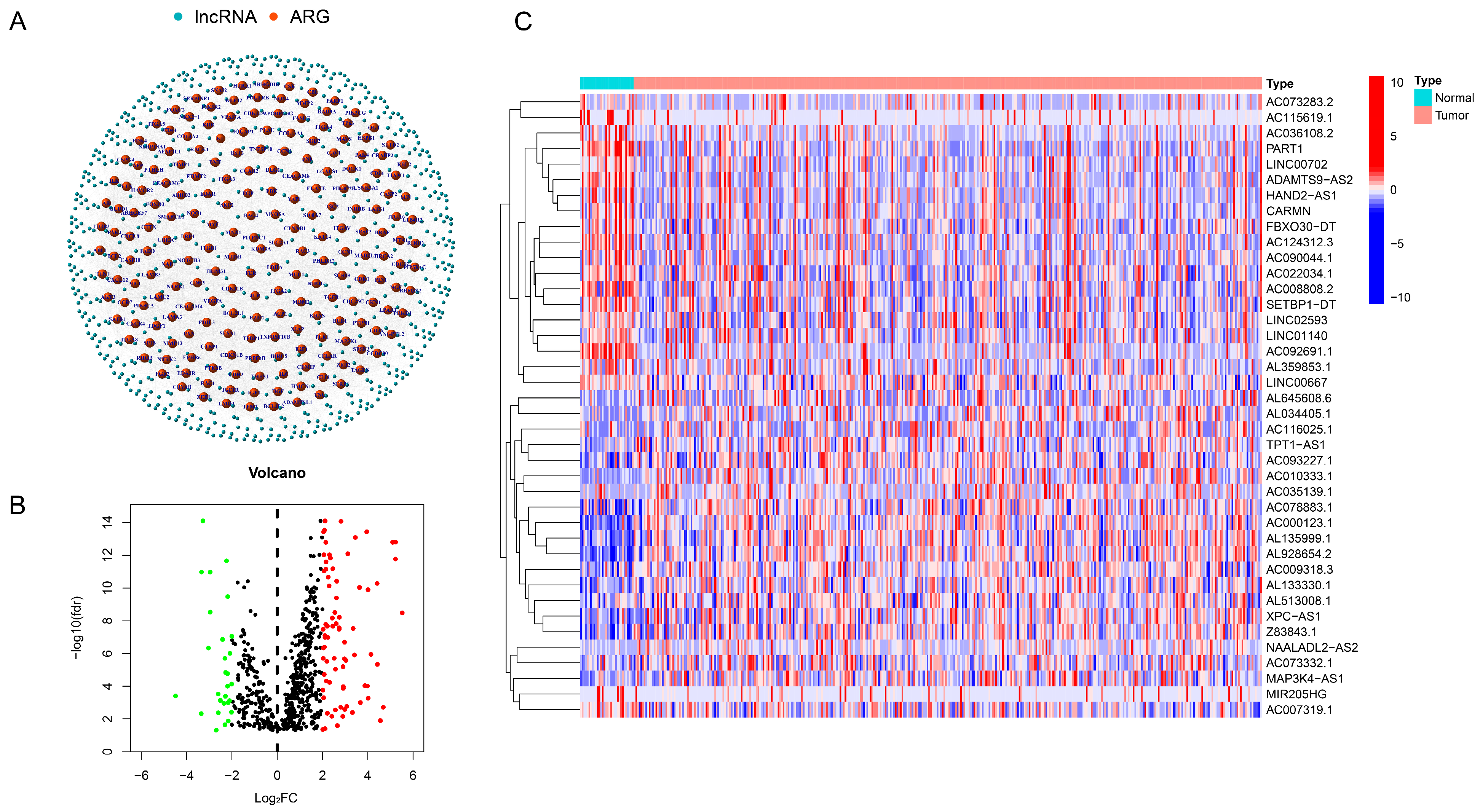
3.1. Identification of Ar-lncRNA
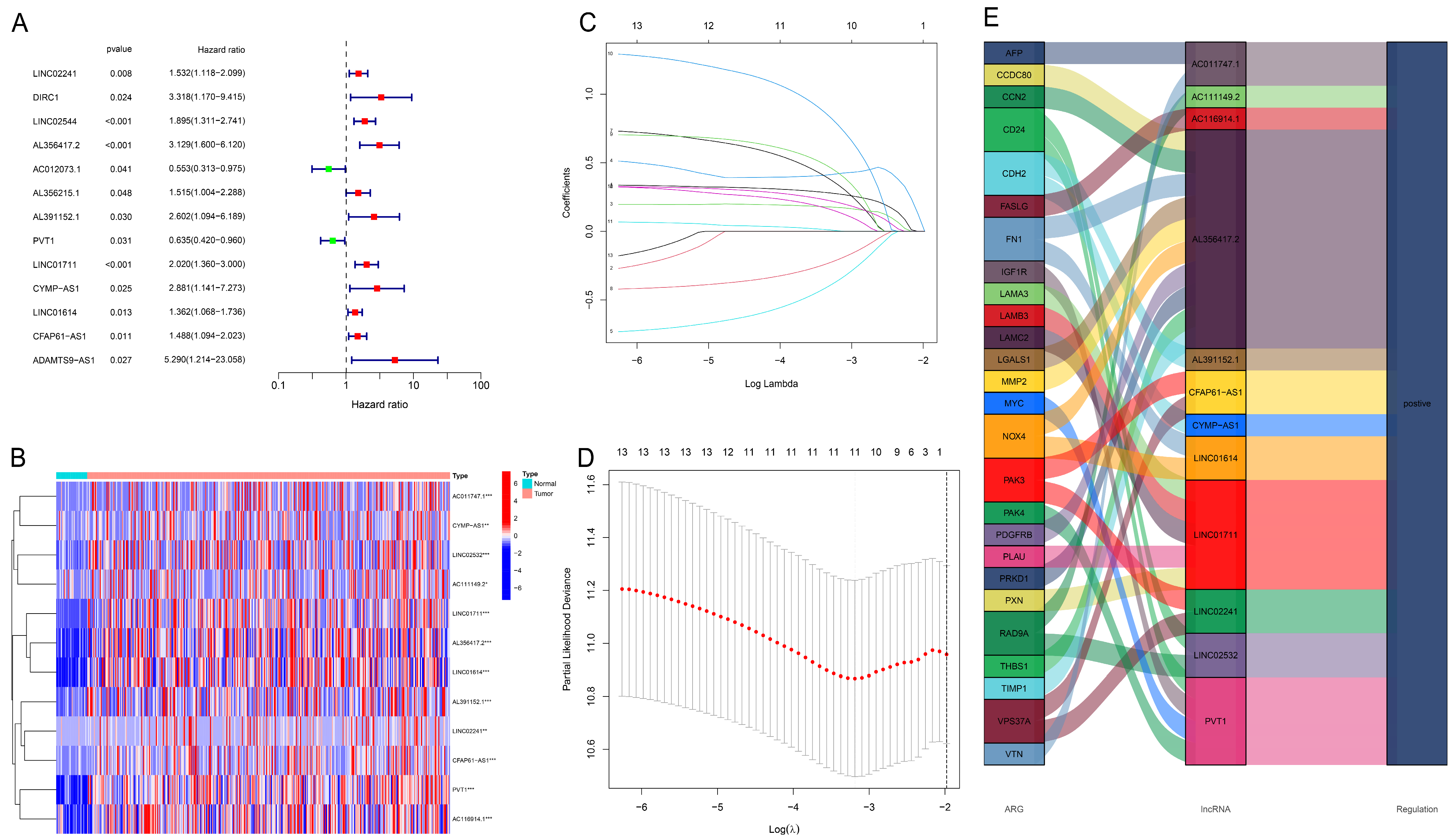
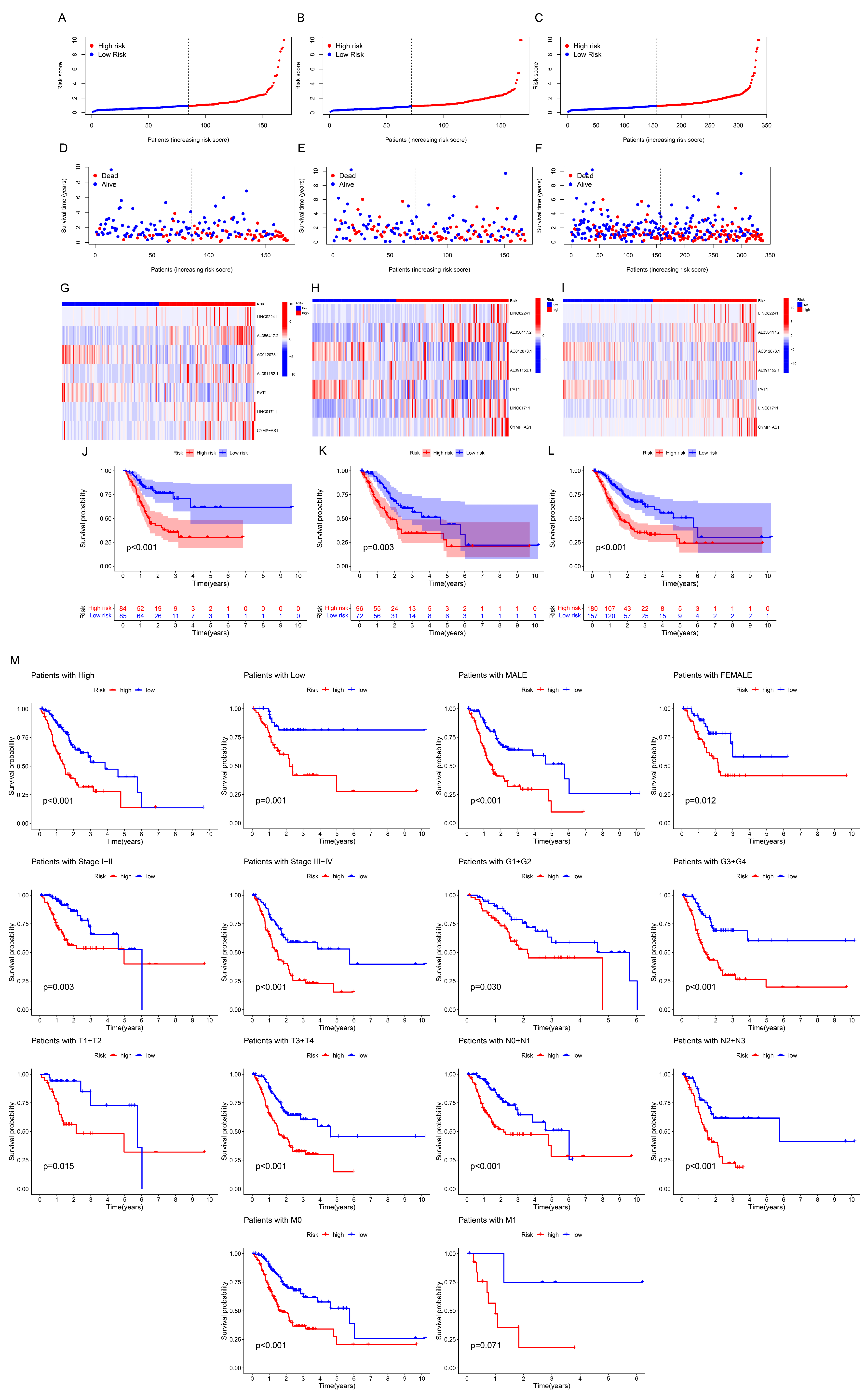
3.2. Construction and Evaluation of Prognostic Model
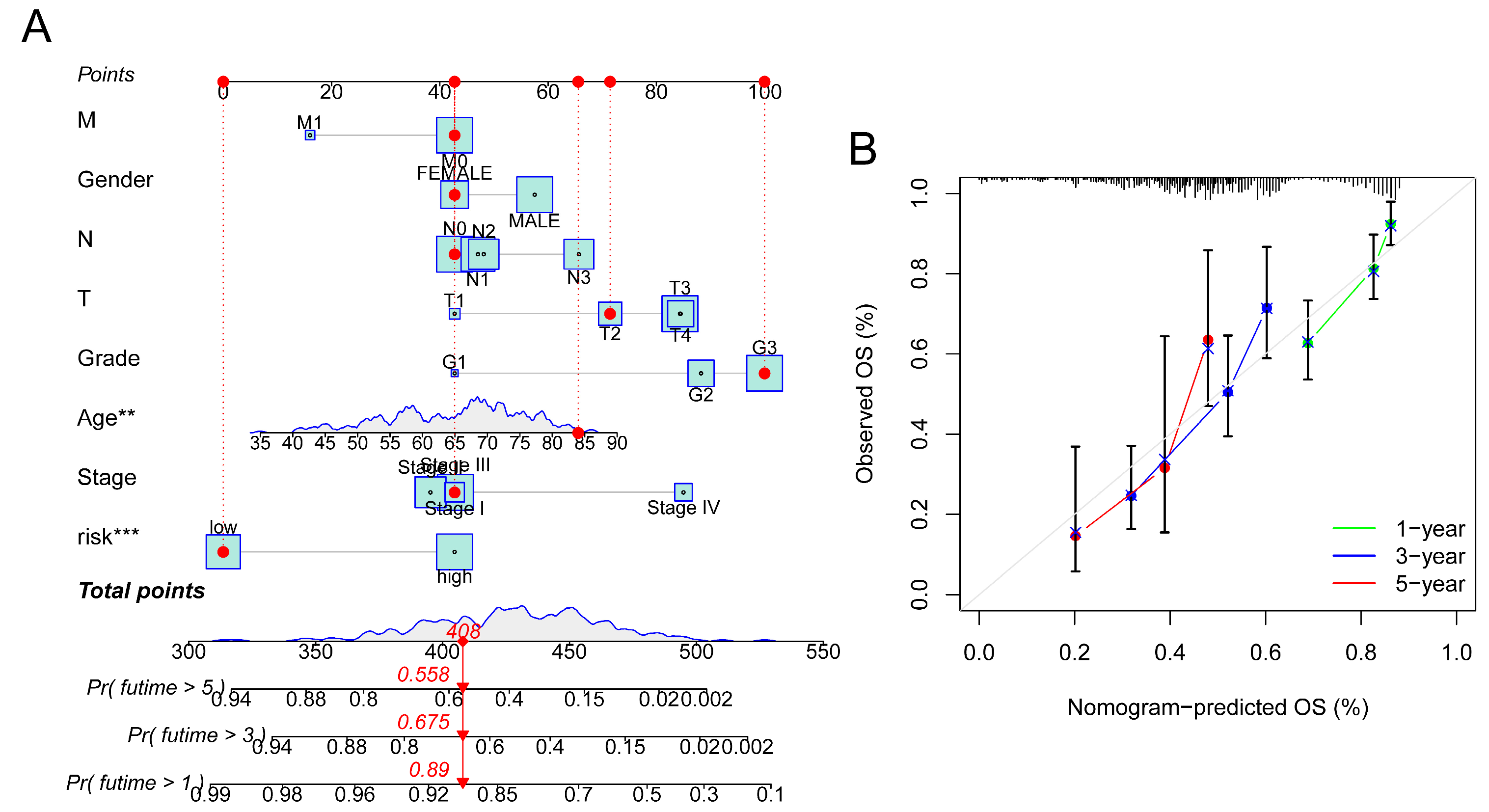
3.3. Construction of Nomogram
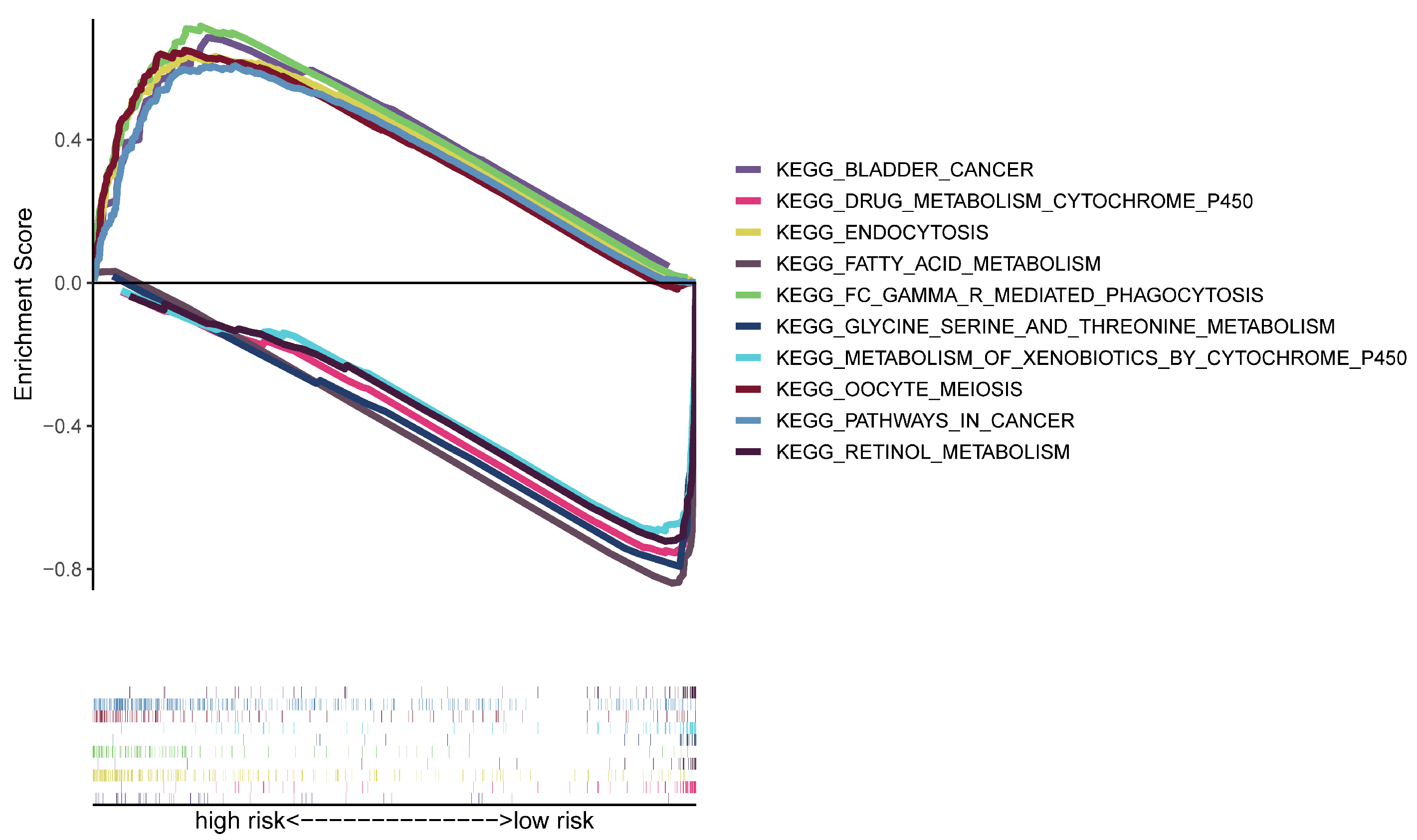
3.4. Analyses of Functional Enrichment
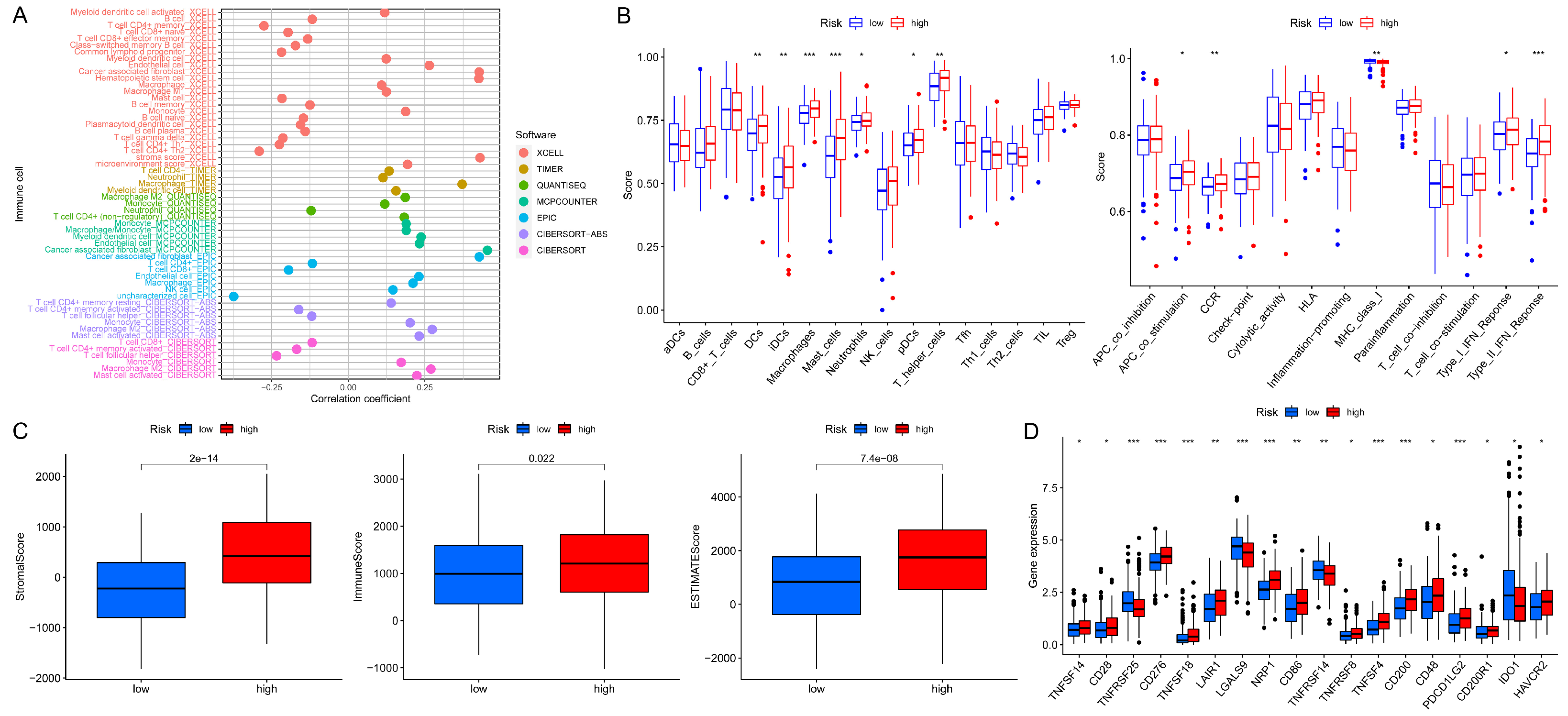
3.5. Analyses of Immune Characteristics and Clinical Treatment in Groups
3.6. Prognosis and Immunotherapy Prospects of Each GC Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [CrossRef] [PubMed]
- Sorscher, S. Helicobacter pylori and Gastric Cancer Screening. J. Clin. Oncol. 2024, Jco2400509. [Google Scholar] [CrossRef]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, Cd004064. [Google Scholar] [CrossRef]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric cancer treatment: Recent progress and future perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current developments in gastric cancer: From molecular profiling to treatment strategy. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155–170. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H.; et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA 2023, 330, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; Yeh, K.H.; et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021, 24, 946–958. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Zhong, Q.; Lv, C.B.; Zhu, J.Y.; Lin, G.T.; Zhang, Z.Q.; Wu, D.; Weng, C.M.; Chen, Q.X.; Lian, M.Q.; et al. The safety and efficacy of neoadjuvant immunochemotherapy following laparoscopic gastrectomy for gastric cancer: A multicenter Real-world clinical study. Int. J. Surg. 2024, 110, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, Z.; Lin, D.; Liu, Y.; Zhou, L.; Wang, D.; Wu, A.; Li, Z. Clinicopathological features of tumor mutation burden, Epstein-Barr virus infection, microsatellite instability and PD-L1 status in Chinese patients with gastric cancer. Diagn. Pathol. 2021, 16, 38. [Google Scholar] [CrossRef]
- Koemans, W.J.; Chalabi, M.; van Sandick, J.W.; van Dieren, J.M.; Kodach, L.L. Beyond the PD-L1 horizon: In search for a good biomarker to predict success of immunotherapy in gastric and esophageal adenocarcinoma. Cancer Lett. 2019, 442, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Di Bartolomeo, M.; Mandala, M.; Ryu, M.H.; Caglevic, C.; Olesinski, T.; Chung, H.C.; Muro, K.; Goekkurt, E.; McDermott, R.S.; et al. Association between gene expression signatures and clinical outcomes of pembrolizumab versus paclitaxel in advanced gastric cancer: Exploratory analysis from the randomized, controlled, phase III KEYNOTE-061 trial. J. Immunother. Cancer 2023, 11, e006920. [Google Scholar] [CrossRef]
- Chiarugi, P.; Giannoni, E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008, 76, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Sung, J.Y.; Kim, S.H.; Yun, U.J.; Kim, H.; Jang, E.J.; Yoo, H.E.; Hong, E.K.; Goh, S.H.; Moon, A.; et al. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021, 508, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Rescorla, F.J. Cell surface adhesion molecules and adhesion-initiated signaling: Understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signal. 2012, 24, 393–401. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Rodrigues, M.F.; Rumjanek, F.D. Mitochondria: Are mitochondria accessory to metastasis? Int. J. Biochem. Cell Biol. 2014, 51, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, E.; Shahbahrami, R.; Goudarzi, H.; Eslami, G.; Faghihloo, E. Anoikis resistance and oncoviruses. J. Cell Biochem. 2018, 119, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Kyprianou, N. Targeting anoikis resistance in prostate cancer metastasis. Mol. Aspects Med. 2010, 31, 205–214. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Du, S.; Yang, Z.; Lu, X.; Yousuf, S.; Zhao, M.; Li, W.; Miao, J.; Wang, X.; Yu, H.; Zhu, X.; et al. Anoikis resistant gastric cancer cells promote angiogenesis and peritoneal metastasis through C/EBPβ-mediated PDGFB autocrine and paracrine signaling. Oncogene 2021, 40, 5764–5779. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Peng, Y.; Liu, R.; Li, Q. Construction of an original anoikis-related prognostic model closely related to immune infiltration in gastric cancer. Front. Genet. 2022, 13, 1087201. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Katebi, M. Autophagy, anoikis, ferroptosis, necroptosis, and endoplasmic reticulum stress: Potential applications in melanoma therapy. J. Cell Physiol. 2019, 234, 19471–19479. [Google Scholar] [CrossRef] [PubMed]
- McCabe, E.M.; Rasmussen, T.P. lncRNA involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin. Cancer Biol. 2021, 75, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, X.; Zhao, Y.; Zhou, T.; Jiang, X.; Wen, Y.; Meng, W.; Li, S. Comprehensive analysis of cuproptosis-related long noncoding RNA immune infiltration and prediction of prognosis in patients with bladder cancer. Front. Genet. 2022, 13, 990326. [Google Scholar] [CrossRef]
- Gao, G.B.; Chen, L.; Pan, J.F.; Lei, T.; Cai, X.; Hao, Z.; Wang, Q.; Shan, G.; Li, J. LncRNA RGMB-AS1 inhibits HMOX1 ubiquitination and NAA10 activation to induce ferroptosis in non-small cell lung cancer. Cancer Lett. 2024, 590, 216826. [Google Scholar] [CrossRef]
- Tyagi, N.; Roy, S.; Vengadesan, K.; Gupta, D. Multi-omics approach for identifying CNV-associated lncRNA signatures with prognostic value in prostate cancer. Noncoding RNA Res. 2024, 9, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, J.; Zhang, Q.; Hu, Y.; Ge, Y. Development of Biomarker Signatures Associated with Anoikis to Predict Prognosis in Endometrial Carcinoma Patients. J. Oncol. 2021, 2021, 3375297. [Google Scholar] [CrossRef]
- Meng, T.; Huang, R.; Zeng, Z.; Huang, Z.; Yin, H.; Jiao, C.; Yan, P.; Hu, P.; Zhu, X.; Li, Z.; et al. Identification of Prognostic and Metastatic Alternative Splicing Signatures in Kidney Renal Clear Cell Carcinoma. Front. Bioeng. Biotechnol. 2019, 7, 270. [Google Scholar] [CrossRef]
- Bunea, F.; She, Y.; Ombao, H.; Gongvatana, A.; Devlin, K.; Cohen, R. Penalized least squares regression methods and applications to neuroimaging. Neuroimage 2011, 55, 1519–1527. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Yuan, M.; Jia, Y.; Xing, Y.; Wang, Y.; Liu, Y.; Liu, X.; Liu, D. Screening and validation of platelet activation-related lncRNAs as potential biomarkers for prognosis and immunotherapy in gastric cancer patients. Front. Genet. 2022, 13, 965033. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Wang, X.; Yang, Q. Identification of a Six-Immune-Related Long Non-coding RNA Signature for Predicting Survival and Immune Infiltrating Status in Breast Cancer. Front. Genet. 2020, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, X.P.; Xu, S.; Hu, M.G.; Zhao, Z.M.; Zhao, G.D.; Xiao, Z.H.; Liu, R. Identification of a CD4+ conventional T cells-related lncRNAs signature associated with hepatocellular carcinoma prognosis, therapy, and tumor microenvironment. Front. Immunol. 2022, 13, 1111246. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; He, L.; Guo, C.; Zhong, Z.; Yan, X.; Cao, J.; Xu, Q.; Zhang, H.; Duan, B. m6A-related lncRNAs predict prognosis and indicate cell cycle in gastric cancer. Front. Genet. 2023, 14, 1140218. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Liao, C.; Zhao, Z.; Gao, H.; Huang, R.; Chen, J.; Wu, F.; Zeng, F.; Zhang, Y.; et al. PVT1 promotes proliferation and macrophage immunosuppressive polarization through STAT1 and CX3CL1 regulation in glioblastoma multiforme. CNS Neurosci. Ther. 2024, 30, e14566. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Wang, Y.; Zuo, Y.; Chen, D.; Guo, X. Comprehensive prediction of immune microenvironment and hot and cold tumor differentiation in cutaneous melanoma based on necroptosis-related lncRNA. Sci. Rep. 2023, 13, 7299. [Google Scholar] [CrossRef]
- Yue, B.; Chen, J.; Bao, T.; Zhang, Y.; Yang, L.; Zhang, Z.; Wang, Z.; Zhu, C. Chromosomal copy number amplification-driven Linc01711 contributes to gastric cancer progression through histone modification-mediated reprogramming of cholesterol metabolism. Gastric Cancer 2024, 27, 308–323. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Jiang, F.; Shen, Y.; Fang, F.; Li, Q.; Yang, C.; Dong, Y.; Shen, X. A prognostic signature of pyroptosis-related lncRNAs verified in gastric cancer samples to predict the immunotherapy and chemotherapy drug sensitivity. Front. Genet. 2022, 13, 939439. [Google Scholar] [CrossRef]
- Sekar, R.; Motzler, K.; Kwon, Y.; Novikoff, A.; Jülg, J.; Najafi, B.; Wang, S.; Warnke, A.L.; Seitz, S.; Hass, D.; et al. Vps37a regulates hepatic glucose production by controlling glucagon receptor localization to endosomes. Cell Metab. 2022, 34, 1824–1842.e1829. [Google Scholar] [CrossRef]
- Magne, N.; Rousseau, V.; Duarte, K.; Poëa-Guyon, S.; Gleize, V.; Mutel, A.; Schmitt, C.; Castel, H.; Idbaih, A.; Huillard, E.; et al. PAK3 is a key signature gene of the glioma proneural subtype and affects its proliferation, differentiation and growth. Cell. Oncol. 2021, 44, 1257–1271. [Google Scholar] [CrossRef]
- Tan, X.; Tong, L.; Li, L.; Xu, J.; Xie, S.; Ji, L.; Fu, J.; Liu, Q.; Shen, S.; Liu, Y.; et al. Loss of Smad4 promotes aggressive lung cancer metastasis by de-repression of PAK3 via miRNA regulation. Nat. Commun. 2021, 12, 4853. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, Z.; Yang, X.; Li, Z.; Sang, S.; Islam, M.T.; Guo, A.A.; Li, Z.; Wang, X.; Wang, J.; et al. Development and interpretation of a pathomics-driven ensemble model for predicting the response to immunotherapy in gastric cancer. J. Immunother. Cancer 2024, 12, e008927. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.-J.; Guo, J.-M.; Huang, L.; Zhang, Y.-Y.; Zhu, Y.-T.; Tang, L.-S.; Wang, J.-L.; Li, H.-S.; Liu, J.-Y. Anoikis-Related Long Non-Coding RNA Signatures to Predict Prognosis and Immune Infiltration of Gastric Cancer. Bioengineering 2024, 11, 893. https://doi.org/10.3390/bioengineering11090893
Meng W-J, Guo J-M, Huang L, Zhang Y-Y, Zhu Y-T, Tang L-S, Wang J-L, Li H-S, Liu J-Y. Anoikis-Related Long Non-Coding RNA Signatures to Predict Prognosis and Immune Infiltration of Gastric Cancer. Bioengineering. 2024; 11(9):893. https://doi.org/10.3390/bioengineering11090893
Chicago/Turabian StyleMeng, Wen-Jun, Jia-Min Guo, Li Huang, Yao-Yu Zhang, Yue-Ting Zhu, Lian-Sha Tang, Jia-Ling Wang, Hong-Shuai Li, and Ji-Yan Liu. 2024. "Anoikis-Related Long Non-Coding RNA Signatures to Predict Prognosis and Immune Infiltration of Gastric Cancer" Bioengineering 11, no. 9: 893. https://doi.org/10.3390/bioengineering11090893
APA StyleMeng, W.-J., Guo, J.-M., Huang, L., Zhang, Y.-Y., Zhu, Y.-T., Tang, L.-S., Wang, J.-L., Li, H.-S., & Liu, J.-Y. (2024). Anoikis-Related Long Non-Coding RNA Signatures to Predict Prognosis and Immune Infiltration of Gastric Cancer. Bioengineering, 11(9), 893. https://doi.org/10.3390/bioengineering11090893







