Towards Complex Tissues Replication: Multilayer Scaffold Integrating Biomimetic Nanohydroxyapatite/Chitosan Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
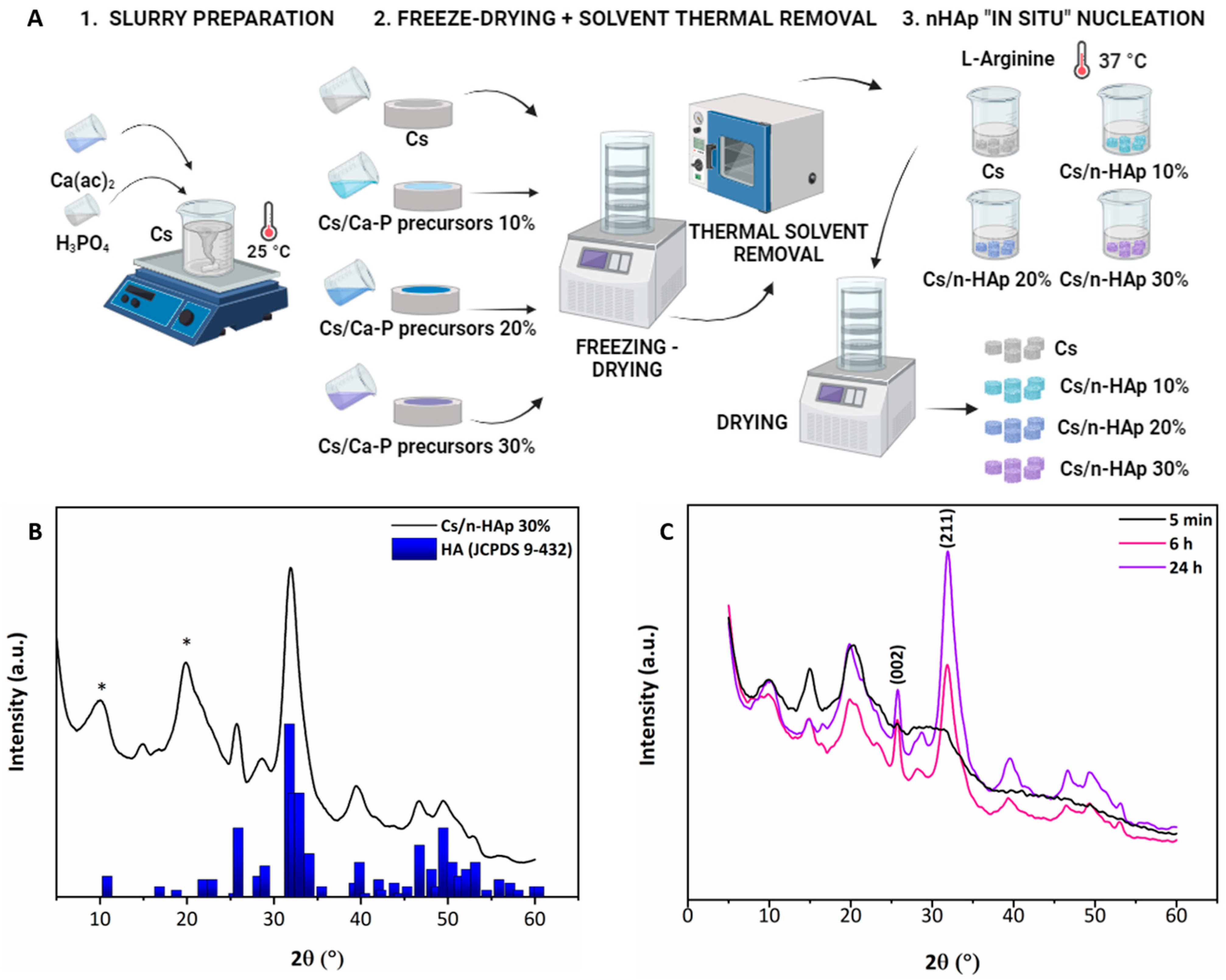
2.2. Cs/n-HAp Scaffold Preparation
2.3. Cs/n-HAp Multi-Layer Scaffold Prototype Preparation: Proof-of-Concept
2.4. Scaffold Physicochemical Characterization
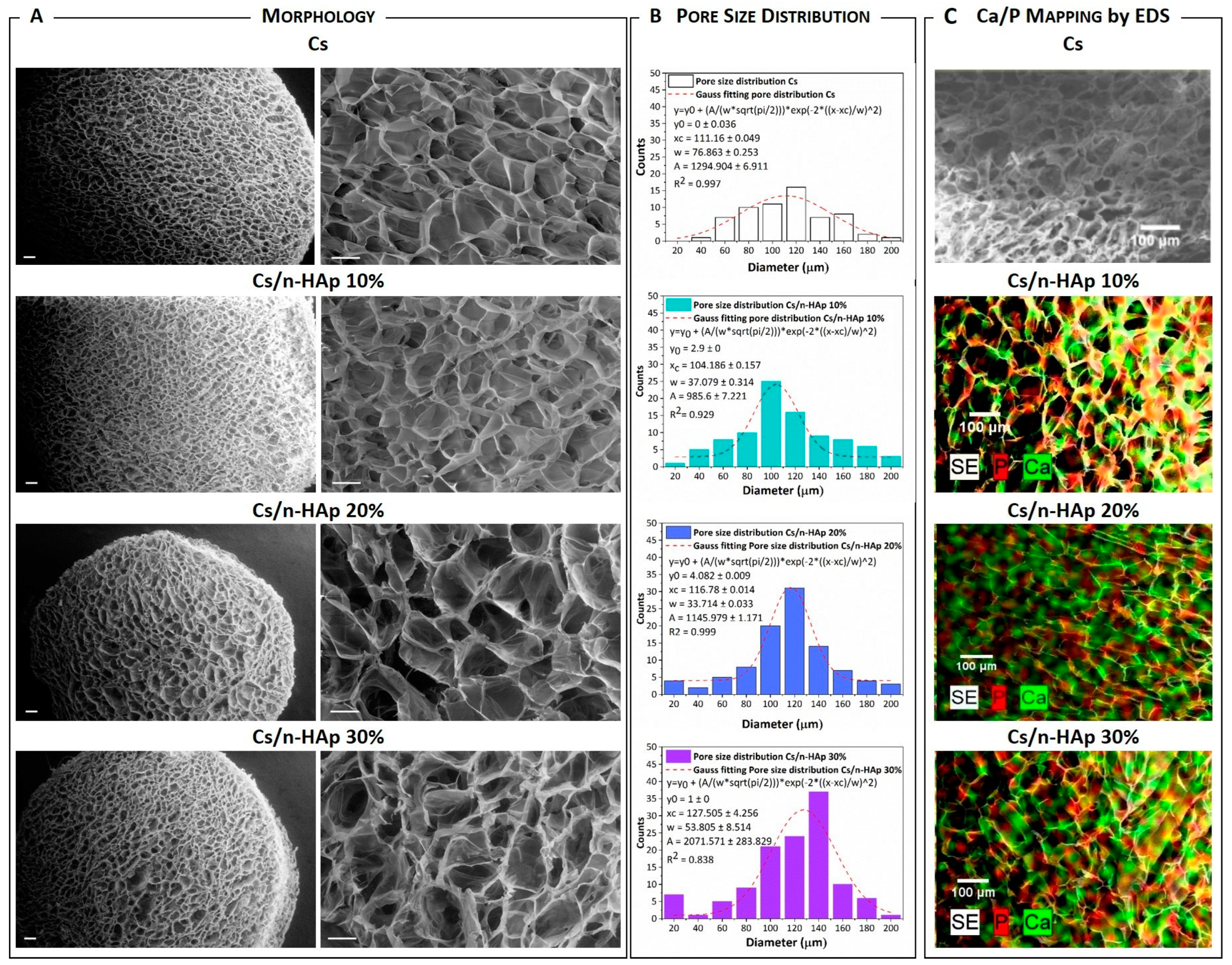
2.4.1. Morphological and Compositional Analysis (SEM-EDS)
2.4.2. Structural Analysis by XRD
2.4.3. FTIR
2.4.4. Thermogravimetric Analysis
2.4.5. Swelling Study
2.4.6. Mechanical Properties
2.5. Preliminary In Vitro Biological Validation
2.5.1. In Vitro Cell Maintenance
2.5.2. Cell Viability Assay
2.5.3. RNA Isolation, Reverse Transcription, and Real-Time PCR for Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. n-HAp Nucleation into Chitosan Matrixes to form Cs/n-HAp Composite: Physicochemical Analysis
3.2. Swelling Behavior
3.3. Mechanical Properties
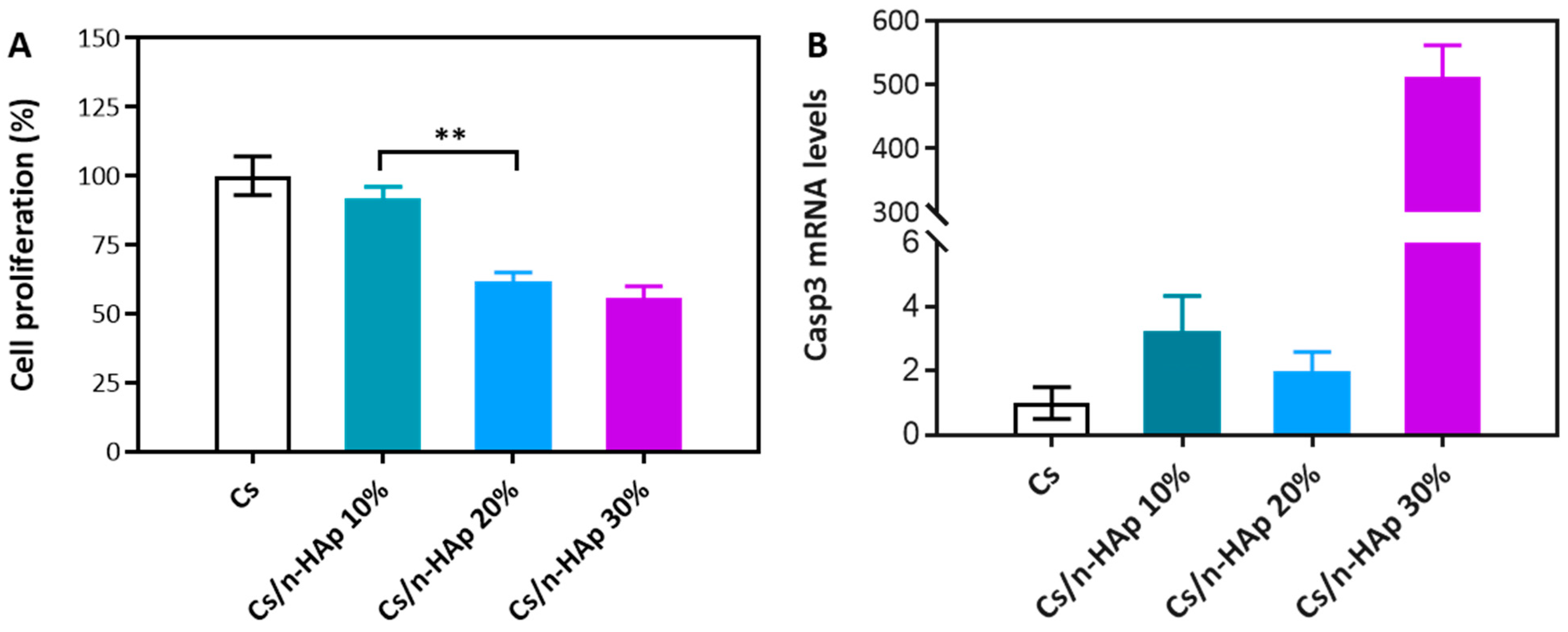
3.4. Viability Assay
3.5. Quantitative Real-Time Polymerase Chain Reaction (PCR)
3.6. Multilayer Assembly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Acetic Acid Removal
- (a)
- T = 100 °C for 3 h, under vacuum conditions (−1 bar);
- (b)
- T = 50 °C for 24 h, under vacuum conditions (−1 bar);
- (c)
- T = 50 °C for 24 h, under vacuum conditions (−1 bar) and successive ethanol treatment at room temperature under bland shaking.
| Sample | Acetic Acid Removal Treatment | ∆pH (Milli-Q) | ∆pH (PBS) | ||
|---|---|---|---|---|---|
| 5′ | 24 h | 5′ | 24 h | ||
| 1 | 100 °C 3 h | +0.01 ± 0.01 | −0.09 ± 0.01 | +0.07 ± 0.01 | 0.25 ± 0.01 |
| 2 | 50 °C 24 h | −0.34 ± 0.04 | −0.47 ± 0.06 | −0.29 ± 0.03 | 0.56 ± 0.01 |
| 3 | 50 °C 24 h + EtOH | −0.37 ± 0.02 | −0.02± 0.01 | +0.06 ± 0.01 | +0.08 ± 0.01 |
| 4 | untreated | −0.37 ± 0.01 | −0.41 ± 0.02 | −0.89 ± 0.11 | 0.72 ± 0.01 |
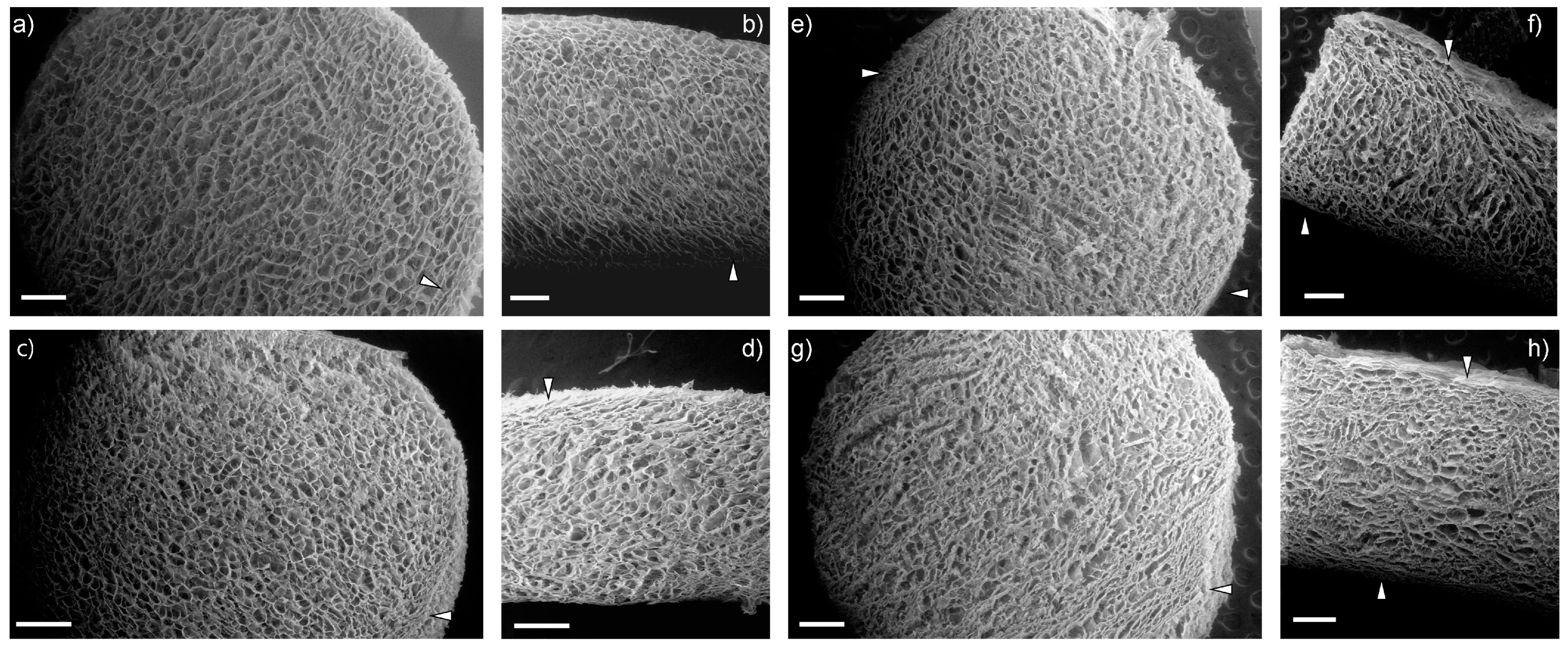
References
- Lee, D.H.; Kim, S.J.; Kim, S.A.; Ju, G.-I. Past, present, and future of cartilage restoration: From localized defect to arthritis. Knee Surg. Relat. Res. 2022, 34, 1. [Google Scholar] [CrossRef]
- Gobbi, A.; Karnatzikos, G.; Kumar, A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg. Sports Traumatol. Arthrosc. 2013, 22, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, P.; Steinwachs, M.; Erggelet, C.; Krause, S.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef]
- Borrelli, J.; Olson, S.A.; Godbout, C.; Schemitsch, E.H.; Stannard, J.P.; Giannoudis, P.V. Understanding Articular Cartilage Injury and Potential Treatments. J. Orthop. Trauma 2019, 33, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.; Jiang, C.-C. Repair of Articular Cartilage Defects: Review and Perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am.—Ser. A 1993, 75, 532–553. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Donaldson, J.; Bentley, G. Current solutions for the treatment of chronic articular cartilage defects in the knee. EFORT Open Rev. 2020, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, F.; Salim, A.; Khan, I. Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine. Stem Cell Rev. Rep. 2023, 19, 1615–1634. [Google Scholar] [CrossRef]
- Scalera, F.; Corcione, C.E.; Montagna, F.; Sannino, A.; Maffezzoli, A. Development and characterization of UV curable epoxy/hydroxyapatite suspensions for stereolithography applied to bone tissue engineering. Ceram. Int. 2014, 40, 15455–15462. [Google Scholar] [CrossRef]
- Scalera, F.; Gervaso, F.; Sanosh, K.; Sannino, A.; Licciulli, A. Influence of the calcination temperature on morphological and mechanical properties of highly porous hydroxyapatite scaffolds. Ceram. Int. 2013, 39, 4839–4846. [Google Scholar] [CrossRef]
- Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Sannino, A.; Licciulli, A. High-performance hydroxyapatite scaffolds for bone tissue engineering applications. Int. J. Appl. Ceram. Technol. 2012, 9, 507–516. [Google Scholar] [CrossRef]
- Gervaso, F.; Padmanabhan, S.K.; Scalera, F.; Sannino, A.; Licciulli, A. Mechanical stability of highly porous hydroxyapatite scaffolds during different stages of in vitro studies. Mater. Lett. 2016, 185, 239–242. [Google Scholar] [CrossRef]
- Corcione, C.E.; Scalera, F.; Gervaso, F.; Montagna, F.; Sannino, A.; Maffezzoli, A. One-step solvent-free process for the fabrication of high loaded PLA/HA composite filament for 3D printing. J. Therm. Anal. Calorim. 2018, 134, 575–582. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Gervaso, F.; Carrozzo, M.; Scalera, F.; Sannino, A.; Licciulli, A. Wollastonite/hydroxyapatite scaffolds with improved mechanical, bioactive and biodegradable properties for bone tissue engineering. Ceram. Int. 2013, 39, 619–627. [Google Scholar] [CrossRef]
- Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Licciulli, A.; Deponti, D.; Di Giancamillo, A.; Domeneghini, C.; Peretti, G.M.; Sannino, A. Development and mechanical characterization of a collagen/hydroxyapatite bilayered scaffold for ostechondral defect re-placement. Key Eng. Mater. 2012, 493–494, 890–895. [Google Scholar] [CrossRef]
- Palazzo, B.; Scalera, F.; Soloperto, G.; Scialla, S.; Gervaso, F. Recent Strategies in Osteochondral Substitutes Design: Towards the Mimicking of a Multifaceted Anatomical Unit from the Nano to the Macro Level. J. Nanomed. Nanotechnol. 2017, 8, 458. [Google Scholar] [CrossRef]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C.; Zhang, D.; Sui, X.; Lin, Y.; Liu, S.; et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Kosik-Kozioł, A.; Heljak, M.; Święszkowski, W. Mechanical properties of hybrid triphasic scaffolds for osteochondral tissue engineering. Mater. Lett. 2020, 261, 126893. [Google Scholar] [CrossRef]
- Marquass, B.; Somerson, J.; Hepp, P.; Aigner, T.; Schwan, S.; Bader, A.; Josten, C.; Zscharnack, M.; Schulz, R. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J. Orthop. Res. 2010, 28, 1586–1599. [Google Scholar] [CrossRef]
- Kang, H.; Zeng, Y.; Varghese, S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater. 2018, 78, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Barron, V.; Neary, M.; Mohamed, K.M.S.; Ansboro, S.; Shaw, G.; O’malley, G.; Rooney, N.; Barry, F.; Murphy, M. Evaluation of the Early In Vivo Response of a Functionally Graded Macroporous Scaffold in an Osteochondral Defect in a Rabbit Model. Ann. Biomed. Eng. 2016, 44, 1832–1844. [Google Scholar] [CrossRef]
- Han, F.; Yang, X.; Zhao, J.; Zhao, Y.; Yuan, X. Photocrosslinked layered gelatin-chitosan hydrogel with graded compositions for osteochondral defect repair. J. Mater. Sci. Mater. Med. 2015, 26, 160. [Google Scholar] [CrossRef]
- Scalera, F.; Pereira, S.; Bucciarelli, A.; Tobaldi, D.; Quarta, A.; Gervaso, F.; Castro, P.; Polini, A.; Piccirillo, C. Chitosan-hydroxyapatite composites made from sustainable sources: A morphology and antibacterial study. Mater. Today Sustain. 2023, 21, 100334. [Google Scholar] [CrossRef]
- Chen, R.; Pye, J.S.; Li, J.; Little, C.B.; Li, J.J. Multiphasic scaffolds for the repair of osteochondral defects: Outcomes of preclinical studies. Bioact. Mater. 2023, 27, 505–545. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Sosio, C.; Di Giancamillo, A.; Deponti, D.; Gervaso, F.; Scalera, F.; Melato, M.; Campagnol, M.; Boschetti, F.; Nonis, A.; Domeneghini, C.; et al. Osteochondral repair by a novel interconnecting collagen-hydroxyapatite substitute: A large-animal study. Tissue Eng. Part A 2015, 21, 704–715. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Guan, J.-J.; Yang, J.; Wang, Y.; Zhang, C.-Q.; Ke, Q.-F. Hybrid nanostructured hydroxyapatite–chitosan composite scaffold: Bioinspired fabrication, mechanical properties and biological properties. J. Mater. Chem. B 2015, 3, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Leonor, I.B.; Azevedo, H.S.; Reis, R.L.; Mano, J.F. Designing biomaterials based on biomineralization of bone. J. Mater. Chem. 2010, 20, 2911. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, B.; Izzo, D.; Scalera, F.; Cancelli, A.; Gervaso, F. Bio-Hybrid Scaffolds for Bone Tissue Engineering: Nano-Hydroxyapatite/Chitosan Composites. Key Eng. Mater. 2014, 631, 300–305. [Google Scholar] [CrossRef]
- Izzo, D.; Palazzo, B.; Scalera, F.; Gullotta, F.; Lapesa, V.; Scialla, S.; Sannino, A.; Gervaso, F. Chitosan scaffolds for cartilage regeneration: Influence of different ionic crosslinkers on biomaterial properties. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 936–945. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, A.; Deng, A.; Yang, Y.; Gao, L.; Zhong, Z.; Yang, S. Pore architecture and cell viability on freeze dried 3D recombinant human collagen-peptide (RHC)–chitosan scaffolds. Mater. Sci. Eng. C 2015, 49, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.-M.; et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.-H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef]
- Palazzo, B.; Gallo, A.; Casillo, A.; Nitti, P.; Ambrosio, L.; Piconi, C. Fabrication, Characterization and Cell Cultures on a Novel Composite Chitosan-Nano-Hydroxyapatite Scaffold. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. S2), 73–78. [Google Scholar] [CrossRef] [PubMed]
- Koc, S.; Baygar, T.; Özarslan, S.; Sarac, N.; Ugur, A. Fabrication and Characterization of a Multifunctional Coating to Promote the Osteogenic Properties of Orthopedic Implants. Materials 2023, 16, 6608. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Tafaghodi, M.; Eisvand, F.; Ahmadi-Soleimani, S.M.; Khajouee, M.; Ghazizadeh, H.; Mosafer, J. Preparation and Evaluation of the In situ Gel-forming Chitosan Hydrogels for Nasal Delivery of Morphine in a Single Unit dose in Rats to Enhance the Analgesic Responses. Curr. Drug Deliv. 2024, 21, 1024–1035. [Google Scholar] [CrossRef]
- Sun, M.T.; O’connor, A.J.; Milne, I.; Biswas, D.; Casson, R.; Wood, J.; Selva, D. Development of Macroporous Chitosan Scaffolds for Eyelid Tarsus Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Cannillo, V. Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests. Materials 2020, 13, 2819. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]


| Scaffold | Acetic Acid (% w/v) | Cs | Ca(ac)2 (mg/mL) | H3PO4 (mg/mL) |
|---|---|---|---|---|
| Cs | 1 | 1.67 | - | - |
| Cs/Ca-P precursor 10 | 1 | 23 | 8.9 | |
| Cs/Ca-P precursor 20 | 2 | 46 | 17.85 | |
| Cs/Ca-P precursor 30 | 3 | 69 | 26.775 |
| Gene | Ref Seq mRNA acc. n. | 5′-3′ Sense | 5′-3′ Antisense | PCR Size (bp) |
|---|---|---|---|---|
| Casp-3 | NM_001284409.1 | CTGGACTGTGGCATTGAGAC | TAACCAGGTGCTGTAGAGTA | 157 |
| 28S | NR_003279.1 | CGTGAGACAGGTTAGTTTTAC | ATCCCACAGATGGTAGCTTC | 143 |
| Scaffold | Mean Diameter (µm) | Porosity (%) | Unn. C. (wt%) | |
|---|---|---|---|---|
| Ca | P | |||
| Cs | 111 ± 1 | 97.88 ± 0.09 | ------ | ------ |
| Cs/n-HAp 10 | 104 ± 1 | 97.80 ± 0.12 | 7.61 ± 1.29 | 2.95 ± 0.64 |
| Cs/n-HAp 20 | 117 ± 1 | 97.54 ± 0.04 | 13.49 ± 1.26 | 5.12 ± 0.80 |
| Cs/n-HAp 30 | 128 ± 4 | 98.10 ± 0.08 | 20.02 ± 0.95 | 7.86 ± 0.76 |
| Scaffold | Elow (kPa) | Ehigh (kPa) | εy |
|---|---|---|---|
| Cs | 2.16 ± 0,16 | 30.26 ± 1.77 | 0.581 ± 0.006 |
| Cs/n-HAp 10 | 3.49 ± 0.13 | 55.55 ± 2.88 | 0.590 ± 0.001 |
| Cs/n-HAp 20 | 7.86 ± 0.41 | 67.78 ± 3.20 | 0.584 ± 0.007 |
| Cs/n-HAp 30 | 8.26 ± 0.50 | 81.94 ± 5.16 | 0.577 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzo, B.; Scialla, S.; Barca, A.; Sercia, L.; Izzo, D.; Gervaso, F.; Scalera, F. Towards Complex Tissues Replication: Multilayer Scaffold Integrating Biomimetic Nanohydroxyapatite/Chitosan Composites. Bioengineering 2024, 11, 471. https://doi.org/10.3390/bioengineering11050471
Palazzo B, Scialla S, Barca A, Sercia L, Izzo D, Gervaso F, Scalera F. Towards Complex Tissues Replication: Multilayer Scaffold Integrating Biomimetic Nanohydroxyapatite/Chitosan Composites. Bioengineering. 2024; 11(5):471. https://doi.org/10.3390/bioengineering11050471
Chicago/Turabian StylePalazzo, Barbara, Stefania Scialla, Amilcare Barca, Laura Sercia, Daniela Izzo, Francesca Gervaso, and Francesca Scalera. 2024. "Towards Complex Tissues Replication: Multilayer Scaffold Integrating Biomimetic Nanohydroxyapatite/Chitosan Composites" Bioengineering 11, no. 5: 471. https://doi.org/10.3390/bioengineering11050471
APA StylePalazzo, B., Scialla, S., Barca, A., Sercia, L., Izzo, D., Gervaso, F., & Scalera, F. (2024). Towards Complex Tissues Replication: Multilayer Scaffold Integrating Biomimetic Nanohydroxyapatite/Chitosan Composites. Bioengineering, 11(5), 471. https://doi.org/10.3390/bioengineering11050471











