Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of GelMA
2.2. 1H-NMR Spectroscopy
2.3. GelMA Hydrogel Fabrication
2.4. PEGDA-GelMA Scaffold Fabrication
2.5. Preparation of Plant-Based Scaffolds
2.6. Decellularization Assessment
2.7. Ultrastructural Analysis
2.8. Biomechanical Tests
2.9. Assessment of Scaffold Degradability
2.10. Assessment of Swelling Degree
2.11. Human Adipose-Derived Stem Cells (hASC) Isolation and Cell Culture
2.12. Viability Assay
2.13. Dead/Live Staining
2.14. Osteochondrogenic Differentiation Assay
2.15. Immunofluorescence and Confocal Imaging
2.16. Statistical Analysis
3. Results
3.1. GelMA Hydrogel Fabrication and Mechanical Properties
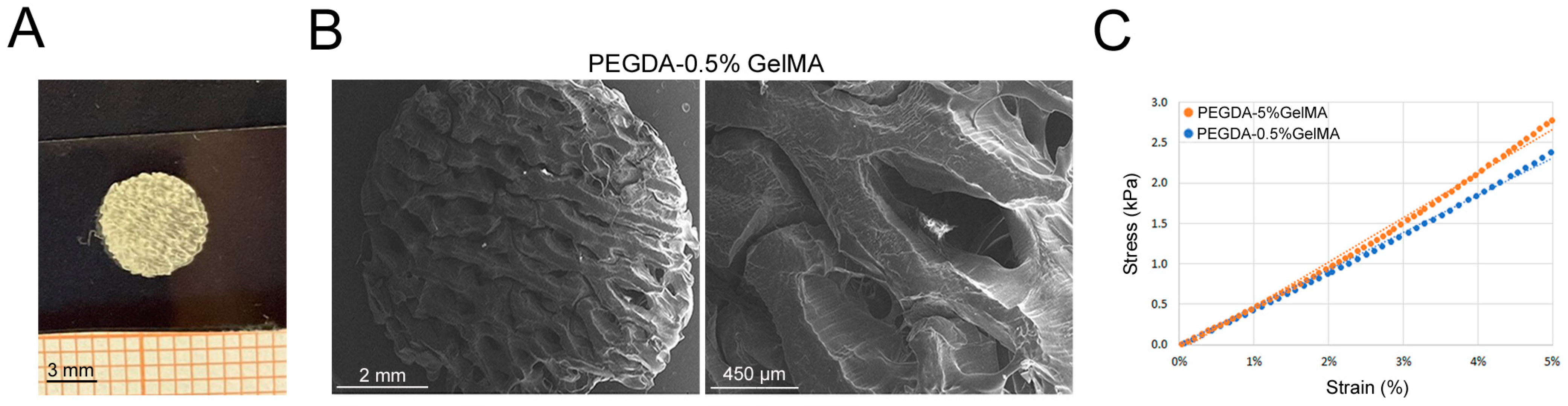
3.2. PEGDA-GelMA Scaffold Characterization and Mechanical Properties
3.3. Plant-Based Scaffold Characterization and Mechanical Properties
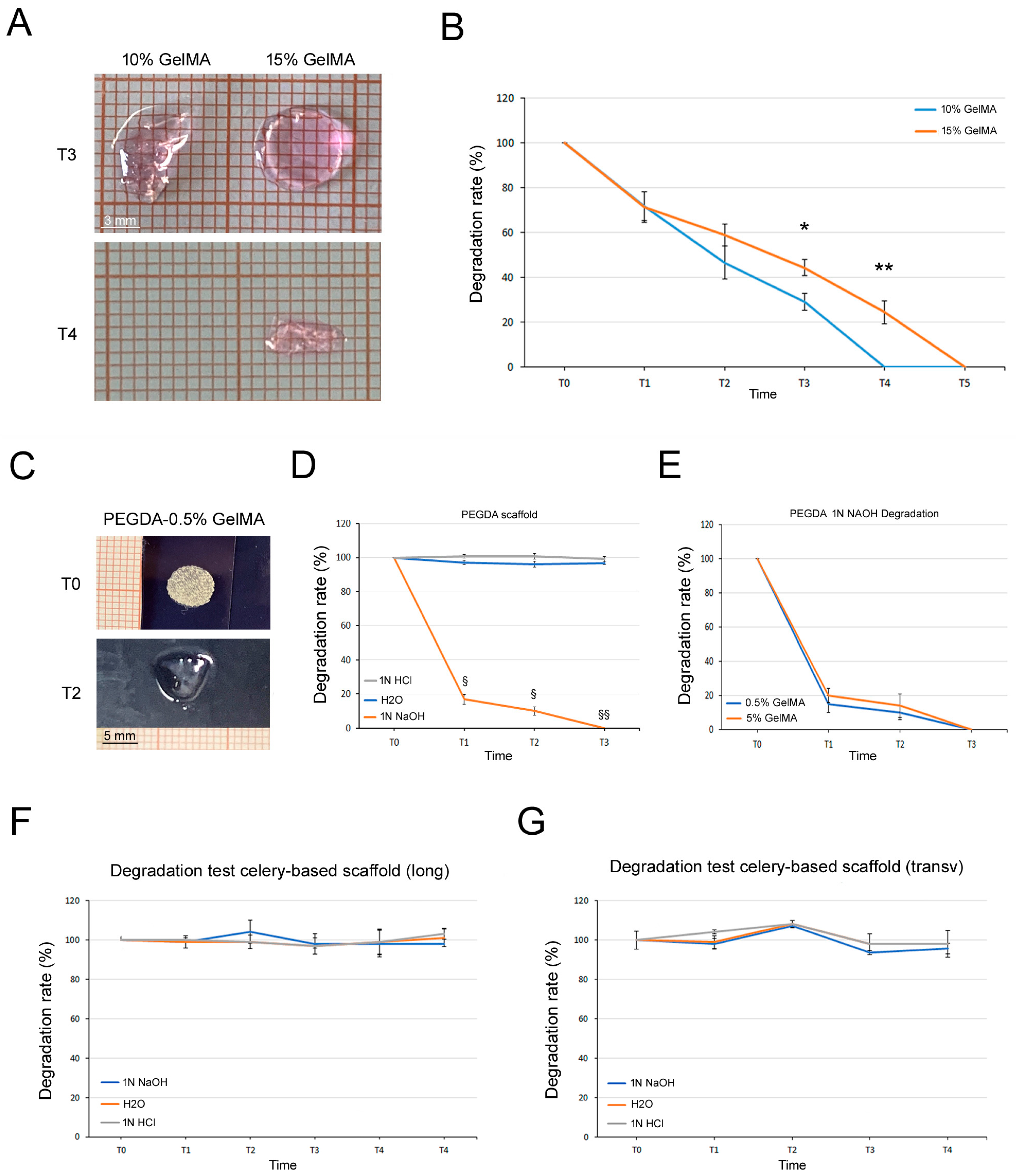
3.4. Degradation Tests
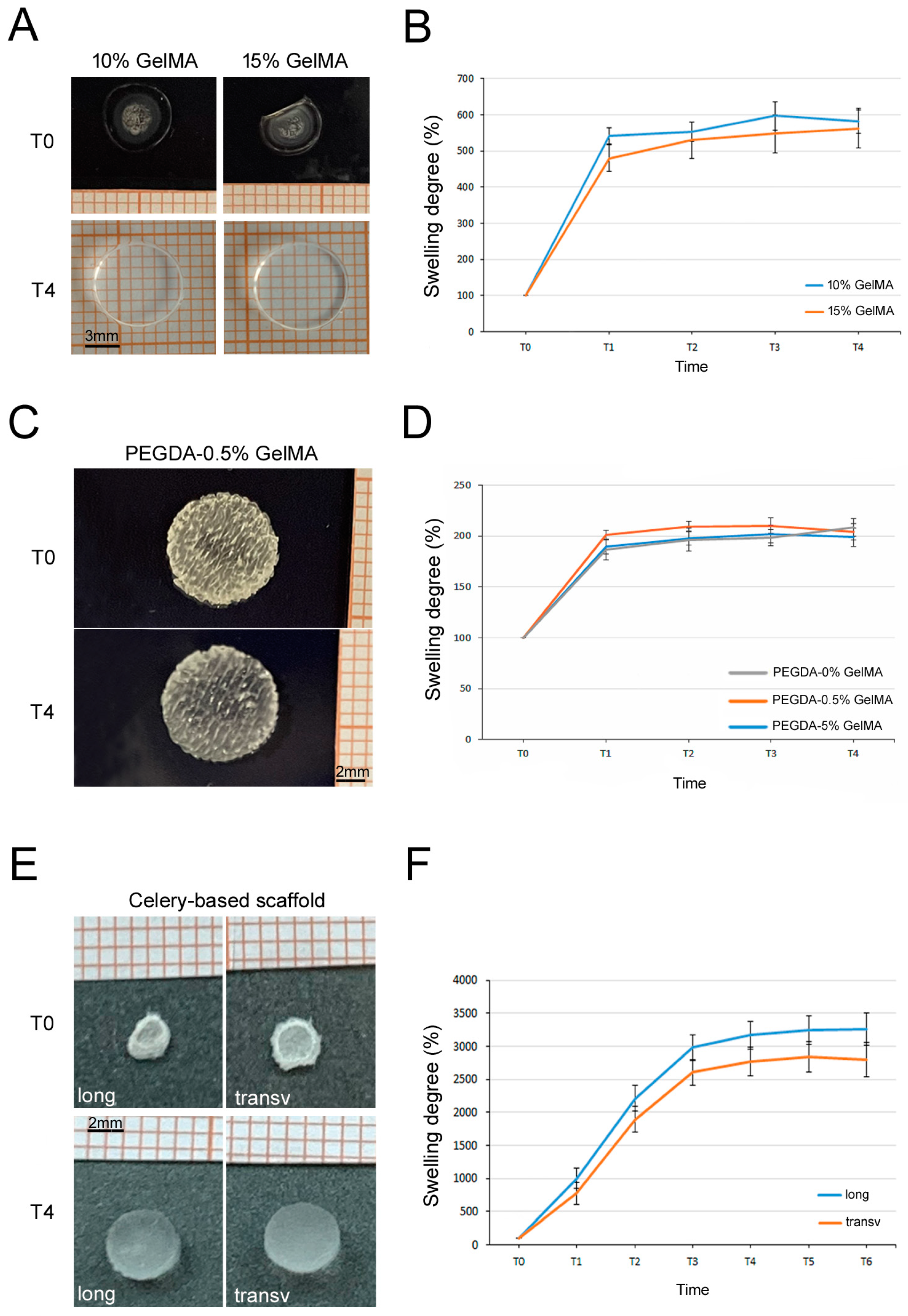
3.5. Swelling Tests
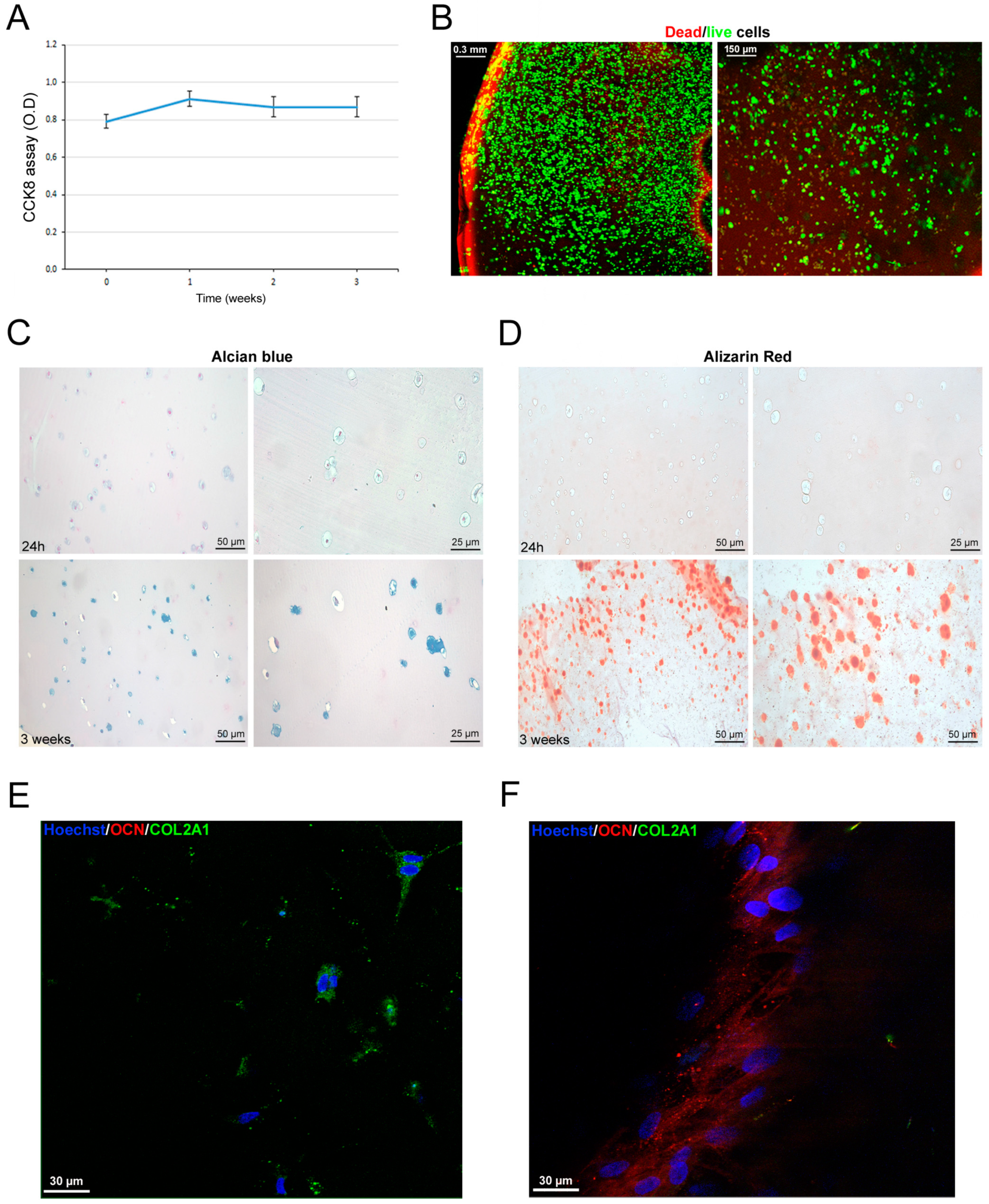
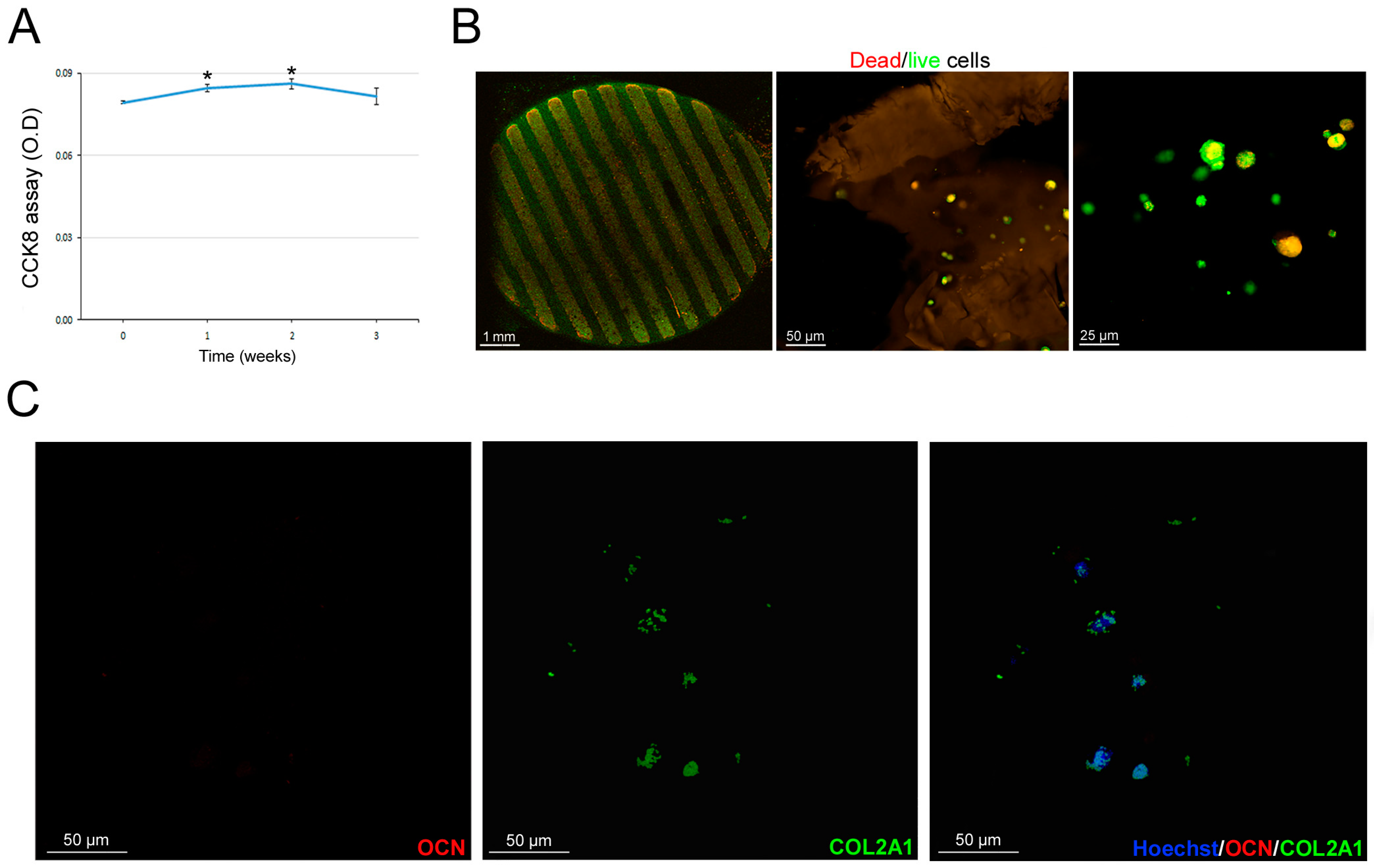
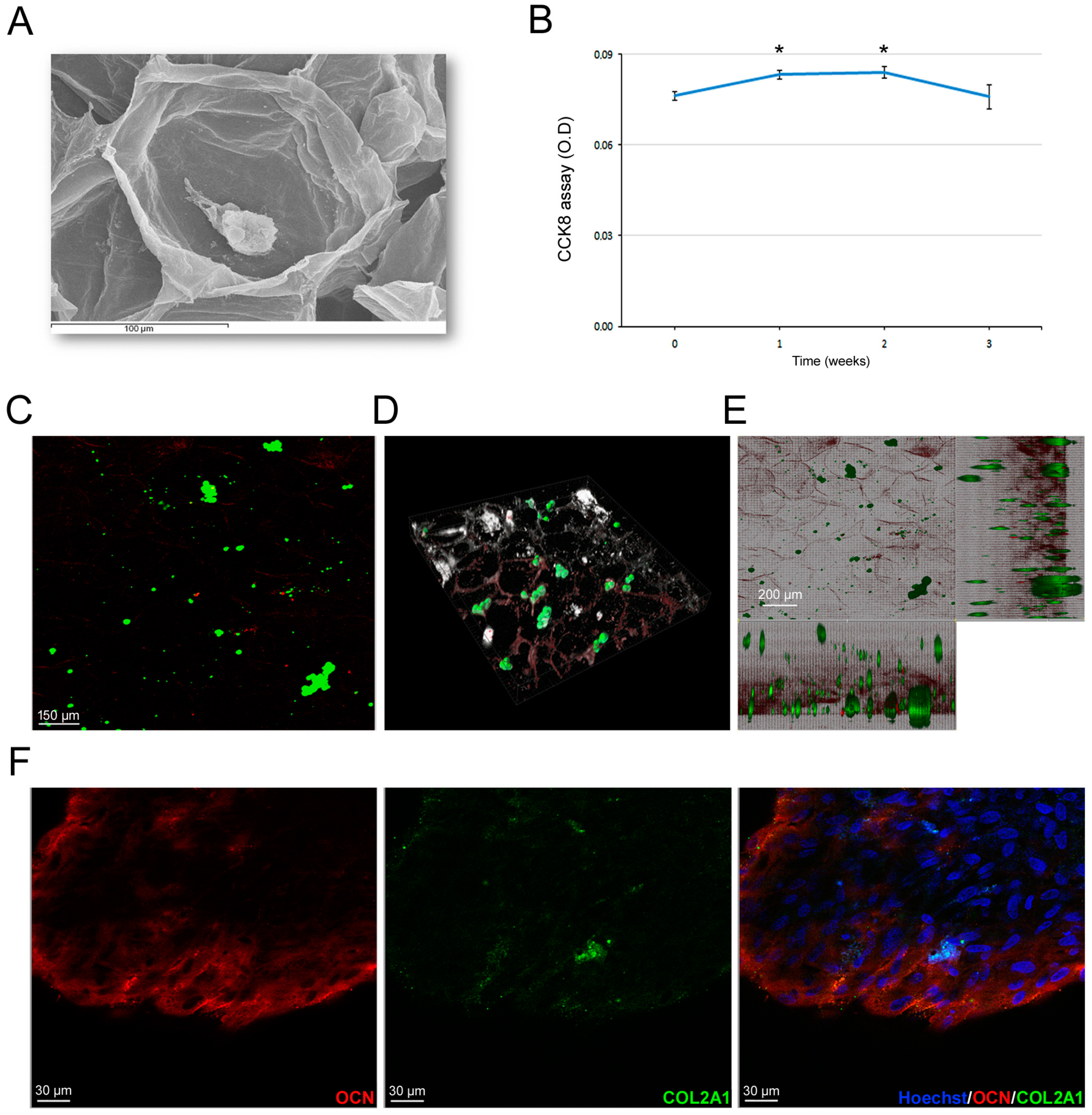
3.6. Scaffold-Dependent hASC Survival and Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falah, M.; Nierenberg, G.; Soudry, M.; Hayden, M.; Volpin, G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 2010, 34, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.M.; Martin, D.F.; Boles, C.A.; Curl, W.W. Chondral and osteochondral injuries. Diagnosis and management. Clin. Sports Med. 2001, 20, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Orth, P.; Cucchiarini, M.; Wagenpfeil, S.; Menger, M.D.; Madry, H. PTH [1-34]-induced alterations of the subchondral bone provoke early osteoarthritis. Osteoarthr. Cartil. 2014, 22, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Dasan, A.; Chandrasekar, A. Special Issue: Bioceramics, Bioglasses, and Gels for Tissue Engineering. Gels 2023, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.E.; Soliman, S.S.; Li, X.; Bilder, D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 2011, 147, 603–614. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Su, T. Adipogenesis or osteogenesis: Destiny decision made by mechanical properties of biomaterials. RSC Adv. 2022, 12, 24501–24510. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, F.; Hu, Q.; Krebsbach, P.H. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials 2014, 35, 1176–1184. [Google Scholar] [CrossRef]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Wang, G.; An, Y.; Zhang, X.; Ding, P.; Bi, H.; Zhao, Z. Chondrocyte Spheroids Laden in GelMA/HAMA Hybrid Hydrogel for Tissue-Engineered Cartilage with Enhanced Proliferation, Better Phenotype Maintenance, and Natural Morphological Structure. Gels 2021, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Hosseinabadi, H.G.; Cecen, B.; Hassan, S.; Zhang, Y.S. Permeability mapping of gelatin methacryloyl hydrogels. Acta Biomater. 2018, 77, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, M.; Cheng, J.; Liu, X.; Liu, Z.; Liu, J.; Tang, P. 3D-Printed GelMA/PEGDA/F127DA Scaffolds for Bone Regeneration. J. Funct. Biomater. 2023, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, K.N.; Hergert, J.E.; Uzcategui, A.C.; Schoonraad, S.A.; Bryant, S.J.; McLeod, R.R.; Ferguson, V.L. Controlled Mechanical Property Gradients Within a Digital Light Processing Printed Hydrogel-Composite Osteochondral Scaffold. Ann. Biomed. Eng. 2024, 52, 2162–2177. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Storti, G.; Scioli, M.G.; Kim, B.S.; Orlandi, A.; Cervelli, V. Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Applications. Stem Cells Int. 2019, 2019, 3673857. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Bracco Gartner, T.C.L.; Deddens, J.C.; Mol, E.A.; Magin Ferrer, M.; van Laake, L.W.; Bouten, C.V.C.; Khademhosseini, A.; Doevendans, P.A.; Suyker, W.J.L.; Sluijter, J.P.G.; et al. Anti-fibrotic Effects of Cardiac Progenitor Cells in a 3D-Model of Human Cardiac Fibrosis. Front. Cardiovasc. Med. 2019, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alfredo, N.; Munar-Bestard, M.; Ramis, J.M.; Monjo, M. Synthesis and Modification of Gelatin Methacryloyl (GelMA) with Antibacterial Quaternary Groups and Its Potential for Periodontal Applications. Gels 2022, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, A.; Montaina, L.; Pescosolido, F.; Carotenuto, F.; Trovalusci, F.; De Matteis, F.; Tamburri, E.; Di Nardo, P. Electrically conductive scaffolds mimicking the hierarchical structure of cardiac myofibers. Sci. Rep. 2023, 13, 2863. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; Bielli, A.; Sanchez, M.; Scimeca, M.; Gimble, J.M.; Cervelli, V.; Orlandi, A. CD146 expression regulates osteochondrogenic differentiation of human adipose-derived stem cells. J. Cell Physiol. 2022, 237, 589–602. [Google Scholar] [CrossRef]
- Orlandi, A.; Hao, H.; Ferlosio, A.; Clément, S.; Hirota, S.; Spagnoli, L.G.; Gabbiani, G.; Chaponnier, C. Alpha actin isoforms expression in human and rat adult cardiac conduction system. Differentiation 2009, 77, 360–368. [Google Scholar] [CrossRef]
- Loessner, D.; Meinert, C.; Kaemmerer, E.; Martine, L.C.; Yue, K.; Levett, P.A.; Klein, T.J.; Melchels, F.P.W.; Khademhosseini, A.; Hutmacher, D.W. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc. 2016, 11, 727–746. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, G.J.; Green, M.; Shah, V.; Leyendecker, K.; Cosgriff-Hernandez, E. A user’s guide to degradation testing of polyethylene glycol-based hydrogels: From in vitro to in vivo studies. J. Biomed. Mater. Res. A 2024, 112, 1200–1212. [Google Scholar] [CrossRef]
- Buonvino, S.; Ciocci, M.; Nanni, F.; Cacciotti, I.; Melino, S. New vegetable-waste biomaterials by Lupin albus L. as cellular scaffolds for applications in biomedicine and food. Biomaterials 2023, 293, 121984. [Google Scholar] [CrossRef]
- Kim, C.; Young, J.L.; Holle, A.W.; Jeong, K.; Major, L.G.; Jeong, J.H.; Aman, Z.; Han, D.-W.; Hwang, Y.; Spatz, J.P.; et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann. Biomed. Eng. 2020, 48, 893–902. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.H. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Jaroszewicz, J.; Walboomers, X.F.; Swieszkowski, W. Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. J. Biomed Mater. Res. A 2018, 106, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tenaglio, S.; Esworthy, T.; Hann, S.Y.; Cui, H.; Webster, T.J.; Fenniri, H.; Zhang, L.G. Three-Dimensional Printing Biologically Inspired DNA-Based Gradient Scaffolds for Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 33219–33228. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, J.; Yu, S.; Mao, Z.; Gao, C.; Zhu, Y.; Mao, C.; Zheng, L. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials 2019, 188, 130–143. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes. Tissue Eng. Regen. Med. 2017, 14, 383–391. [Google Scholar] [CrossRef]
- Walker, M.; Luo, J.; Pringle, E.W.; Cantini, M. ChondroGELesis: Hydrogels to harness the chondrogenic potential of stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111822. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent advances in GelMA hydrogel transplantation for musculoskeletal disorders and related disease treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef] [PubMed]


| Scaffold Type | Fabrication Method |
|---|---|
| GelMA 10% | UV crosslinking |
| GelMA 15% | UV crosslinking |
| PEGDA + GelMA 0.5% | 3D-printed PEGDA scaffolds with UV crosslinked 0.5% GelMA |
| PEGDA + GelMA 5% | 3D-printed PEGDA scaffolds with UV crosslinked 5% GelMA |
| Celery 24 h transv | Celery sections sliced transversally and decellularized for 24 h |
| Celery 24 h long | Celery sections sliced longitudinally and decellularized for 24 h |
| Celery 72 h transv | Celery sections sliced transversally and decellularized for 72 h |
| Celery 72 h long | Celery sections sliced longitudinally and decellularized for 72 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorelli, E.; Scioli, M.G.; Terriaca, S.; Ul Haq, A.; Storti, G.; Madaghiele, M.; Palumbo, V.; Pashaj, E.; De Matteis, F.; Ribuffo, D.; et al. Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells. Bioengineering 2024, 11, 920. https://doi.org/10.3390/bioengineering11090920
Fiorelli E, Scioli MG, Terriaca S, Ul Haq A, Storti G, Madaghiele M, Palumbo V, Pashaj E, De Matteis F, Ribuffo D, et al. Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells. Bioengineering. 2024; 11(9):920. https://doi.org/10.3390/bioengineering11090920
Chicago/Turabian StyleFiorelli, Elena, Maria Giovanna Scioli, Sonia Terriaca, Arsalan Ul Haq, Gabriele Storti, Marta Madaghiele, Valeria Palumbo, Ermal Pashaj, Fabio De Matteis, Diego Ribuffo, and et al. 2024. "Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells" Bioengineering 11, no. 9: 920. https://doi.org/10.3390/bioengineering11090920
APA StyleFiorelli, E., Scioli, M. G., Terriaca, S., Ul Haq, A., Storti, G., Madaghiele, M., Palumbo, V., Pashaj, E., De Matteis, F., Ribuffo, D., Cervelli, V., & Orlandi, A. (2024). Comparison of Bioengineered Scaffolds for the Induction of Osteochondrogenic Differentiation of Human Adipose-Derived Stem Cells. Bioengineering, 11(9), 920. https://doi.org/10.3390/bioengineering11090920








