Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer
Abstract
1. Introduction
2. Materials and Methods
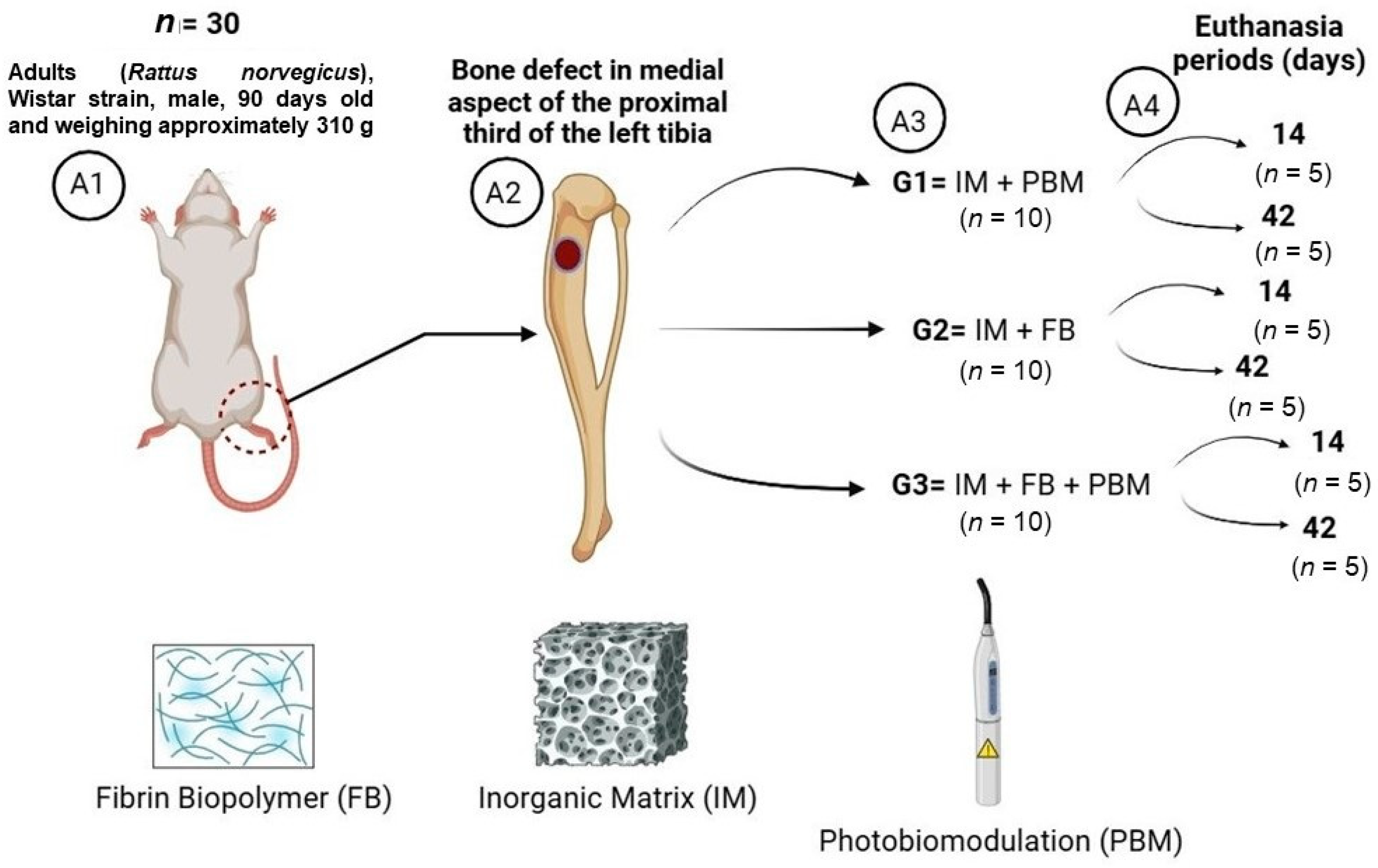
2.1. Experimental Design
- (1)
- G1 (n = 10): Inorganic Matrix + Photobiomodulation (IM + PBM);
- (2)
- G2 (n = 10): Inorganic Matrix + fibrin biopolymer (IM + FB);
- (3)
- G3 (n = 10): Inorganic Matrix + fibrin biopolymer + Photobiomodulation (IM + FB + PBM).
2.2. Experimental Surgery
2.3. Euthanasia and Tissue Collection
2.4. Computerized Microtomography
2.5. Histological Processing
2.6. Immunohistochemical Analysis
2.7. Morphometric Evaluation
2.8. Statistical Analysis
3. Results
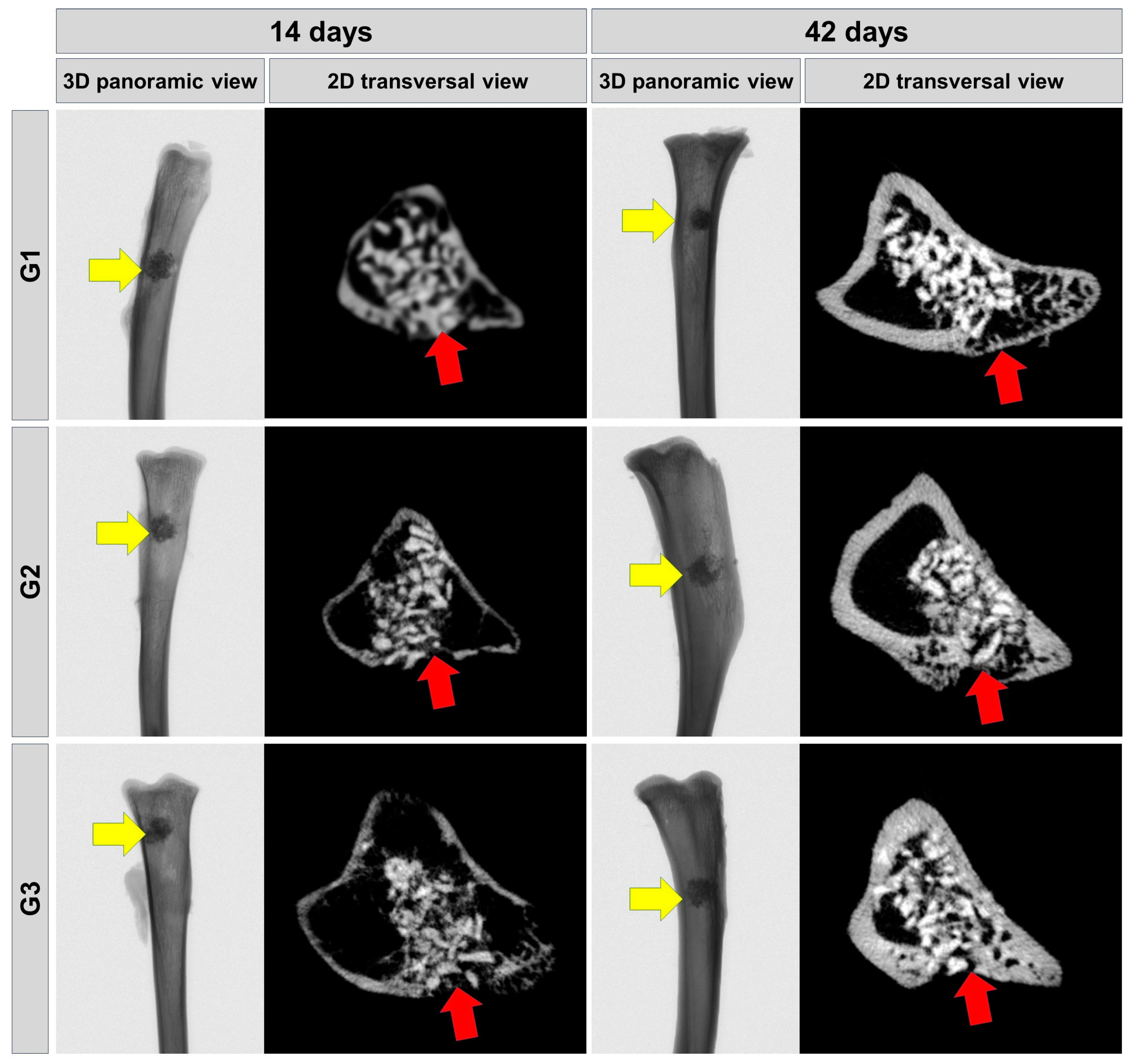
3.1. Microtomographic Evaluation
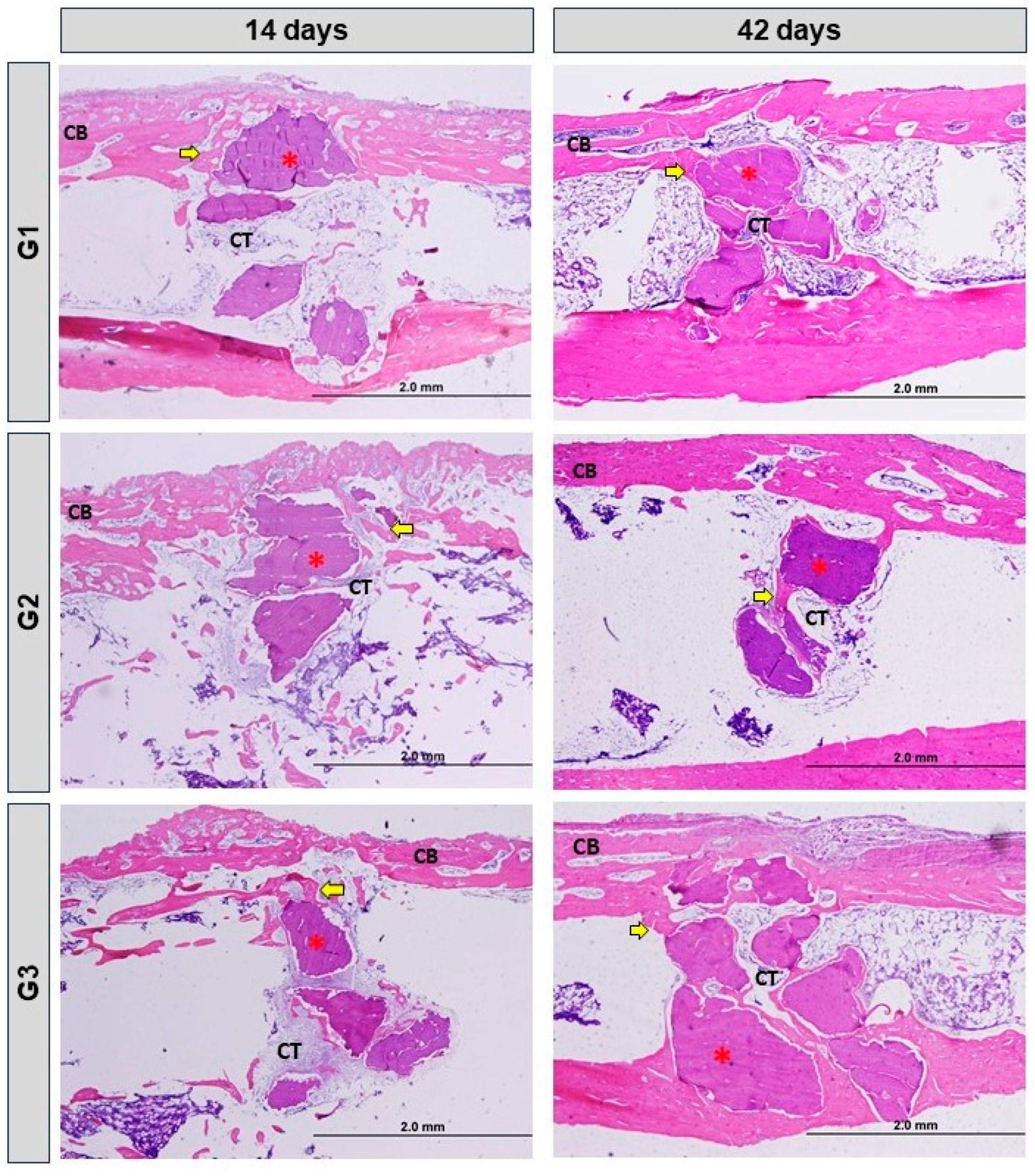
3.2. Histomorphological Analysis
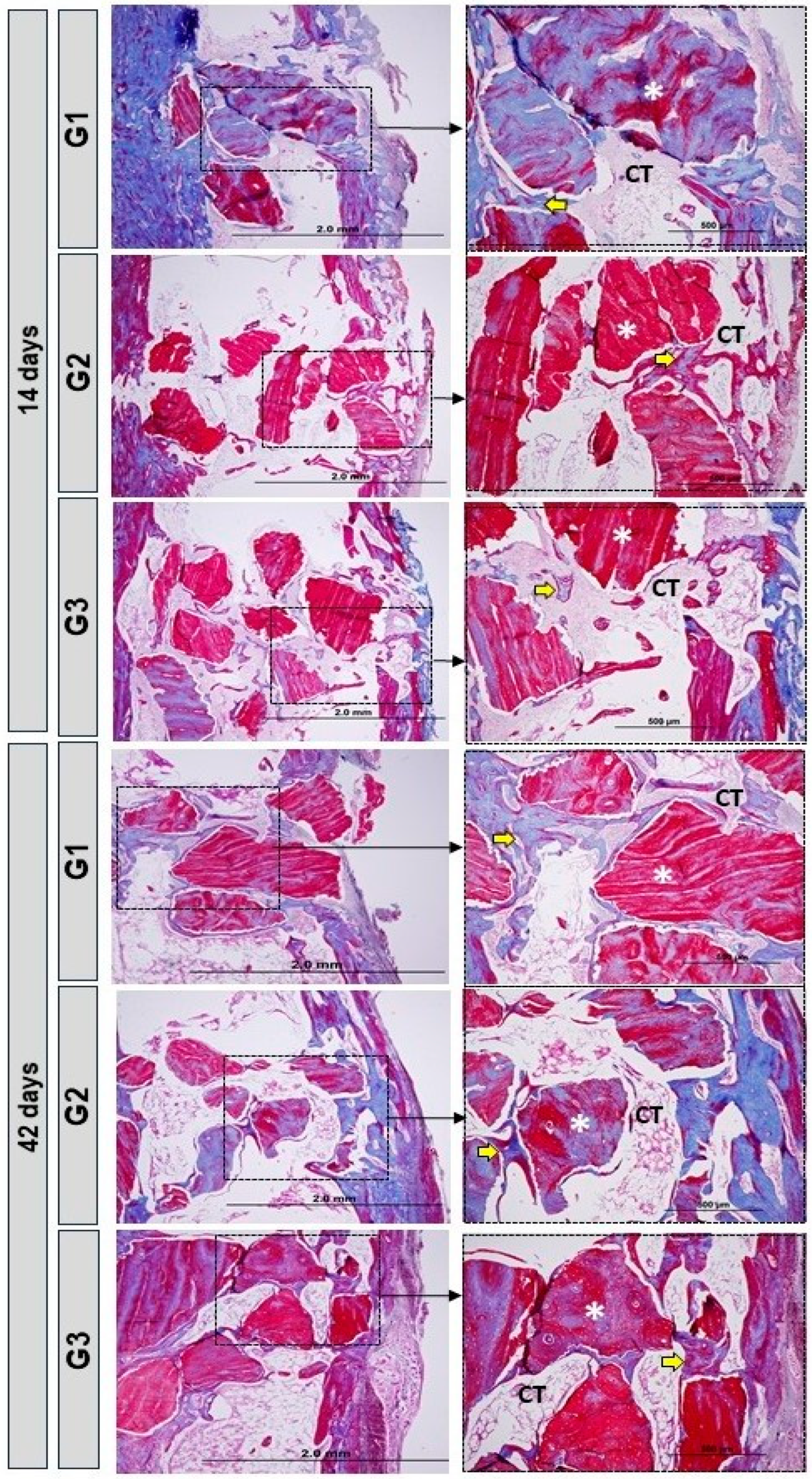
3.3. Birefringence Analysis of Collagen Fibers
3.4. Immunohistochemical Analysis
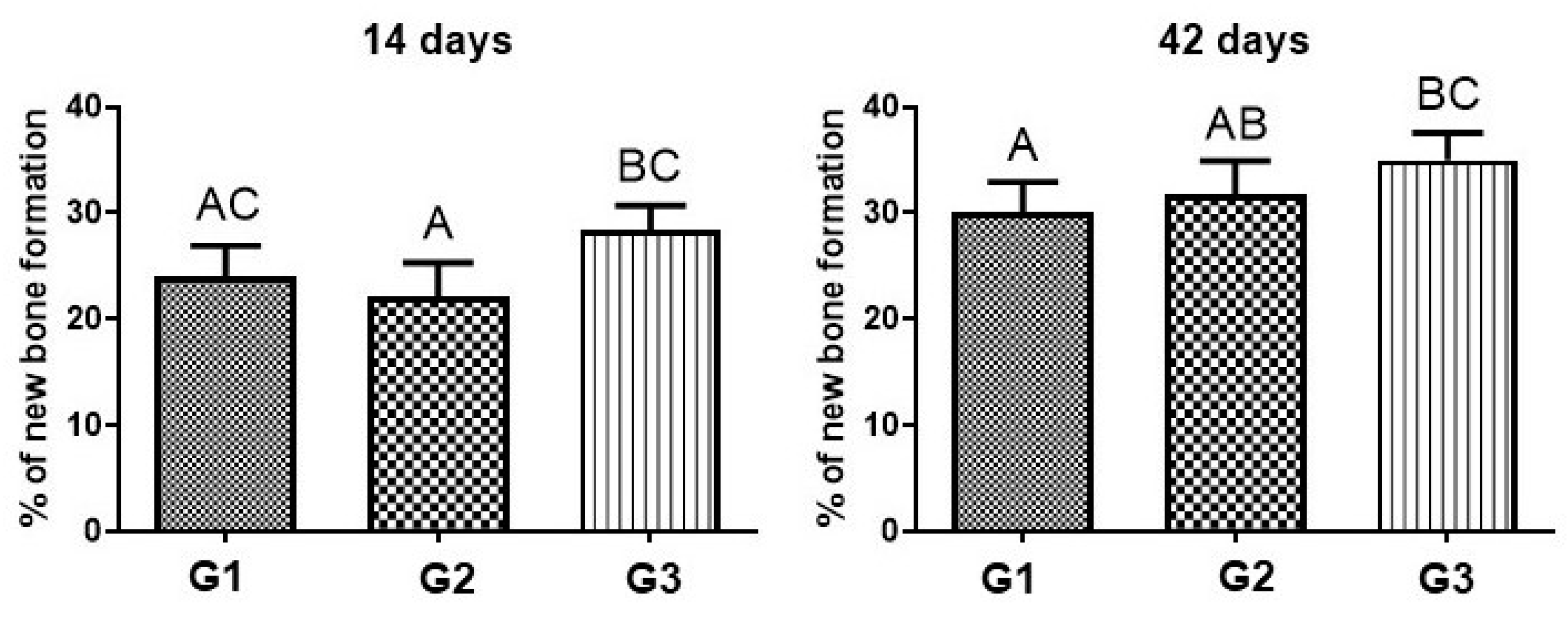
3.5. Morphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wang, X.; Lee, Y.-W.; Feng, L.; Wang, B.; Pan, Q.; Meng, X.; Cao, H.; Li, L.; Wang, H.; et al. Bioactive Scaffold Fabricated by 3D Printing for Enhancing Osteoporotic Bone Regeneration. Bioengineering 2022, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Chrzanowski, W.; Salih, V.M.; Kim, H.W.; Knowles, J.C. Tissue Engineering in Dentistry. J. Dent. 2014, 42, 915–992. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic Osteoinductivity of Porous Titanium Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef] [PubMed]
- Crovace, A.M.; Giancamillo, A.D.; Gervaso, F.; Mangiavini, L.; Zani, D.; Scalera, F.; Palazzo, B.; Izzo, D.; Agnoletto, M.; Domenicucci, M.; et al. Evaluation of in Vivo Response of Three Biphasic Scaffolds for Osteochondral Tissue Regeneration in a Sheep Model. Vet. Sci. 2019, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Boccaccio, A. Design of Materials for Bone Tissue Scaffolds. Materials 2021, 14, 5985. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Flores, M.; Madureira, S.; Zanotto, F.; Monteiro, F.J.; Laranjeira, M.S. Magnetic Bone Tissue Engineering: Reviewing the Effects of Magnetic Stimulation on Bone Regeneration and Angiogenesis. Pharmaceutics 2023, 15, 1045. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.G.; Yun, P.Y.; Yeo, I.S.; Jin, S.C.; Oh, J.S.; Kim, H.J.; Yu, S.K.; Lee, S.Y.; Kim, J.S.; et al. Autogenous Teeth Used for Bone Grafting: A Comparison with Traditional Grafting Materials. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.D.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.D.B.; Pereira, E.D.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 3150. [Google Scholar] [CrossRef]
- de Azambuja Carvalho, P.H.; Al-Maawi, S.; Dohle, E.; Sader, R.A.; Pereira-Filho, V.A.; Ghanaati, S. Cellular Response of Human Osteoblasts to Different Presentations of Deproteinized Bovine Bone. Materials 2022, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Crist, B.D.; Morshed, S.; Watson, J.T.; Pape, H.-C. Management of Aseptic Nonunions and Severe Bone Defects: Let Us Get This Thing Healed! OTA Int. 2023, 6, e258. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, F.; Wang, R.; Shang, X.; Yang, P.; Zhu, Y.; Tsoi, J.K.H.; Chan, K.; Wang, S. Guided Bone Regeneration in a Periodontally Compromised Individual with Autogenous Tooth Bone Graft: A Radiomics Analysis. J. Funct. Biomater. 2023, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Mauffrey, C.; Vasario, G.; Battiston, B.; Lewis, C.; Beazley, J.; Seligson, D. Tibial pilon fractures: A review of incidence, diagnosis, treatment, and complications. Acta Orthop. Belg. 2011, 77, 432–440. [Google Scholar]
- Egol, K.A.; Nauth, A.; Lee, M.; Pape, H.C.; Watson, J.T.; Borrelli, J. Bone Grafting: Sourcing, Timing, Strategies, and Alternatives. J. Orthop. Trauma. 2015, 29 (Suppl. S12), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Ben Bouzid, Y.; Bassir, R.-A.; Boufettal, M.; Mekkaoui, J.; Kharmaz, M.; Lamrani, M.O.; Berrada, M.S. Minimally Invasive Technique in the Management of Tibial Pilon Fractures: New Approach and Promising Results. Adv. Orthop. 2023, 2023, 1272490. [Google Scholar] [CrossRef]
- Hampel, G.A.; Yilmaz, E.; Massrey, C.; Clifton, W.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. History of Bone Grafts in Spine Surgery. Cureus 2022, 14, e24655. [Google Scholar] [CrossRef]
- Trevisiol, C.H.; Turner, R.T.; Pfaff, J.E.; Hunter, J.C.; Menagh, P.J.; Hardin, K.; Ho, E.; Iwaniec, U.T. Impaired Osteoinduction in a Rat Model for Chronic Alcohol Abuse. Bone 2007, 41, 175–180. [Google Scholar] [CrossRef]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef]
- Nguyen-Thanh, T.; Nguyen-Tran, B.-S.; Cruciani, S.; Nguyen-Thi, T.-D.; Dang-Cong, T.; Maioli, M. Osteochondral Regeneration Ability of Uncultured Bone Marrow Mononuclear Cells and Platelet-Rich Fibrin Scaffold. Bioengineering 2023, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.P.D.O.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.V.T.C.; Ferreira Júnior, R.S.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.D.B.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Iwase, D.; Metoki, Y.; Kusumoto, Y.; Aikawa, J.; Fukushima, K.; Takano, S.; Mukai, M.; Uchida, K.; Inoue, G.; Takaso, M. Using Allogenous Structural Bone Graft for Uncontained Tibial Bone Defects ≥ 10 Mm in Depth in Primary Total Knee Arthroplasty. BMC Musculoskelet. Disord. 2022, 23, 528. [Google Scholar] [CrossRef] [PubMed]
- Melek, L.N. Tissue Engineering in Oral and Maxillofacial Reconstruction. Tanta Dent. J. 2015, 12, 211–223. [Google Scholar] [CrossRef]
- Magri, A.M.P.; Parisi, J.R.; de Andrade, A.L.M.; Rennó, A.C.M. Bone Substitutes and Photobiomodulation in Bone Regeneration: A Systematic Review in Animal Experimental Studies. J. Biomed. Mater. Res. A 2021, 109, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brum, I.; Frigo, L.; Lana Devita, R.; da Silva Pires, J.L.; Hugo Vieira de Oliveira, V.; Rosa Nascimento, A.L.; de Carvalho, J.J. Histomorphometric, Immunohistochemical, Ultrastructural Characterization of a Nano-Hydroxyapatite/Beta-Tricalcium Phosphate Composite and a Bone Xenograft in Sub-Critical Size Bone Defect in Rat Calvaria. Materials 2020, 13, 4598. [Google Scholar] [CrossRef]
- Pollock, R.; Alcelik, I.; Bhatia, C.; Chuter, G.; Lingutla, K.; Budithi, C.; Krishna, M. Donor Site Morbidity Following Iliac Crest Bone Harvesting for Cervical Fusion: A Comparison between Minimally Invasive and Open Techniques. Eur. Spine J. 2008, 17, 845–852. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of Photobiomodulation on Bone Defects Grafted with Bone Substitutes: A Systematic Review of in Vivo Animal Studies. J. Biophotonics 2021, 14, e202000267. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef]
- Chen, K.Y.; Shyu, P.C.; Dong, G.C.; Chen, Y.S.; Kuo, W.W.; Yao, C.H. Reconstruction of Calvarial Defect Using a Tricalcium Phosphate-Oligomeric Proanthocyanidins Cross-Linked Gelatin Composite. Biomaterials 2009, 30, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Qi, Y.; Ma, X.; Qiao, S.; Cai, H.X.; Zhao, B.C.; Jiang, H.B.; Lee, E.S. Comparison of Autogenous Tooth Materials and Other Bone Grafts. Tissue Eng. Regen. Med. 2021, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Savadori, P.; Patano, A.; Inchingolo, A.D.; Rapone, B.; Malcangi, G.; Inchingolo, F.; Dipalma, G.; Tartaglia, F.C.; et al. Socket Preservation Using Dentin Mixed with Xenograft Materials: A Pilot Study. Materials 2023, 16, 4945. [Google Scholar] [CrossRef]
- Carbonari, M.; Ludtke, J.; Santos, P.C.V.; Amaral, N.T.; Gehrke, S.A. Characterization physical-chemical and biological the bovine Characterization physical-chemical and biological the bovine bone graft, Bonefill in biogical assays—Part 1. ImplantNews 2010, 7, 103–110. [Google Scholar]
- Chisci, G.; Hatia, A.; Chisci, E.; Chisci, D.; Gennaro, P.; Gabriele, G. Socket Preservation after Tooth Extraction: Particulate Autologous Bone vs. Deproteinized Bovine Bone. Bioengineering 2023, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF—Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Morello, G.; Polini, A.; Scalera, F.; Rizzo, R.; Gigli, G.; Gervaso, F. Preparation and Characterization of Salt-Mediated Injectable Thermosensitive Chitosan/Pectin Hydrogels for Cell Embedding and Culturing. Polymers 2021, 13, 2674. [Google Scholar] [CrossRef]
- Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Advances in Regenerative Medicine and Biomaterials. Methods Mol. Biol. 2023, 2575, 127–152. [Google Scholar] [CrossRef]
- Chon, J.G.; Kang, J.W.; Kim, C.U.; Jeong, U.; Md, J.G. Treatment of 10-Mm-Deep or Greater Uncontained Tibial Bone Defects in Primary Total Knee Reconstruction without Metal Augmentation: Autologous Oblique Structural Peg Bone and Cancellous Chip Bone Grafting. CiOS Clin. Orthop. Surg. 2021, 13, 168–174. [Google Scholar] [CrossRef]
- Fernandes, Y.; Mantovani, R.; Reino, D.; Novaes, A.; Messora, M.; Gustavo Sousa, L.; Palioto, D.; Scombatti de Souza, S. Evaluation of a New Porcine Bone Graft on the Repair of Surgically Created Critical Bone Defects in Rat Calvaria: Histomorphometric and Microtomographic Study. J. Funct. Biomater. 2022, 13, 124. [Google Scholar] [CrossRef]
- German, I.J.S.; Pomini, K.T.; Bighetti, A.C.C.; Andreo, J.C.; Reis, C.H.B.; Shinohara, A.L.; Rosa, G.M.; de Bortoli Teixeira, D.; de Oliveira Rosso, M.P.; Buchaim, D.V.; et al. Evaluation of the Use of an Inorganic Bone Matrix in the Repair of Bone Defects in Rats Submitted to Experimental Alcoholism. Materials 2020, 13, 695. [Google Scholar] [CrossRef] [PubMed]
- Francisco, I.; Basílio, Â.; Ribeiro, M.P.; Nunes, C.; Travassos, R.; Marques, F.; Pereira, F.; Paula, A.B.; Carrilho, E.; Marto, C.M.; et al. Three-Dimensional Impression of Biomaterials for Alveolar Graft: Scoping Review. J. Funct. Biomater. 2023, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Chadha, V.; Donos, N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: A randomized, controlled clinical trial. Clin. Oral. Implant. Res. 2010, 21, 688–698. [Google Scholar] [CrossRef]
- Gao, X.; Cheng, H.; Sun, X.; Lu, A.; Ruzbarsky, J.J.; Wang, B.; Huard, J. Comparison of Autologous Blood Clots with Fibrin Sealant as Scaffolds for Promoting Human Muscle-Derived Stem Cell-Mediated Bone Regeneration. Biomedicines 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Goubran, H.A.; Chen, T.M.; Ou, K.L.; El-Ekiaby, M.; Radosevic, M. Blood-Derived Biomaterials and Platelet Growth Factors in Regenerative Medicine. Blood Rev. 2013, 27, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Rodrigues, A.D.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; de Souza Bueno, C.R.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The New Heterologous Fibrin Sealant in Combination with Low-Level Laser Therapy (LLLT) in the Repair of the Buccal Branch of the Facial Nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef]
- Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; Lima, A.B.B.D.C.O.; Haddad, G.R.; Gatti, M.A.N.; Medolago, N.B.; Rigotto Carneiro, M.T.; dos Santos, L.D.; Ferreira, R.S.; et al. Treatment of Chronic Venous Ulcers with Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 627541. [Google Scholar] [CrossRef]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; Dos Santos, L.D.; Barraviera, B. Heterologous Fibrin Sealant Derived from Snake Venom: From Bench to Bedside—An Overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef]
- de Oliveira, L.S.D.S.; de Araújo, A.A.; de Araújo Júnior, R.F.; Barboza, C.A.G.; Borges, B.C.D.; da Silva, J.S.P. Low-Level Laser Therapy (780 Nm) Combined with Collagen Sponge Scaffold Promotes Repair of Rat Cranial Critical-Size Defects and Increases TGF-β, FGF-2, OPG/RANK and Osteocalcin Expression. Int. J. Exp. Pathol. 2017, 98, 75–85. [Google Scholar] [CrossRef]
- de Oliveira, F.L.D.; Nagato, A.C.; Aarestrup, F.M.; Aarestrup, B.J.V. Bone Neoformation Induced by Low-Level Laser and Methylene Blue Suggests Early Ossification in Rats. J. Lasers Med. Sci. 2022, 13, e48. [Google Scholar] [CrossRef]
- Hauck, M.; Schardong, J.; Donini, G.; Normann, T.C.; Plentz, R.D.M. Effects of Photobiomodulation Therapy (PBMT) over Endothelial Function in Healthy Individuals: A Preliminary Crossover Clinical Trial. Lasers Med. Sci. 2023, 38, 104. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-S.; Fierro, C.; Bravo, L.; Perez-Flores, A. Benefits of Using Low-Level Laser Therapy in the Rapid Maxillary Expansion: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2021, 14, S101–S106. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.V.; dos Reis, T.; Amorim, J.; Rocha, F.S.; Marques, M.M.; Guerra, E.S.; Hanna, R.; Gallo, C.B. Efficacy of Photobiomodulation Therapy on Healing of Ionizing Irradiated Bone: A Systematic Review of in Vivo Animal Studies. Lasers Med. Sci. 2022, 37, 3379–3392. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Sadeghi Ghuchani, M.; Eslaminejad, M.B.; Taghiyar, L.; Kalhori, K.A.M.; Pedram, M.S.; Shayan, A.M.; Aghdami, N.; Abrahamse, H. The Effects of Combined Low Level Laser Therapy and Mesenchymal Stem Cells on Bone Regeneration in Rabbit Calvarial Defects. J. Photochem. Photobiol. B 2015, 151, 180–185. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Venken, K.; Callewaert, F.; Boonen, S.; Vanderschueren, D. Sex Hormones, Their Receptors and Bone Health. Osteoporos. Int. 2008, 19, 1517–1525. [Google Scholar] [CrossRef]
- Oury, F. A Crosstalk between Bone and Gonads. Ann. N. Y. Acad. Sci. 2012, 1260, 1–7. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Andreo, J.C.; Rosso, M.P.D.O.; Della Coletta, B.B.; German, Í.J.S.; Biguetti, A.C.C.; Shinohara, A.L.; Rosa, G.M., Jr.; Cosin Shindo, J.V.T.; et al. Fibrin Sealant Derived from Human Plasma as a Scaffold for Bone Grafts Associated with Photobiomodulation Therapy. Int. J. Mol. Sci. 2019, 20, 1761. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Bighetti, A.C.C.; Hamzé, A.L.; Reis, C.H.B.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Júnior, R.S.F.; de Souza, A.T.; et al. Tissue Bioengineering with Fibrin Scaffolds and Deproteinized Bone Matrix Associated or Not with the Transoperative Laser Photobiomodulation Protocol. Molecules 2023, 28, 407. [Google Scholar] [CrossRef]
- Barros, L.C.; Ferreira, R.S.; Barraviera, S.R.C.S.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.S.; Toscano, E.; Barraviera, B. A New Fibrin Sealant from Crotalus Durissus Terrificus Venom: Applications in Medicine. J. Toxicol. Env. Health B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, J.B.; Buchaim, D.V.; de Souza Bueno, C.R.; Pomini, K.T.; Barraviera, B.; Júnior, R.S.F.; Andreo, J.C.; de Castro Rodrigues, A.; Cestari, T.M.; Buchaim, R.L. Effects of low-level laser therapy on autogenous bone graft stabilized with a new heterologous fibrin sealant. J. Photochem. Photobiol. B 2016, 162, 663–668. [Google Scholar] [CrossRef]
- Bosco, A.F.; Faleiros, P.L.; Carmona, L.R.; Garcia, V.G.; Theodoro, L.H.; de Araujo, N.J.; Nagata, M.J.H.; de Almeida, J.M. Effects of Low-Level Laser Therapy on Bone Healing of Critical-Size Defects Treated with Bovine Bone Graft. J. Photochem. Photobiol. B 2016, 163, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Freitas, N.R.D.; Guerrini, L.B.; Esper, L.A.; Sbrana, M.C.; Santos, C.C.V.D.; Almeida, A.L.P.F.D. Photobiomodulation and Inorganic Bovine Bone in Guided Bone Regeneration: Histomorphometric Analysis in Rats. J. Funct. Biomater. 2023, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- de Lima Barbosa, R.; Stellet Lourenço, E.; de Azevedo dos Santos, J.V.; Rodrigues Santiago Rocha, N.; Mourão, C.F.; Alves, G.G. The Effects of Platelet-Rich Fibrin in the Behavior of Mineralizing Cells Related to Bone Tissue Regeneration—A Scoping Review of In Vitro Evidence. J. Funct. Biomater. 2023, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.D.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.D.S.B.M.; Roque, D.D.; Rosso, M.P.D.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef] [PubMed]
- Ramires, G.A.D.; Helena, J.T.; De Oliveira, J.C.S.; Faverani, L.P.; Bassi, A.P.F. Evaluation of Guided Bone Regeneration in Critical Defects Using Bovine and Porcine Collagen Membranes: Histomorphometric and Immunohistochemical Analyses. Int. J. Biomater. 2021, 2021, 8828194. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Doering, H.; Schmidt, T.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Histological Results after Maxillary Sinus Augmentation with Straumann® BoneCeramic, Bio-Oss®, Puros®, and Autologous Bone. A Randomized Controlled Clinical Trial. Clin. Oral. Implant. Res. 2013, 24, 576–585. [Google Scholar] [CrossRef]
- Kapogianni, E.; Barbeck, M.; Jung, O.; Arslan, A.; Kuhnel, L.; Xiong, X.; Krastev, R.; Friedrich, R.; Schnettler, R.; Fienitz, T.; et al. Comparison of Material-Mediated Bone Regeneration Capacities of Sintered and Non-Sintered Xenogeneic Bone Substitutes via 2D and 3D Data. In Vivo 2019, 33, 2169–2180. [Google Scholar] [CrossRef]
- Kim, Y.; Brodt, M.D.; Tang, S.Y.; Silva, M.J. MicroCT for Scanning and Analysis of Mouse Bones. Methods Mol. Biol. 2021, 2230, 169–198. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Optimization of Digital Volume Correlation Computation in SR-MicroCT Images of Trabecular Bone and Bone-Biomaterial Systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef]
- Fernández, M.P.; Witte, F.; Tozzi, G. Applications of X-Ray Computed Tomography for the Evaluation of Biomaterial-Mediated Bone Regeneration in Critical-Sized Defects. J. Microsc. 2020, 277, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.; Momesso, G.A.C.; Ramalho-Ferreira, G.; Consolaro, R.B.; De Carvalho, P.S.P.; Faverani, L.P.; Bassi, A.P.F. Bone Healing Evaluation in Critical-Size Defects Treated with Xenogenous Bone Plus Porcine Collagen. Implant. Dent. 2017, 26, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. [Google Scholar] [CrossRef]
- Bueno, C.R.D.S.; Tonin, M.C.C.; Buchaim, D.V.; Barraviera, B.; Ferreira Junior, R.S.; Santos, P.S.D.S.; Reis, C.H.B.; Pastori, C.M.; Pereira, E.D.S.B.M.; Nogueira, D.M.B.; et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals 2023, 16, 653. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Brito, A.F.; Brazete, D.; Pereira, I.C.; Carrilho, E.; Abrantes, A.M.; Pires, A.S.; Aguiar, M.J.; Carvalho, L.; Botelho, M.F.; et al. Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats. Materials 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.D.C. Biocompatibility of anioniccollagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chung, T.Y.; Baxter, G.D. Laser Photobiomodulation of Proliferation of Cells in Culture: A Review of Human and Animal Studies. Photomed. Laser Surg. 2010, 28 (Suppl. S1), S3–S40. [Google Scholar] [CrossRef]
- Klassmann, F.-A.; Ervolino, E.; Kluppel, L.-E.; Theodoro, L.-H.; Mulinari-Santos, G.; Garcia, V.-G. A Randomised Trial of the Bone Formation after Maxillary Sinus Floor Augmentation with Bovine Hydroxyapatite (Cerabone®) and Photobiomodulation: Histomorphometric and Immunohistochemical Analysis. J. Clin. Exp. Dent. 2023, 15, e542–e550. [Google Scholar] [CrossRef]
- Nogueira, D.M.B.; Figadoli, A.L.D.F.; Alcantara, P.L.; Pomini, K.T.; Santos German, I.J.; Reis, C.H.B.; Rosa Júnior, G.M.; Rosso, M.P.D.O.; Santos, P.S.D.S.; Zangrando, M.S.R.; et al. Biological Behavior of Xenogenic Scaffolds in Alcohol-Induced Rats: Histomorphometric and Picrosirius Red Staining Analysis. Polymers 2022, 14, 584. [Google Scholar] [CrossRef]
- Marques, L.; Holgado, L.A.; Francischone, L.A.; Ximenez, J.P.B.; Okamoto, R.; Kinoshita, A. New LLLT Protocol to Speed up the Bone Healing Process-Histometric and Immunohistochemical Analysis in Rat Calvarial Bone Defect. Lasers Med. Sci. 2015, 30, 1225–1230. [Google Scholar] [CrossRef]
- De Marco, A.C.; Torquato, L.C.; Gonçalves, P.R.; Ribeiro, T.C.; Nunes, C.M.; Bernardo, D.V.; Gomes, M.F.; Jardini, M.A.N.; Santamaria, M.P. The Effect of Photobiomodulation Therapy in Different Doses on Bone Repair of Critical Size Defects in Rats: A Histomorphometric Study. J. Lasers Med. Sci. 2021, 12, e53. [Google Scholar] [CrossRef] [PubMed]
- Asteinza Castro, I.M.; Morga, A.A.; Johnson, D.S. Photobiomodulation Therapy Combined with Static Magnetic Field in Tibial Fracture Healing of a Dog: A Case Report. Vet. Med. Sci. 2023, 9, 591–599. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue—A Review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Laurinavicius, A.; Plancoulaine, B.; Herlin, P.; Laurinaviciene, A. Comprehensive Immunohistochemistry: Digital, Analytical and Integrated. Pathobiology 2016, 83, 156–163. [Google Scholar] [CrossRef]
- Pires, J.L.D.S.; de Carvalho, J.J.; Pereira, M.J.D.S.; Brum, I.D.S.; Nascimento, A.L.R.; dos Santos, P.G.P.; Frigo, L.; Fischer, R.G. Repair of Critical Size Bone Defects Using Synthetic Hydroxyapatite or Xenograft with or without the Bone Marrow Mononuclear Fraction: A Histomorphometric and Immunohistochemical Study in Rat Calvaria. Materials 2021, 14, 2854. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.A.; Cestari, T.M.; Vidotti, H.A.; De Assis, G.F.; Garlet, G.P.; Taga, R. Sintered Anorganic Bone Graft Increases Autocrine Expression of VEGF, MMP-2 and MMP-9 during Repair of Critical-Size Bone Defects. J. Mol. Histol. 2014, 45, 447–461. [Google Scholar] [CrossRef]
- De Marco, A.C.; Cavassini Torquato, L.; Camacho Ribeiro, T.; Moretto Nunes, C.; Vicensotto Bernardo, A.; Martins Maciel, C.C.; Alberto Pereira, K.; Neves Jardini, M.A.; Pedrine Santamaria, M. Effect of Photobiomodulation Therapy Associated withBiphasic Phosphate Calcium on Bone Repair: A Histomorphometric Study in Rats. J. Lasers Med. Sci. 2022, 13, e33. [Google Scholar] [CrossRef]
- Silva, S.K.; Plepis, A.M.G.; Martins, V.d.C.A.; Horn, M.M.; Buchaim, D.V.; Buchaim, R.L.; Pelegrine, A.A.; Silva, V.R.; Kudo, M.H.M.; Fernandes, J.F.R.; et al. Suitability of Chitosan Scaffolds with Carbon Nanotubes for Bone Defects Treated with Photobiomodulation. Int. J. Mol. Sci. 2022, 23, 6503. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.F.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The Stimulation of Healing within a Rat Calvarial Defect by MPCL-TCP/Collagen Scaffolds Loaded with RhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Nagata, M.J.H.; Messora, M.; Pola, N.; Campos, N.; Vieira, R.; Esper, L.A.; Sbrana, M.; Fucini, S.; Garcia, V.; Bosco, A. Influence of the Ratio of Particulate Autogenous Bone Graft/Platelet-Rich Plasma on Bone Healing in Critical-Size Defects: A Histologic and Histometric Study in Rat Calvaria. J. Orthop. Res. 2010, 28, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular Mechanosensors in Osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A. Tartrate-Resistant Acid Phosphatase (TRAP) and the Osteoclast/Immune Cell Dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.G.; Issa, J.P.M.; de Figueiredo, F.A.T.; Santos dos, G.R.; Galdeano, E.A.; Alves, M.C.; Chacon, E.L.; Ferreira Junior, R.S.; Barraviera, B.; da Cunha, M.R. da A New Heterologous Fibrin Sealant as Scaffold to Recombinant Human Bone Morphogenetic Protein-2 (RhBMP-2) and Natural Latex Proteins for the Repair of Tibial Bone Defects. Acta Histochem. 2015, 117, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol. 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Andreo, J.C.; Pomini, K.T.; Barraviera, B.; Ferreira, R.S.; Duarte, M.A.H.; Alcalde, M.P.; Reis, C.H.B.; de Bortoli Teixeira, D.; de Souza Bueno, C.R.; et al. A Biocomplex to Repair Experimental Critical Size Defects Associated with Photobiomodulation Therapy. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210056. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Bronnikova, I.I.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Linkova, D.D.; Aleynik, D.Y. Aspects of In Vitro Biodegradation of Hybrid Fibrin–Collagen Scaffolds. Polymers 2021, 13, 3470. [Google Scholar] [CrossRef]
- Manfro, R.; Fonseca, F.S.; Bortoluzzi, M.C.; Sendyk, W.R. Comparative, Histological and Histomorphometric Analysis of Three Anorganic Bovine Xenogenous Bone Substitutes: Bio-Oss, Bone-Fill and Gen-Ox Anorganic. J. Maxillofac. Oral. Surg. 2014, 13, 464–470. [Google Scholar] [CrossRef]
- Grossi-Oliveira, G.; Faverani, L.P.; Mendes, B.C.; Braga Polo, T.O.; Batista Mendes, G.C.; De Lima, V.N.; Ribeiro Júnior, P.D.; Okamoto, R.; Magro-Filho, O. Comparative Evaluation of Bone Repair with Four Different Bone Substitutes in Critical Size Defects. Int. J. Biomater. 2020, 2020, 5182845. [Google Scholar] [CrossRef]
- Santos, M.A.; Silva, D.N.; Rovaris, K.; Sousa, F.B.; Dantas, E.L.A.; Loureiro, L.A.; Pereira, T.M.C.; Meyrelles, S.S.; Bertollo, R.M.; Vasquez, E.C. Optimal Parameters of Laser Therapy to Improve Critical Calvarial Defects. Front. Physiol. 2022, 13, 841146. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Patano, A.; Palumbo, I.; Guglielmo, M.; Trilli, I.; Netti, A.; Ferrara, I.; Viapiano, F.; Inchingolo, A.D.; et al. Photobiomodulation and Growth Factors in Dentistry: A Systematic Review. Photonics 2023, 10, 1095. [Google Scholar] [CrossRef]
- Farzan, A.; Khaleghi, K.; Pirayesh, Z. Effect of Low-Level Laser Therapy on Bone Formation in Rapid Palatal Expansion: A Systematic Review. J. Lasers Med. Sci. 2022, 13, e13. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-C.; Hang, N.-L.-T.; Colley, M.M.S.; Chang, J.; Hsiao, Y.-C.; Lu, L.-S.; Li, B.-S.; Chang, C.-J.; Yang, T.-S. Single Cell Effects of Photobiomodulation on Mitochondrial Membrane Potential and Reactive Oxygen Species Production in Human Adipose Mesenchymal Stem Cells. Cells 2022, 11, 972. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Moganan, M.; Hamid, M.S.A.; Sulaiman, N.; Moorthy, U.; Hasnan, N.; Yusof, A. Comparison between Low-Level and High-Intensity Laser Therapy as an Adjunctive Treatment for Knee Osteoarthritis: A Randomized, Double-Blind Clinical Trial. Life 2023, 13, 1519. [Google Scholar] [CrossRef]
- Pomini, K.T.; Andreo, J.C.; Rodrigues, A.; de C. Rodrigues, A.; de O. Gonçalves, J.B.; Daré, L.R.; German, I.J.; Rosa, G.M., Jr.; Buchaim, R.L. Effect of low-intensity pulsed ultrasound on bone regeneration: Biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Lisboa-Filho, P.N.; da Silva, A.C.; Bim-Júnior, O.; de Souza Batista, F.R.; Ervolino-Silva, A.C.; Garcia-Junior, I.R.; Okamoto, R. Sonochemical time standardization for bioactive materials used in periimplantar defects filling. Ultrason. Sonochem 2019, 56, 437–446. [Google Scholar] [CrossRef]
- Lirani, A.P.; Lazaretti-Castro, M. Evidences of physical agents action on bone metabolism and their potential clinical use. Arq. Bras. Endocrinol. Metabol. 2005, 49, 891–896. [Google Scholar] [CrossRef][Green Version]
- Buchaim, D.V.; Bueno, P.C.D.S.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Zilio, M.G.; Salatin, J.A.; Kawano, N.; Furlanette, G.; Buchaim, R.L. Action of a deproteinized xenogenic biomaterial in the process of bone repair in rats submitted to inhalation of cigarette smoke. Acta Cir. Bras. 2018, 33, 324–332. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Ferreira, S.; Rangel Garcia, I.; Caneva, M.; Botticelli, D. Healing at implants installed concurrently to maxillary sinus floor elevation with Bio-Oss® or autologous bone grafts. A histo-morphometric study in rabbits. Clin. Oral Implants Res. 2017, 28, 503–511. [Google Scholar] [CrossRef] [PubMed]

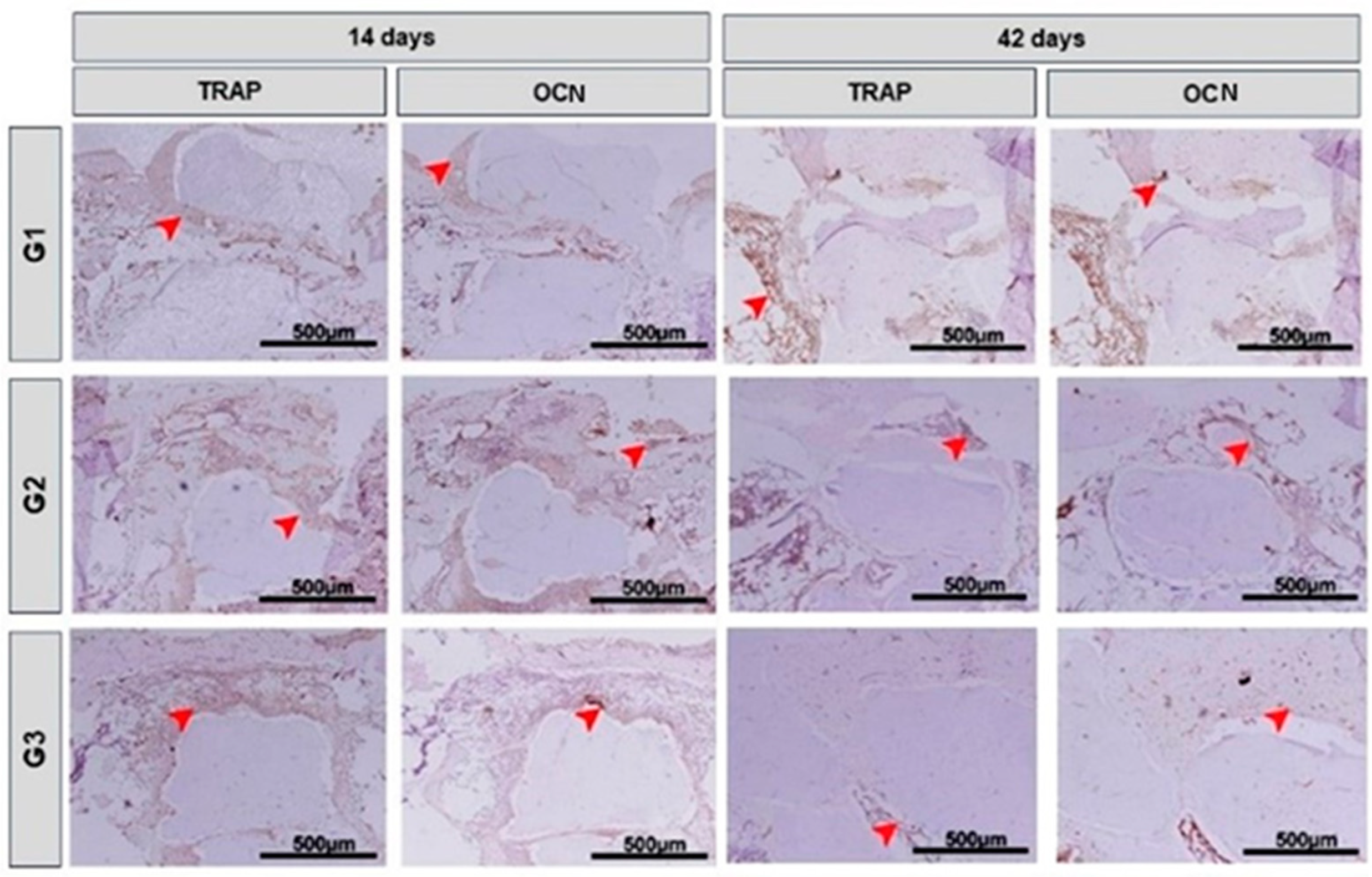

| Parameter | Description |
|---|---|
| Type of laser | GaAlAs (gallium-aluminum-arsenide) Manufacturer: Ibramed, Amparo, Brazil |
| Wavelength (nm) | 830 |
| Output power (mW) | 30 |
| Beam area (cm2) | 0.116 |
| Irradiance (mW/cm2) | 258.62 |
| Treatment time of irradiation, per point (s) | 24 |
| Number of points of the application LLLT | 2 (One above and one below the defect) |
| Energy per point (J) | 0.72 |
| Total energy (J) | 2.88 |
| Energy density of irradiation, per point (J/cm2) | 6.20 |
| Application method | Positioned for laser irradiation at perpendicular incidence to the tibia |
| Emission mode | Continuous |
| Frequency | Immediately after surgery and three times a week until euthanasia |
| 14 Days | 42 Days | p-Value | |
|---|---|---|---|
| G1 | 24.1 ± 2.91 aAC | 30.1 ± 2.9 bA | p = 0.0116 |
| G2 | 22.2 ± 3.11 aA | 31.8 ± 3.12 bAB | p = 0.0012 |
| G3 | 28.4 ± 2.3 aBC | 35.1 ± 2.55 bBC | p = 0.0026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigliar, M.F.R.; Marega, L.F.; Duarte, M.A.H.; Alcalde, M.P.; Rosso, M.P.d.O.; Ferreira Junior, R.S.; Barraviera, B.; Reis, C.H.B.; Buchaim, D.V.; Buchaim, R.L. Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer. Bioengineering 2024, 11, 78. https://doi.org/10.3390/bioengineering11010078
Vigliar MFR, Marega LF, Duarte MAH, Alcalde MP, Rosso MPdO, Ferreira Junior RS, Barraviera B, Reis CHB, Buchaim DV, Buchaim RL. Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer. Bioengineering. 2024; 11(1):78. https://doi.org/10.3390/bioengineering11010078
Chicago/Turabian StyleVigliar, Maria Fernanda Rossi, Lais Furlaneto Marega, Marco Antonio Hungaro Duarte, Murilo Priori Alcalde, Marcelie Priscila de Oliveira Rosso, Rui Seabra Ferreira Junior, Benedito Barraviera, Carlos Henrique Bertoni Reis, Daniela Vieira Buchaim, and Rogerio Leone Buchaim. 2024. "Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer" Bioengineering 11, no. 1: 78. https://doi.org/10.3390/bioengineering11010078
APA StyleVigliar, M. F. R., Marega, L. F., Duarte, M. A. H., Alcalde, M. P., Rosso, M. P. d. O., Ferreira Junior, R. S., Barraviera, B., Reis, C. H. B., Buchaim, D. V., & Buchaim, R. L. (2024). Photobiomodulation Therapy Improves Repair of Bone Defects Filled by Inorganic Bone Matrix and Fibrin Heterologous Biopolymer. Bioengineering, 11(1), 78. https://doi.org/10.3390/bioengineering11010078










