Quantitative Assessment of Acetabular Defects in Revision Hip Arthroplasty Based on 3D Modeling: The Area Increase Ratio (AIR) Method
Abstract
1. Introduction
2. Material and Methods
2.1. Pre-Processing and Mirroring
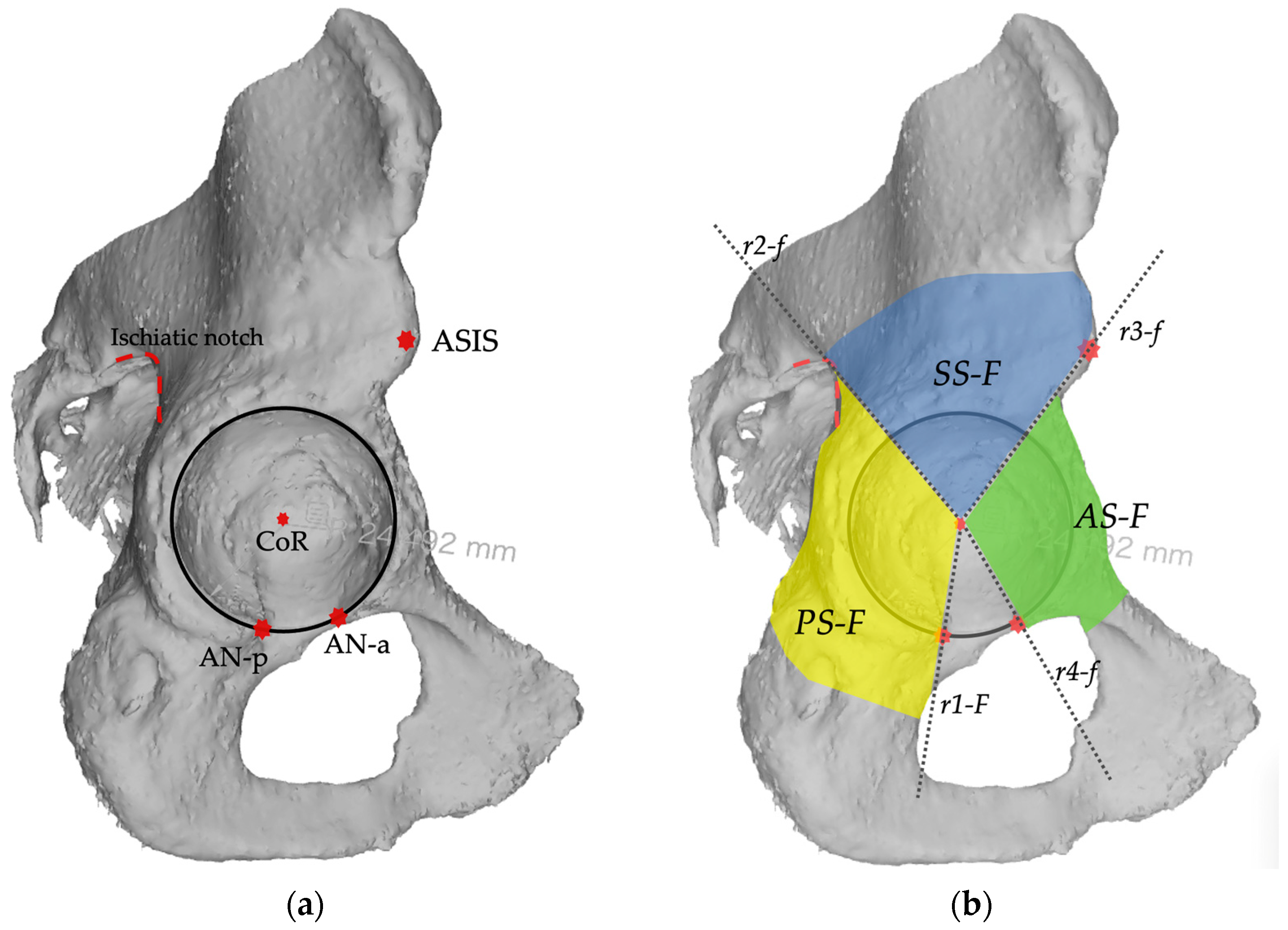
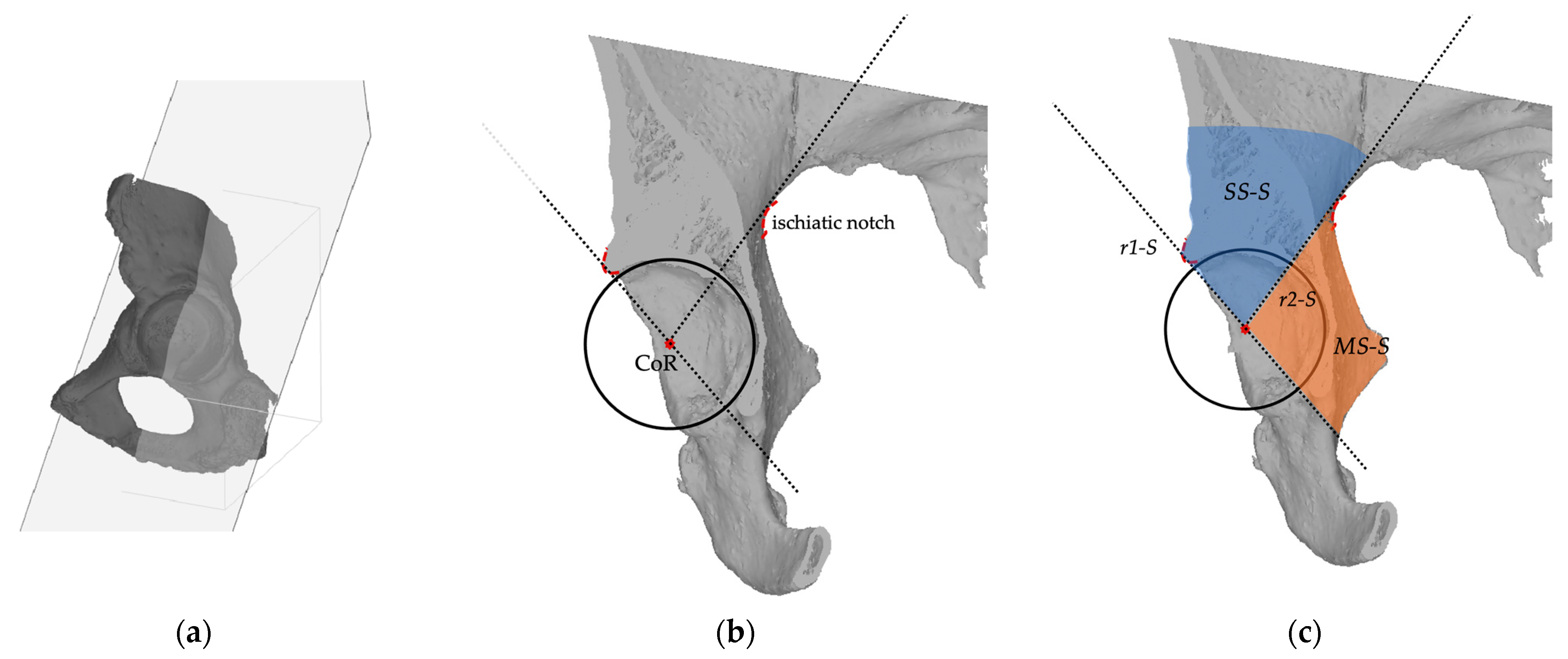
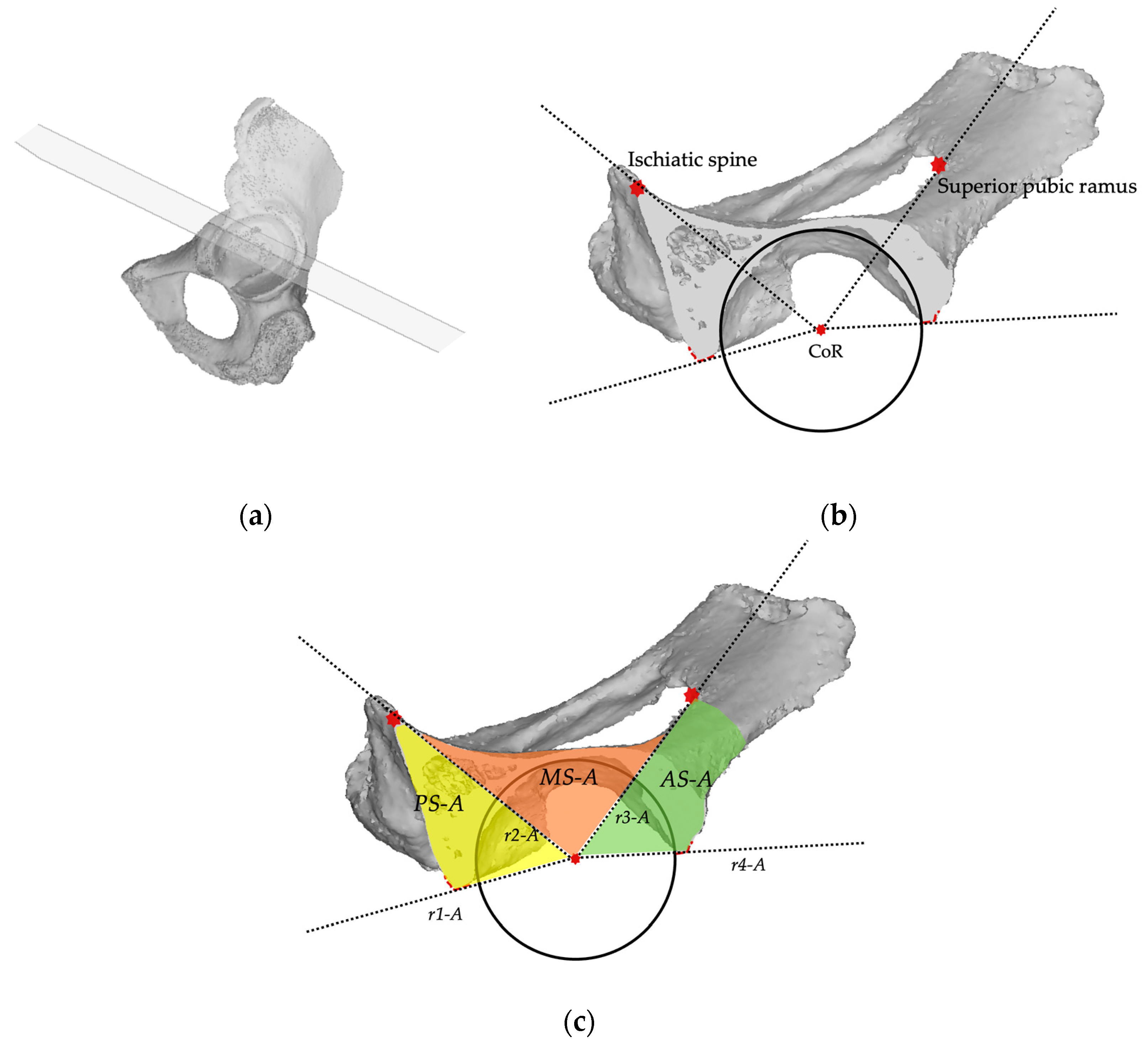
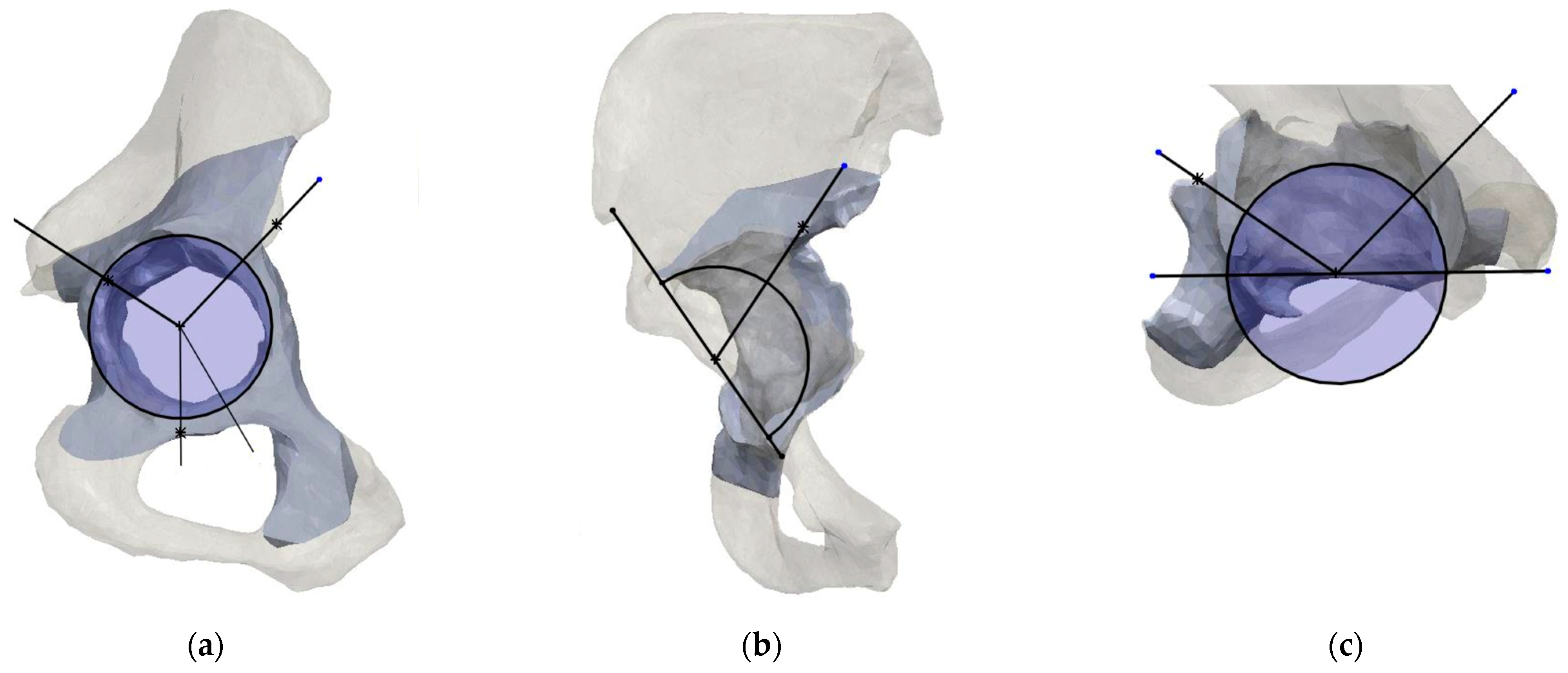
2.2. Planes and Sectors Definition
2.3. Analysis
3. Results
3.1. Quantitative Defect Assessment
3.2. Qualitative Defect Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, A.M.; Farley, K.X.; Guild, G.N.; Bradbury, T.L. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2030. J. Arthroplast. 2020, 35, S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Erivan, R.; Villatte, G.; Dartus, J.; Reina, N.; Descamps, S.; Boisgard, S. Progression and Projection for Hip Surgery in France, 2008–2070: Epidemiologic Study with Trend and Projection Analysis. Orthop. Traumatol. Surg. Res. 2019, 105, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Saleh, H.; Bolz, N.; Buza, J.; Iorio, R.; Rathod, P.A.; Schwarzkopf, R.; Deshmukh, A.J. Re-Revision Total Hip Arthroplasty: Epidemiology and Factors Associated with Outcomes. J. Clin. Orthop. Trauma 2020, 11, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Askew, N.; Habibi, A.; Nherera, L.; Macaulay, W.; Seyler, T.; Schwarzkopf, R. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040–2060. Arthroplast. Today 2023, 21, 101152. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, G.; Podda, D.; Mastio, M.; Capone, A. Long-Term Results of Isolated Acetabular Revisions with Reinforcement Rings: A 10- to 15-Year Follow-Up. HIP Int. 2019, 29, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gu, J.; Zhou, Y. Primary Total Hip Arthroplasty Failure: Aseptic Loosening Remains the Most Common Cause of Revision. Am. J. Transl. Res. 2022, 14, 7080–7089. [Google Scholar] [PubMed]
- Paprosky, W.G.; Perona, P.G.; Lawrence, J.M. Acetabular Defect Classification and Surgical Reconstruction in Revision Arthroplasty. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, J.A.; Capello, W.N.; Borden, L.S.; Bargar, W.L.; Bierbaum, B.F.; Boettcher, W.G.; Steinberg, M.E.; Stulberg, S.D.; Wedge, J.H. Classification and Management of Acetabular Abnormalities in Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1989, 243, 126–137. [Google Scholar] [CrossRef]
- Gross, A.E.; Allan, D.G.; Catre, M.; Garbuz, D.S.; Stockley, I. Bone Grafts in Hip Replacement Surgery. The Pelvic Side. Orthop. Clin. N. Am. 1993, 24, 679–695. [Google Scholar] [CrossRef]
- Saleh, K.J.; Holtzman, J.; Gafni, A.; Saleh, L.; Jaroszynski, G.; Wong, P.; Woodgate, I.; Davis, A.; Gross, A.E. Development, Test Reliability and Validation of a Classification for Revision Hip Arthroplasty. J. Orthop. Res. 2001, 19, 50–56. [Google Scholar] [CrossRef]
- Engh, C.A.; Glassman, A.H.; Griffin, W.L.; Mayer, J.G. Results of Cementless Revision for Failed Cemented Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 1988, 235, 91–110. [Google Scholar] [CrossRef]
- Gustilo, R.B.; Pasternak, H.S. Revision Total Hip Arthroplasty with Titanium Ingrowth Prosthesis and Bone Grafting for Failed Cemented Femoral Component Loosening. Clin. Orthop. Relat. Res. 1988, 235, 111–119. [Google Scholar] [CrossRef]
- Yu, R.; Hofstaetter, J.G.; Sullivan, T.; Costi, K.; Howie, D.W.; Solomon, L.B. Validity and Reliability of the Paprosky Acetabular Defect Classification. Clin. Orthop. Relat. Res. 2013, 471, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Gozzard, C.; Blom, A.; Taylor, A.; Smith, E.; Learmonth, I. A Comparison of the Reliability and Validity of Bone Stock Loss Classification Systems Used for Revision Hip Surgery. J. Arthroplast. 2003, 18, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Adriani, M.; Malahias, M.-A.; Fassihi, S.C.; Nocon, A.A.; Bostrom, M.P.; Sculco, P.K. Reliability and Validity of Acetabular and Femoral Bone Loss Classification Systems in Total Hip Arthroplasty: A Systematic Review. HSS J. 2020, 16, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Horas, K.; Arnholdt, J.; Steinert, A.F.; Hoberg, M.; Rudert, M.; Holzapfel, B.M. Acetabular Defect Classification in Times of 3D Imaging and Patient-Specific Treatment Protocols. Orthopäde 2017, 46, 168–178. [Google Scholar] [CrossRef]
- Marongiu, G.; Prost, R.; Capone, A. A New Diagnostic Approach for Periprosthetic Acetabular Fractures Based on 3D Modeling: A Study Protocol. Diagnostics 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos-Vaquinhas, A.; López-Torres, I.I.; Matas-Diez, J.A.; Calvo-Haro, J.A.; Vaquero, J.; Sanz-Ruiz, P. Improvement of Surgical Time and Functional Results after Do-It-Yourself 3D-Printed Model Preoperative Planning in Acetabular Defects Paprosky IIA-IIIB. Orthop. Traumatol. Surg. Res. 2022, 108, 103277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Zeng, Y.; Zhai, Z.; Mao, Y.; Yu, D.; Wang, L.; Yan, M.; Zhu, Z.; Li, H. Comparison of 3D Printing Rapid Prototyping Technology with Traditional Radiographs in Evaluating Acetabular Defects in Revision Hip Arthroplasty: A Prospective and Consecutive Study. Orthop. Surg. 2021, 13, 1773–1780. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Ying, H.; Mao, Y.; Zhu, Z.; Li, H. Reliability and Validity Test of a Novel Three-Dimensional Acetabular Bone Defect Classification System Aided with Additive Manufacturing. BMC Musculoskelet. Disord. 2022, 23, 432. [Google Scholar] [CrossRef]
- Meynen, A.; Vles, G.; Zadpoor, A.A.; Mulier, M.; Scheys, L. The Morphological Variation of Acetabular Defects in Revision Total Hip Arthroplasty—A Statistical Shape Modeling Approach. J. Orthop. Res. 2021, 39, 2419–2427. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, D.C.; Jaenisch, M.; Osterhaus, T.A.; Gathen, M.; Wimmer, M.; Randau, T.M.; Schildberg, F.A.; Rössler, P.P. Acetabular Defects in Revision Hip Arthroplasty: A Therapy-Oriented Classification. Arch. Orthop. Trauma Surg. 2020, 140, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Meynen, A.; Vles, G.; Roussot, M.; Van Eemeren, A.; Wafa, H.; Mulier, M.; Scheys, L. Advanced Quantitative 3D Imaging Improves the Reliability of the Classification of Acetabular Defects. Arch. Orthop. Trauma Surg. 2022, 143, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Gelaude, F.; Clijmans, T.; Delport, H. Quantitative Computerized Assessment of the Degree of Acetabular Bone Deficiency: Total Radial Acetabular Bone Loss (TrABL). Adv. Orthop. 2011, 2011, 494382. [Google Scholar] [CrossRef] [PubMed]
- Hettich, G.; Schierjott, R.A.; Ramm, H.; Graichen, H.; Jansson, V.; Rudert, M.; Traina, F.; Grupp, T.M. Method for Quantitative Assessment of Acetabular Bone Defects. J. Orthop. Res. 2019, 37, 181–189. [Google Scholar] [CrossRef]
- Schierjott, R.A.; Hettich, G.; Graichen, H.; Jansson, V.; Rudert, M.; Traina, F.; Weber, P.; Grupp, T.M. Quantitative Assessment of Acetabular Bone Defects: A Study of 50 Computed Tomography Data Sets. PLoS ONE 2019, 14, e0222511. [Google Scholar] [CrossRef]



| Spec | Age | Sex | Side | Diagnosis | Paprosky Type |
|---|---|---|---|---|---|
| 1 | 67 | F | right | Aseptic loosening | IIC |
| 2 | 78 | F | right | Aseptic loosening | IIIB |
| 3 | 84 | M | left | Aseptic loosening | IIC |
| 4 | 78 | F | left | Aseptic loosening | IIA |
| 5 | 74 | M | right | Aseptic loosening | IIIA |
| 6 | 69 | M | right | Aseptic loosening | IIIB |
| Minimal | Moderate | Severe | Massive |
|---|---|---|---|
| 0–20% | >20–50% | >50–100% | >100% |
| Frontal Plane | Sagittal Plane | Axial Plane | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Spec. | Acetabular Diameter | PS-F | SS-F | AS-F | SS-S | MS-S | PS-A | MS-A | AS-A |
| 1 | 66 mm | 1239.78 mm2 | 1038.92 mm2 | 1020.5 mm2 | 976.34 mm2 | 1989.53 mm2 | 338.73 mm2 | 1723.69 mm2 | 457.5 mm2 |
| +5.91% | +8.85% | +0.93% | +50.75% | +87.17% | +1.84% | +81.38% | +6.98% | ||
| 2 | 56 mm | 1194.42 mm2 | 1033.49 mm2 | 1046.81 mm2 | 1196.34 mm2 | 1443.84 mm2 | 369.56 mm2 | 1569.34 mm2 | 572.02 mm2 |
| +47.42% | +35.43% | +53.11% | +171.35% | +82.62% | +28.61% | +136,47% | +109.02% | ||
| 3 | 56 mm | 963.31 mm2 | 892.65 mm2 | 1020.97 mm2 | 494.59 mm2 | 870.91 mm2 | 320.26 mm2 | 696.71 mm2 | 273.66 mm2 |
| +36.53% | +30.93% | +17.75% | +5.15% | +114.42% | +4.02% | +117.19% | +0.00% | ||
| 4 | 52 mm | 1039.78 mm2 | 868.92 mm2 | 680.5 mm2 | 580.34 mm2 | 989.53 mm2 | 300.73 mm2 | 829.69 mm2 | 307.5 mm2 |
| +43.09% | +46.66% | +8.43% | +44.35% | +49.97% | +45.65% | +40.64% | +15.83% | ||
| 5 | 54 mm | 1189.42 mm2 | 1220.49 mm2 | 867.81 mm2 | 706.34 mm2 | 1143.84 mm2 | 469.56 mm2 | 978.34 mm2 | 332.02 mm2 |
| +57.88% | +72.00% | +36.51% | +72.30% | +55.59% | +75.74% | +58.4% | +30.48% | ||
| 6 | 58 mm | 1363.31 mm2 | 1642.65 mm2 | 1220.97 mm2 | 1194.59 mm2 | 1830.91 mm2 | 640.26 mm2 | 1596.71 mm2 | 433.66 mm2 |
| +80.12% | +124.61% | +31.28% | +136.76% | +124.25% | +93.87% | +129.01% | +47.72% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marongiu, G.; Campacci, A.; Capone, A. Quantitative Assessment of Acetabular Defects in Revision Hip Arthroplasty Based on 3D Modeling: The Area Increase Ratio (AIR) Method. Bioengineering 2024, 11, 341. https://doi.org/10.3390/bioengineering11040341
Marongiu G, Campacci A, Capone A. Quantitative Assessment of Acetabular Defects in Revision Hip Arthroplasty Based on 3D Modeling: The Area Increase Ratio (AIR) Method. Bioengineering. 2024; 11(4):341. https://doi.org/10.3390/bioengineering11040341
Chicago/Turabian StyleMarongiu, Giuseppe, Antonio Campacci, and Antonio Capone. 2024. "Quantitative Assessment of Acetabular Defects in Revision Hip Arthroplasty Based on 3D Modeling: The Area Increase Ratio (AIR) Method" Bioengineering 11, no. 4: 341. https://doi.org/10.3390/bioengineering11040341
APA StyleMarongiu, G., Campacci, A., & Capone, A. (2024). Quantitative Assessment of Acetabular Defects in Revision Hip Arthroplasty Based on 3D Modeling: The Area Increase Ratio (AIR) Method. Bioengineering, 11(4), 341. https://doi.org/10.3390/bioengineering11040341






