Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine
Abstract
1. Introduction
2. Methods
3. Bioprinting in Plastic and Reconstructive Surgery
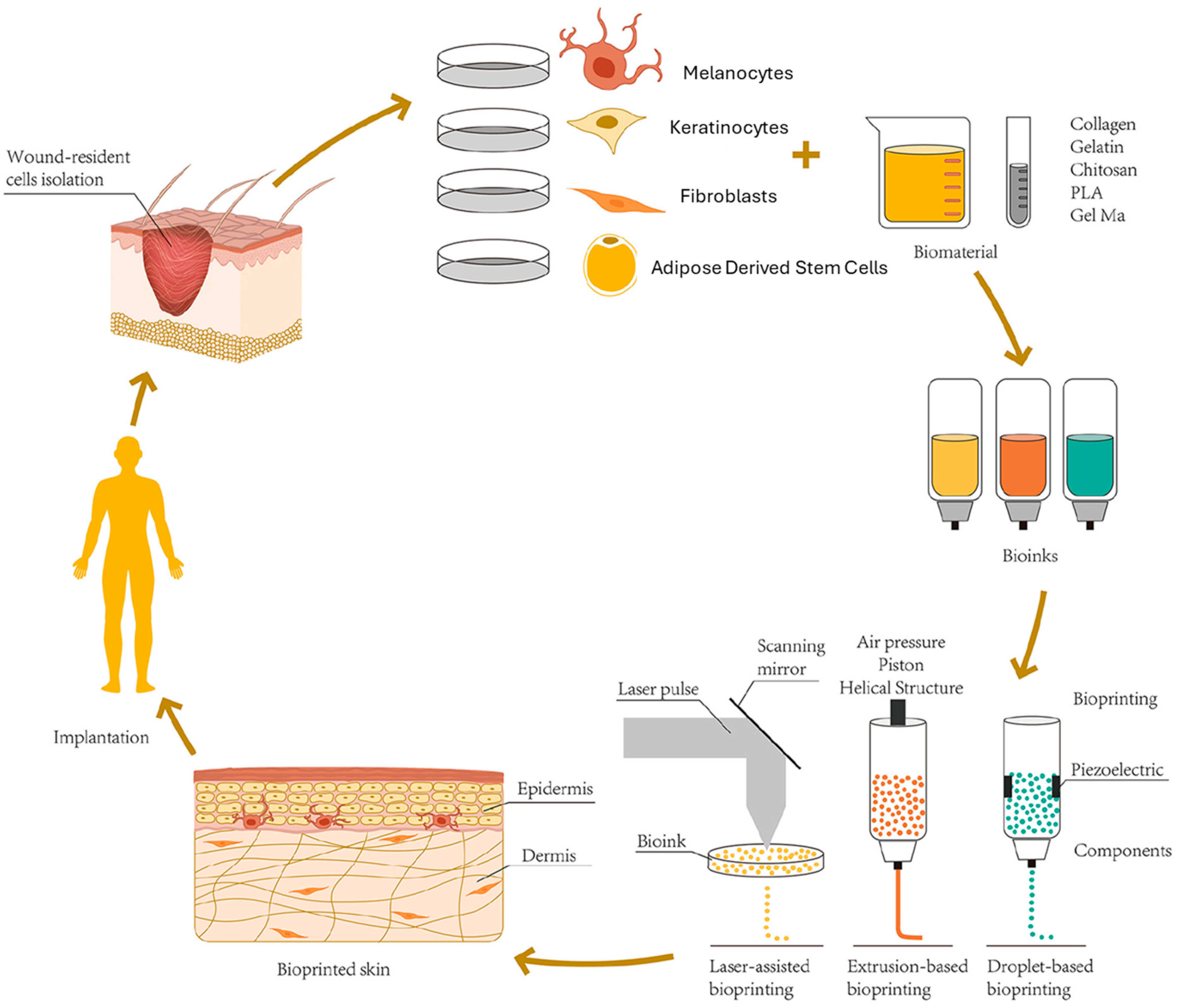
3.1. Skin and Wound Healing
3.2. Epidermal Appendages
3.2.1. Melanocytes
3.2.2. Hair Follicles and Sweat Glands
3.3. Craniofacial Reconstruction
3.3.1. Auricular and Nasal Reconstruction
3.3.2. Tracheal Reconstruction
3.4. Peripheral Nerve Reconstruction
3.5. Other Soft Tissue Reconstruction Considerations
3.6. State of the Art in Plastic and Reconstructive Surgery
4. Bioprinting in Orthopedic Surgery
4.1. Bone Tissue Reconstruction
4.1.1. Bioink and Additive Selection
4.1.2. Scaffold Drug Delivery and Antimicrobials
4.1.3. Higher Complexity 3D Printing Designs
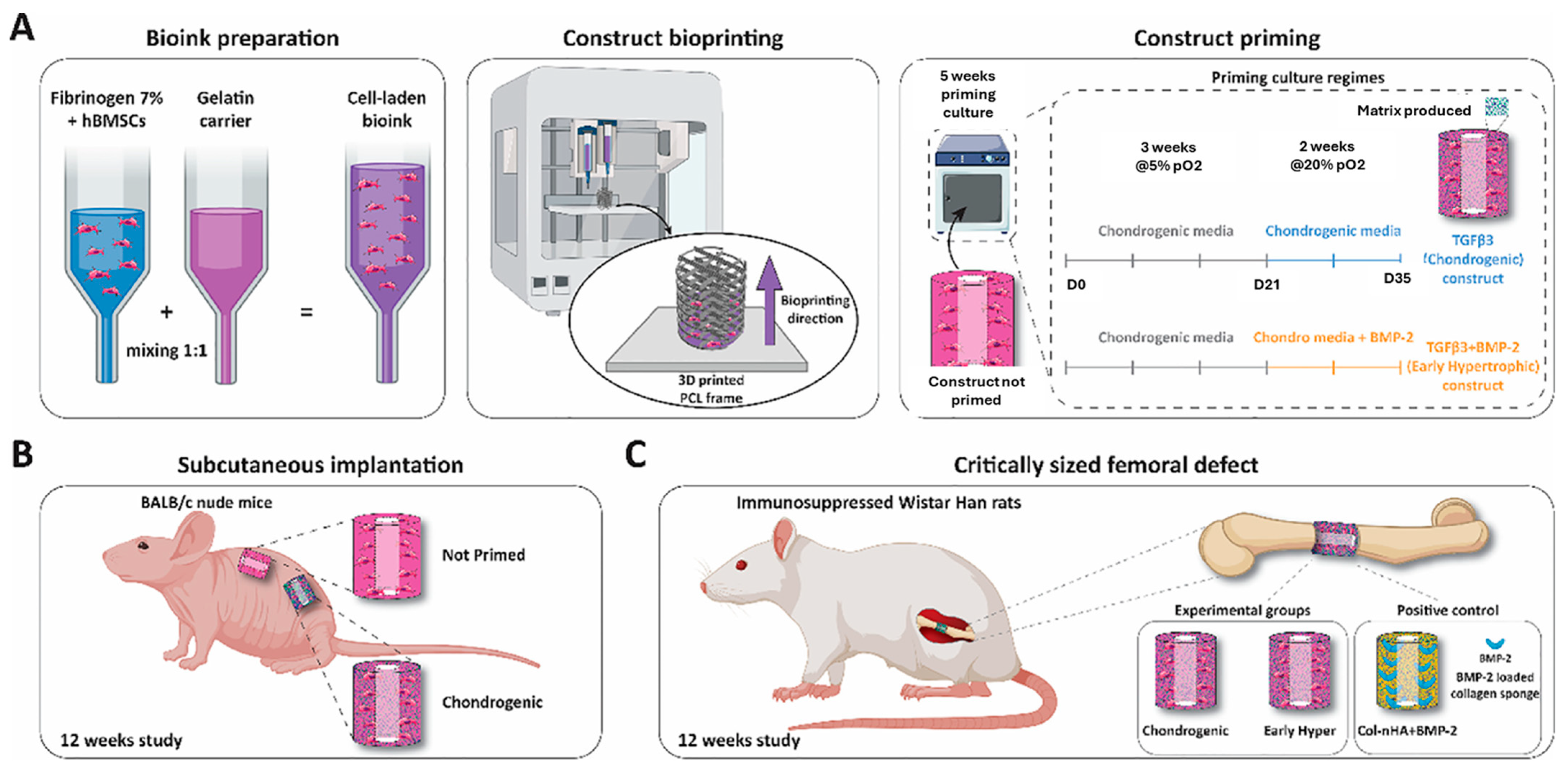
4.2. Cartilage Reconstruction
Bioink and Additive Selection
4.3. State of the Art in Orthopedics
5. Bioprinting in Ophthalmology
5.1. Corneal Reconstruction
5.2. Retinal Reconstruction
5.3. Lacrimal Gland Reconstruction
5.4. State of the Art in Ophthalmology
6. Future Directions and Challenges
6.1. In Situ Bioprinting
6.2. Regulatory Oversight
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M. The bioprinting roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, Y.; Zhu, Y.; Lin, Z.; Chen, L.; Zhang, Y.; Xia, H.; Mao, C. 3D printed personalized titanium plates improve clinical outcome in microwave ablation of bone tumors around the knee. Sci. Rep. 2017, 7, 7626. [Google Scholar] [CrossRef]
- Guillemot, F.; Mironov, V.; Nakamura, M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2010, 2, 010201. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Guillotin, B.; Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 2011, 29, 183–190. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef]
- Stanco, D.; Urbán, P.; Tirendi, S.; Ciardelli, G.; Barrero, J. 3D bioprinting for orthopaedic applications: Current advances, challenges and regulatory considerations. Bioprinting 2020, 20, e00103. [Google Scholar] [CrossRef]
- Poomathi, N.; Singh, S.; Prakash, C.; Patil, R.V.; Perumal, P.; Barathi, V.A.; Balasubramanian, K.K.; Ramakrishna, S.; Maheshwari, N. Bioprinting in ophthalmology: Current advances and future pathways. Rapid Prototyp. J. 2019, 25, 496–514. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Himdani, S.; Clement, M.; Whitaker, I.S. The challenge for reconstructive surgeons in the twenty-first century: Manufacturing tissue-engineered solutions. Front. Surg. 2015, 2, 52. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef]
- Goddard, E.; Dodds, S. Ethics and policy for bioprinting. In 3D Bioprinting: Principles and Protocols; Humana: New York, NY USA, 2020; pp. 43–64. [Google Scholar]
- Hunsberger, J.; Neubert, J.; Wertheim, J.A.; Allickson, J.; Atala, A. Bioengineering priorities on a path to ending organ shortage. Curr. Stem Cell Rep. 2016, 2, 118–127. [Google Scholar] [CrossRef]
- Wu, S.D.; Dai, N.T.; Liao, C.Y.; Kang, L.Y.; Tseng, Y.W.; Hsu, S.H. Planar-/Curvilinear-Bioprinted Tri-Cell-Laden Hydrogel for Healing Irregular Chronic Wounds. Adv. Health Mater. 2022, 11, e2201021. [Google Scholar] [CrossRef]
- Zhang, D.; Lai, L.; Fu, H.; Fu, Q.; Chen, M. 3D-Bioprinted Biomimetic Multilayer Implants Comprising Microfragmented Adipose Extracellular Matrix and Cells Improve Wound Healing in a Murine Model of Full-Thickness Skin Defects. ACS Appl. Mater. Interfaces 2023, 15, 29713–29728. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, T.; Hu, Y.; Jiang, S.; Luo, Y.; Liu, C.; Wang, G.; Zhang, J.; Xu, T.; Zhu, L. 3D bioprinting of integral ADSCs-NO hydrogel scaffolds to promote severe burn wound healing. Regen. Biomater. 2021, 8, rbab014. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, A.; Mao, Z.; Li, F.; Su, T.; Cao, L.; Wang, W.; Liu, Y.; Wang, C. Silk fibroin-gelatin photo-crosslinked 3D-bioprinted hydrogel with MOF-methylene blue nanoparticles for infected wound healing. Int. J. Bioprint. 2023, 9, 773. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110578. [Google Scholar] [CrossRef]
- Zielinska, D.; Fisch, P.; Moehrlen, U.; Finkielsztein, S.; Linder, T.; Zenobi-Wong, M.; Biedermann, T.; Klar, A.S. Combining bioengineered human skin with bioprinted cartilage for ear reconstruction. Sci. Adv. 2023, 9, eadh1890. [Google Scholar] [CrossRef]
- Torsello, M.; Salvati, A.; Borro, L.; Meucci, D.; Tropiano, M.L.; Cialente, F.; Secinaro, A.; Del Fattore, A.; Emiliana, C.M.; Francalanci, P.; et al. 3D bioprinting in airway reconstructive surgery: A pilot study. Int. J. Pediatr. Otorhinolaryngol. 2022, 161, 111253. [Google Scholar] [CrossRef]
- Huo, Y.; Xu, Y.; Wu, X.; Gao, E.; Zhan, A.; Chen, Y.; Zhang, Y.; Hua, Y.; Swieszkowski, W.; Zhang, Y.S.; et al. Functional Trachea Reconstruction Using 3D-Bioprinted Native-Like Tissue Architecture Based on Designable Tissue-Specific Bioinks. Adv. Sci. 2022, 9, e2202181. [Google Scholar] [CrossRef]
- Hooper, R.; Cummings, C.; Beck, A.; Vazquez-Armendariz, J.; Rodriguez, C.; Dean, D. Sheet-based extrusion bioprinting: A new multi-material paradigm providing mid-extrusion micropatterning control for microvascular applications. Biofabrication 2024, 16, 025032. [Google Scholar] [CrossRef]
- Min, K.; Kong, J.S.; Kim, J.; Kim, J.; Gao, G.; Cho, D.-W.; Han, H.H. Three-Dimensional Microfilament Printing of a Decellularized Extracellular Matrix (dECM) Bioink Using a Microgel Printing Bath for Nerve Graft Fabrication and the Effectiveness of dECM Graft Combined with a Polycaprolactone Conduit. ACS Appl. Bio Mater. 2022, 5, 1591–1603. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Alawi, S.A.; Matschke, J.; Muallah, D.; Gelinksy, M.; Dragu, A. 3D bioprinting in plastic and reconstructive surgery: Current concepts, progress, and clinical application. Eur. J. Plast. Surg. 2023, 46, 833–843. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Bay, C.; Chizmar, Z.; Reece, E.M.; Jessie, Z.Y.; Winocour, J.; Vorstenbosch, J.; Winocour, S. Comparison of skin substitutes for acute and chronic wound management. Semin. Plast. Surg. 2021, 35, 171–180. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Franck, C.L.; Senegaglia, A.C.; Leite, L.M.B.; de Moura, S.A.B.; Francisco, N.F.; Ribas Filho, J.M. Influence of adipose tissue-derived stem cells on the burn wound healing process. Stem Cells Int. 2019, 2019, 2340725. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Yi, H.-G.; Kim, S.-W.; Cho, D.-W. 3D cell printed tissue analogues: A new platform for theranostics. Theranostics 2017, 7, 3118. [Google Scholar] [CrossRef] [PubMed]
- Zahid, A.A.; Ahmed, R.; ur Rehman, S.R.; Augustine, R.; Tariq, M.; Hasan, A. Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int. J. Biol. Macromol. 2019, 136, 901–910. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric oxide therapy for diabetic wound healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Romero, F.R.; Haddad, G.R.; Miot, H.A.; Cataneo, D.C. Palmar hyperhidrosis: Clinical, pathophysiological, diagnostic and therapeutic aspects. An. Bras. Dermatol. 2016, 91, 716–725. [Google Scholar] [CrossRef]
- Tajima, T.; Morikawa, S.; Nakamura, A. Clinical features and molecular basis of pseudohypoaldosteronism type 1. Clin. Pediatr. Endocrinol. 2017, 26, 109–117. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Danczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D bioprinting in skin related research: Recent achievements and application perspectives. ACS Synth. Biol. 2021, 11, 26–38. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Min, D.; Lee, W.; Bae, I.H.; Lee, T.R.; Croce, P.; Yoo, S.S. Bioprinting of biomimetic skin containing melanocytes. Exp. Dermatol. 2018, 27, 453–459. [Google Scholar] [CrossRef]
- Nuutila, K. Hair follicle transplantation for wound repair. Adv. Wound Care 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Perez-Valle, A.; Del Amo, C.; Andia, I. Overview of current advances in extrusion bioprinting for skin applications. Int. J. Mol. Sci. 2020, 21, 6679. [Google Scholar] [CrossRef]
- Zhang, Y.; Enhejirigala, n.; Yao, B.; Li, Z.; Song, W.; Li, J.; Zhu, D.; Wang, Y.; Duan, X.; Yuan, X. Using bioprinting and spheroid culture to create a skin model with sweat glands and hair follicles. Burn. Trauma 2021, 9, tkab013. [Google Scholar] [CrossRef]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef]
- Song, W.; Yao, B.; Zhu, D.; Zhang, Y.; Li, Z.; Huang, S.; Fu, X. 3D-bioprinted microenvironments for sweat gland regeneration. Burn. Trauma 2022, 10, tkab044. [Google Scholar] [CrossRef]
- Huang, S.; Yao, B.; Xie, J.; Fu, X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016, 32, 170–177. [Google Scholar] [CrossRef]
- Yao, B.; Wang, R.; Wang, Y.; Zhang, Y.; Hu, T.; Song, W.; Li, Z.; Huang, S.; Fu, X. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci. Adv. 2020, 6, eaaz1094. [Google Scholar] [CrossRef]
- Dwivedi, R.; Mehrotra, D. 3D bioprinting and craniofacial regeneration. J. Oral. Biol. Craniofacial Res. 2020, 10, 650–659. [Google Scholar] [CrossRef]
- Shen, Z.; Xia, T.; Zhao, J.; Pan, S. Current status and future trends of reconstructing a vascularized tissue-engineered trachea. Connect. Tissue Res. 2023, 64, 428–444. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, Y.; Ran, X.; Chen, H.; Pan, Q.; Chen, Y.; Zhang, Y.; Ren, W.; Wang, X.; Zhou, G.; et al. Instant trachea reconstruction using 3D-bioprinted C-shape biomimetic trachea based on tissue-specific matrix hydrogels. Bioact. Mater. 2024, 32, 52–65. [Google Scholar] [CrossRef]
- Selim, O.A.; Lakhani, S.; Midha, S.; Mosahebi, A.; Kalaskar, D.M. Three-Dimensional Engineered Peripheral Nerve: Toward a New Era of Patient-Specific Nerve Repair Solutions. Tissue Eng. Part B Rev. 2021, 28, 295–335. [Google Scholar] [CrossRef]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.; Burrell, J.C.; Zeng, J.; Shi, S.; Shanti, R.M.; Kulischak, G.; Cullen, D.K.; Le, A.D. Harnessing 3D collagen hydrogel-directed conversion of human GMSCs into SCP-like cells to generate functionalized nerve conduits. NPJ Regen. Med. 2021, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.; Lee, E.; Shih, W.; Koljaka, S.; Wang, A.; Jorgensen, C.; Hurr, R.; Dave, A.; Sudheendra, K.; Hibino, N. 3D bioprinting for vascularization. Bioengineering 2023, 10, 606. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Chang, C.-W.; Wang, J.; Bupphathong, S.; Huang, W.; Lin, C.-H. 3D-Bioprinted GelMA Scaffold with ASCs and HUVECs for Engineering Vascularized Adipose Tissue. ACS Appl. Bio Mater. 2024, 7, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Benmeridja, L.; De Moor, L.; De Maere, E.; Vanlauwe, F.; Ryx, M.; Tytgat, L.; Vercruysse, C.; Dubruel, P.; Van Vlierberghe, S.; Blondeel, P.; et al. High-throughput fabrication of vascularized adipose microtissues for 3D bioprinting. J. Tissue Eng. Regen. Med. 2020, 14, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Donnely, E.; Griffin, M.; Butler, P.E. Breast Reconstruction with a Tissue Engineering and Regenerative Medicine Approach (Systematic Review). Ann. Biomed. Eng. 2020, 48, 9–25. [Google Scholar] [CrossRef]
- Conci, C.; Bennati, L.; Bregoli, C.; Buccino, F.; Danielli, F.; Gallan, M.; Gjini, E.; Raimondi, M.T. Tissue engineering and regenerative medicine strategies for the female breast. J. Tissue. Eng. Regen. Med. 2020, 14, 369–387. [Google Scholar] [CrossRef]
- Grounds, M.D. Obstacles and challenges for tissue engineering and regenerative medicine: Australian nuances. Clin. Exp. Pharmacol. Physiol. 2018, 45, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Abu Awwad, H.A.M.; Thiagarajan, L.; Kanczler, J.M.; Amer, M.H.; Bruce, G.; Lanham, S.; Rumney, R.M.H.; Oreffo, R.O.C.; Dixon, J.E. Genetically-programmed, mesenchymal stromal cell-laden & mechanically strong 3D bioprinted scaffolds for bone repair. J. Control. Release 2020, 325, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Bhagania, M.; Kaur, K. Tissue engineering in plastic and reconstructive surgery: Fostering advances in the 21st century via an understanding of the present state of the art and future possibilities. Arch. Aesthetic Plast. Surg. 2023, 29, 64–75. [Google Scholar] [CrossRef]
- Bülow, A.; Schäfer, B.; Beier, J.P. Three-Dimensional Bioprinting in Soft Tissue Engineering for Plastic and Reconstructive Surgery. Bioengineering 2023, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Bekisz, J.M.; Flores, R.L.; Witek, L.; Lopez, C.D.; Runyan, C.M.; Torroni, A.; Cronstein, B.N.; Coelho, P.G. Dipyridamole enhances osteogenesis of three-dimensionally printed bioactive ceramic scaffolds in calvarial defects. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2018, 46, 237–244. [Google Scholar] [CrossRef] [PubMed]
- DeMitchell-Rodriguez, E.M.; Shen, C.; Nayak, V.V.; Tovar, N.; Witek, L.; Torroni, A.; Yarholar, L.M.; Cronstein, B.N.; Flores, R.L.; Coelho, P.G. Engineering 3D Printed Bioceramic Scaffolds to Reconstruct Critical-Sized Calvaria Defects in a Skeletally Immature Pig Model. Plast. Reconstr. Surg. 2023, 152, 270e–280e. [Google Scholar] [CrossRef] [PubMed]
- Fama, C.; Kaye, G.J.; Flores, R.; Lopez, C.D.; Bekisz, J.M.; Torroni, A.; Tovar, N.; Coelho, P.G.; Witek, L. Three-Dimensionally-Printed Bioactive Ceramic Scaffolds: Construct Effects on Bone Regeneration. J. Craniofac. Surg. 2021, 32, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.D.; Coelho, P.G.; Witek, L.; Torroni, A.; Greenberg, M.I.; Cuadrado, D.L.; Guarino, A.M.; Bekisz, J.M.; Cronstein, B.N.; Flores, R.L. Regeneration of a Pediatric Alveolar Cleft Model Using Three-Dimensionally Printed Bioceramic Scaffolds and Osteogenic Agents: Comparison of Dipyridamole and rhBMP-2. Plast. Reconstr. Surg. 2019, 144, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.D.; Diaz-Siso, J.R.; Witek, L.; Bekisz, J.M.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Rodriguez, E.D.; Coelho, P.G. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. J. Surg. Res. 2018, 223, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.D.; Diaz-Siso, J.R.; Witek, L.; Bekisz, J.M.; Gil, L.F.; Cronstein, B.N.; Flores, R.L.; Torroni, A.; Rodriguez, E.D.; Coelho, P.G. Dipyridamole Augments Three-Dimensionally Printed Bioactive Ceramic Scaffolds to Regenerate Craniofacial Bone. Plast. Reconstr. Surg. 2019, 143, 1408–1419. [Google Scholar] [CrossRef]
- Shen, C.; Wang, M.M.; Witek, L.; Tovar, N.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Coelho, P.G. Transforming the Degradation Rate of β-tricalcium Phosphate Bone Replacement Using 3-Dimensional Printing. Ann. Plast. Surg. 2021, 87, e153–e162. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Witek, L.; Atria, P.; Sobieraj, M.; Bowers, M.; Lopez, C.D.; Cronstein, B.N.; Coelho, P.G. Form and functional repair of long bone using 3D-printed bioactive scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Flores, R.L.; Witek, L.; Torroni, A.; Ibrahim, A.; Wang, Z.; Liss, H.A.; Cronstein, B.N.; Lopez, C.D.; Maliha, S.G.; et al. Dipyridamole-loaded 3D-printed bioceramic scaffolds stimulate pediatric bone regeneration in vivo without disruption of craniofacial growth through facial maturity. Sci. Rep. 2019, 9, 18439. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Alifarag, A.M.; Tovar, N.; Lopez, C.D.; Cronstein, B.N.; Rodriguez, E.D.; Coelho, P.G. Repair of Critical-Sized Long Bone Defects Using Dipyridamole-Augmented 3D-Printed Bioactive Ceramic Scaffolds. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Zabala, A.; Galvez-Martin, P.; Marchal, J.A.; Vazquez-Lasa, B.; Gallego, I.; Saenz-Del-Burgo, L.; Pedraz, J.L. Chondroitin and Dermatan Sulfate Bioinks for 3D Bioprinting and Cartilage Regeneration. Macromol. Biosci. 2022, 22, e2100435. [Google Scholar] [CrossRef] [PubMed]
- Kiyotake, E.A.; Thomas, E.E.; Iribagiza, C.; Detamore, M.S. High-stiffness, fast-crosslinking, cartilage matrix bioinks. J. Biomech. 2023, 148, 111471. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Garcia-Villen, F.; Zabala, A.; de Retana, A.M.O.; Gallego, I.; Saenz-Del-Burgo, L.; Pedraz, J.L. 3D Bioprinted Hydroxyapatite or Graphene Oxide Containing Nanocellulose-Based Scaffolds for Bone Regeneration. Macromol. Biosci. 2022, 22, e2200236. [Google Scholar] [CrossRef] [PubMed]
- Behan, K.; Dufour, A.; Garcia, O.; Kelly, D. Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules 2022, 12, 216. [Google Scholar] [CrossRef]
- Olmos-Juste, R.; Larranaga-Jaurrieta, G.; Larraza, I.; Ramos-Diez, S.; Camarero-Espinosa, S.; Gabilondo, N.; Eceiza, A. Alginate-waterborne polyurethane 3D bioprinted scaffolds for articular cartilage tissue engineering. Int. J. Biol. Macromol. 2023, 253, 127070. [Google Scholar] [CrossRef]
- Mainardi, V.L.; Rubert, M.; Sabato, C.; de Leeuw, A.; Arrigoni, C.; Dubini, G.; Candrian, C.; Muller, R.; Moretti, M. Culture of 3D bioprinted bone constructs requires an increased fluid dynamic stimulation. Acta Biomater. 2022, 153, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gaihre, B.; George, M.N.; Miller, A.L., 2nd; Xu, H.; Waletzki, B.E.; Lu, L. 3D bioprinting of oligo(poly[ethylene glycol] fumarate) for bone and nerve tissue engineering. J. Biomed. Mater. Res. A 2021, 109, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Rathan, S.; Dejob, L.; Schipani, R.; Haffner, B.; Mobius, M.E.; Kelly, D.J. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv. Health Mater. 2019, 8, e1801501. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, P.; Sadowska, J.M.; O’Brien, F.J.; Kelly, D.J. 3D bioprinting of cartilaginous templates for large bone defect healing. Acta Biomater. 2023, 156, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Theus, A.S.; Kamalakar, A.; Ning, L.; Cao, C.; Tomov, M.L.; Kaiser, J.M.; Goudy, S.; Willett, N.J.; Jang, H.W.; et al. 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers 2021, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Mao, X.; Cao, Y.; Liu, Z.; Shen, Z.; Li, M.; Jia, W.; Guo, Z.; Wang, Z.; Xiang, C.; et al. 3D Bioprinting Using Synovium-Derived MSC-Laden Photo-Cross-Linked ECM Bioink for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 8895–8913. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Liu, Y.; Peng, L.; Lu, D.; Wang, P.; Ke, D.; Yang, H.; Zhu, X.; Ruan, C. 3D-bioprinted anisotropic bicellular living hydrogels boost osteochondral regeneration via reconstruction of cartilage-bone interface. Innovation 2024, 5, 100542. [Google Scholar] [CrossRef] [PubMed]
- Nulty, J.; Burdis, R.; Kelly, D.J. Biofabrication of Prevascularised Hypertrophic Cartilage Microtissues for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 661989. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiao, X.; Yang, X.; Ma, J.; Wang, T.; Jin, W.; Li, W.; Yang, H.; Mao, Y.; Gan, Y.; et al. 3D bioprinting of osteon-mimetic scaffolds with hierarchical microchannels for vascularized bone tissue regeneration. Biofabrication 2022, 14, 035008. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef]
- Hu, G.; Liang, Z.; Fan, Z.; Yu, M.; Pan, Q.; Nan, Y.; Zhang, W.; Wang, L.; Wang, X.; Hua, Y.; et al. Construction of 3D-Bioprinted cartilage-mimicking substitute based on photo-crosslinkable Wharton’s jelly bioinks for full-thickness articular cartilage defect repair. Mater. Today Bio 2023, 21, 100695. [Google Scholar] [CrossRef] [PubMed]
- Nulty, J.; Freeman, F.E.; Browe, D.C.; Burdis, R.; Ahern, D.P.; Pitacco, P.; Lee, Y.B.; Alsberg, E.; Kelly, D.J. 3D bioprinting of prevascularised implants for the repair of critically-sized bone defects. Acta Biomater. 2021, 126, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Browe, D.C.; Nulty, J.; Von Euw, S.; Grayson, W.L.; Kelly, D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cell Mater. 2019, 38, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3D Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Griesbach, J.; Ganeyev, M.; Zehnder, A.K.; Zeng, P.; Schadli, G.N.; Leeuw, A.; Lai, Y.; Rubert, M.; Muller, R. Long-term mechanical loading is required for the formation of 3D bioprinted functional osteocyte bone organoids. Biofabrication 2022, 14, 035018. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.K.; Dinkelborg, P.H.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Stolarov, P.; de Vries, J.; Stapleton, S.; Morris, L.; Martyniak, K.; Kean, T.J. Suitability of Gelatin Methacrylate and Hydroxyapatite Hydrogels for 3D-Bioprinted Bone Tissue. Materials 2024, 17, 1218. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-instructive materials for musculoskeletal regeneration. Acta Biomater. 2019, 96, 20–34. [Google Scholar] [CrossRef]
- Chrungoo, S.; Bharadwaj, T.; Verma, D. Nanofibrous polyelectrolyte complex incorporated BSA-alginate composite bioink for 3D bioprinting of bone mimicking constructs. Int. J. Biol. Macromol. 2024, 266, 131123. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.J.; Davydov, A.V.; Frukhtbeyen, S.; Seppala, J.E.; Takagi, S.; Chow, L.; Alimperti, S. Biofabrication of 3D printed hydroxyapatite composite scaffolds for bone regeneration. Biomed. Mater. 2021, 16, 045002. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ji, M.; Wu, B.; Xu, X.; Wang, G.; Zhang, Y.; Xia, Y.; Li, Z.; Zhang, T.; Sun, W.; et al. Engineering Highly Vascularized Bone Tissues by 3D Bioprinting of Granular Prevascularized Spheroids. ACS Appl. Mater. Interfaces 2023, 15, 43492–43502. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Eichholz, K.; Chariyev-Prinz, F.; Pitacco, P.; Aydin, H.M.; Kelly, D.J.; Vargel, I. Biofabrication of Poly(glycerol sebacate) Scaffolds Functionalized with a Decellularized Bone Extracellular Matrix for Bone Tissue Engineering. Bioengineering 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D bioprinting of HAP/collagen scaffold in gelation bath for bone tissue engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Liu, S.; Kilian, D.; Ahlfeld, T.; Hu, Q.; Gelinsky, M. Egg white improves the biological properties of an alginate-methylcellulose bioink for 3D bioprinting of volumetric bone constructs. Biofabrication 2023, 15, 025013. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Huang, Y.; Yang, S.; Ouyang, J.; Bu, S.; Zhong, W.; Liu, Z.; Xing, M.M.Q. Polydopamine-coated paper-stack nanofibrous membranes enhancing adipose stem cells’ adhesion and osteogenic differentiation. J. Mater. Chem. B 2014, 2, 6917–6923. [Google Scholar] [CrossRef] [PubMed]
- Ojansivu, M.; Rashad, A.; Ahlinder, A.; Massera, J.; Mishra, A.; Syverud, K.; Finne-Wistrand, A.; Miettinen, S.; Mustafa, K. Wood-based nanocellulose and bioactive glass modified gelatin-alginate bioinks for 3D bioprinting of bone cells. Biofabrication 2019, 11, 035010. [Google Scholar] [CrossRef]
- Pant, S.; Thomas, S.; Loganathan, S.; Valapa, R.B. 3D bioprinted poly(lactic acid)/mesoporous bioactive glass based biomimetic scaffold with rapid apatite crystallization and in-vitro Cytocompatability for bone tissue engineering. Int. J. Biol. Macromol. 2022, 217, 979–997. [Google Scholar] [CrossRef]
- Zhu, H.; Monavari, M.; Zheng, K.; Distler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 2022, 18, e2104996. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.J.; Sarker, B.; Zehnder, T.; Silva, R.; Mano, J.F.; Boccaccini, A.R. Bioplotting of a bioactive alginate dialdehyde-gelatin composite hydrogel containing bioactive glass nanoparticles. Biofabrication 2016, 8, 035005. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kuth, S.; Distler, T.; Gogele, C.; Stolzel, K.; Detsch, R.; Boccaccini, A.R.; Schulze-Tanzil, G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111189. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.A.; Lee, J.Y. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar] [CrossRef] [PubMed]
- Raslan, A.; Saenz Del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair. Bone 2022, 154, 116198. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Pan, C.C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, T.; Borciani, G.; Avnet, S.; Rubini, K.; Baldini, N.; Graziani, G.; Boanini, E. Incorporation/Enrichment of 3D Bioprinted Constructs by Biomimetic Nanoparticles: Tuning Printability and Cell Behavior in Bone Models. Nanomaterials 2023, 13, 2040. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kumar, S.B.; Park, C.H.; Kim, C.S. Development of cell-laden photopolymerized constructs with bioactive amorphous calcium magnesium phosphate for bone tissue regeneration via 3D bioprinting. Int. J. Biol. Macromol. 2024, 267, 131412. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Kim, J.; Kim, H.S.; Lim, S.Y.; Han, D.; Huser, A.J.; Lee, S.B.; Gim, Y.; Ji, J.H.; Kim, D.; et al. Acceleration of bone formation by octacalcium phosphate composite in a rat tibia critical-sized defect. J. Orthop. Transl. 2022, 37, 100–112. [Google Scholar] [CrossRef]
- Chiesa, I.; De Maria, C.; Lapomarda, A.; Fortunato, G.M.; Montemurro, F.; Di Gesu, R.; Tuan, R.S.; Vozzi, G.; Gottardi, R. Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication 2020, 12, 025013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B Biointerfaces 2021, 197, 111420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.H.; Rubert, M.; Muller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Bacabac, R.G.; Mullender, M.G. Mechanobiology of bone tissue. Pathol. Biol. 2005, 53, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mondal, P.; Tyeb, S.; Chatterjee, K. Visible light-based 3D bioprinted composite scaffolds of kappa-carrageenan for bone tissue engineering applications. J. Mater. Chem. B 2024, 12, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, R.; Deng, X.; Shao, Z.; Deganello, D.; Yan, C.; Xia, Z. Three-dimensional biofabrication of an aragonite-enriched self-hardening bone graft substitute and assessment of its osteogenicity in vitro and in vivo. Biomater. Transl. 2020, 1, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Kiranda, H.K.; Mahmud, R.; Abubakar, D.; Zakaria, Z.A. Fabrication, Characterization and Cytotoxicity of Spherical-Shaped Conjugated Gold-Cockle Shell Derived Calcium Carbonate Nanoparticles for Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.; Wolf, P.; Mehler, D.; Gotz, H.; Ruzgar, M.; Baranowski, A.; Henrich, D.; Rommens, P.M.; Ritz, U. Biofabrication of SDF-1 Functionalized 3D-Printed Cell-Free Scaffolds for Bone Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 2175. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; van der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012, 8, 2047–2055. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Yang, Y.; Yuan, K.; Zhou, F.; Liu, H.; Zhou, Q.; Yang, S.; Tang, T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021, 6, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Sun, Y.; Choi, Y.J.; Ha, D.H.; Jeon, I.; Cho, D.W. 3D cell-printing of tendon-bone interface using tissue-derived extracellular matrix bioinks for chronic rotator cuff repair. Biofabrication 2021, 13, 035005. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, S.; Yu, J.; Gu, Z.; Zhang, Y. Advances in Translational 3D Printing for Cartilage, Bone, and Osteochondral Tissue Engineering. Small 2022, 18, e2201869. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Murata, D.; Fujimoto, R.; Tamaki, S.; Nagata, S.; Ikeya, M.; Toguchida, J.; Nakayama, K. Bio-3D printing iPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication 2021, 13, 044103. [Google Scholar] [CrossRef] [PubMed]
- Laternser, S.; Keller, H.; Leupin, O.; Rausch, M.; Graf-Hausner, U.; Rimann, M. A Novel Microplate 3D Bioprinting Platform for the Engineering of Muscle and Tendon Tissues. SLAS Technol. 2018, 23, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef] [PubMed]
- Toprakhisar, B.; Nadernezhad, A.; Bakirci, E.; Khani, N.; Skvortsov, G.A.; Koc, B. Development of Bioink from Decellularized Tendon Extracellular Matrix for 3D Bioprinting. Macromol. Biosci. 2018, 18, e1800024. [Google Scholar] [CrossRef] [PubMed]
- Baena, J.M.; Jimenez, G.; Lopez-Ruiz, E.; Antich, C.; Grinan-Lison, C.; Peran, M.; Galvez-Martin, P.; Marchal, J.A. Volume-by-volume bioprinting of chondrocytes-alginate bioinks in high temperature thermoplastic scaffolds for cartilage regeneration. Exp. Biol. Med. 2019, 244, 13–21. [Google Scholar] [CrossRef] [PubMed]
- van Loo, B.; Schot, M.; Gurian, M.; Kamperman, T.; Leijten, J. Single-Step Biofabrication of In Situ Spheroid-Forming Compartmentalized Hydrogel for Clinical-Sized Cartilage Tissue Formation. Adv. Health Mater. 2024, 13, e2300095. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Kruger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef]
- Gorronogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jimenez, G.; Chocarro, C.; Carrillo, E.; Montanez, E.; Galvez-Martin, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Barcelo, X.; Eichholz, K.F.; Garcia, O.; Kelly, D.J. Tuning the Degradation Rate of Alginate-Based Bioinks for Bioprinting Functional Cartilage Tissue. Biomedicines 2022, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, B.L.; Lord, M.S.; Whitelock, J.M.; Melrose, J. Harnessing chondroitin sulphate in composite scaffolds to direct progenitor and stem cell function for tissue repair. Biomater. Sci. 2018, 6, 947–957. [Google Scholar] [CrossRef]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Ozturk, E.; Arlov, O.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed. Mater. Res. A 2022, 110, 884–898. [Google Scholar] [CrossRef]
- Chakraborty, J.; Fernandez-Perez, J.; van Kampen, K.A.; Roy, S.; Ten Brink, T.; Mota, C.; Ghosh, S.; Moroni, L. Development of a biomimetic arch-like 3D bioprinted construct for cartilage regeneration using gelatin methacryloyl and silk fibroin-gelatin bioinks. Biofabrication 2023, 15, 035009. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Liu, M.; Zhang, Y.; Cao, Y.; Pei, R. 3D Bioprinting of Bone Marrow Mesenchymal Stem Cell-Laden Silk Fibroin Double Network Scaffolds for Cartilage Tissue Repair. Bioconjug. Chem. 2020, 31, 1938–1947. [Google Scholar] [CrossRef]
- Kosik-Koziol, A.; Costantini, M.; Mroz, A.; Idaszek, J.; Heljak, M.; Jaroszewicz, J.; Kijenska, E.; Szoke, K.; Frerker, N.; Barbetta, A.; et al. 3D bioprinted hydrogel model incorporating beta-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication 2019, 11, 035016. [Google Scholar] [CrossRef]
- Li, M.; Wu, Y.; Yuan, T.; Su, H.; Qin, M.; Yang, X.; Mi, S. Biofabrication of Composite Tendon Constructs with the Fibrous Arrangement, High Cell Density, and Enhanced Cell Alignment. ACS Appl. Mater. Interfaces 2023, 15, 47989–48000. [Google Scholar] [CrossRef]
- Henrionnet, C.; Pourchet, L.; Neybecker, P.; Messaoudi, O.; Gillet, P.; Loeuille, D.; Mainard, D.; Marquette, C.; Pinzano, A. Combining Innovative Bioink and Low Cell Density for the Production of 3D-Bioprinted Cartilage Substitutes: A Pilot Study. Stem Cells Int. 2020, 2020, 2487072. [Google Scholar] [CrossRef]
- Majumder, N.; Roy, C.; Doenges, L.; Martin, I.; Barbero, A.; Ghosh, S. Covalent Conjugation of Small Molecule Inhibitors and Growth Factors to a Silk Fibroin-Derived Bioink to Develop Phenotypically Stable 3D Bioprinted Cartilage. ACS Appl. Mater. Interfaces 2024, 16, 9925–9943. [Google Scholar] [CrossRef]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Valerio, M.S.; Edwards, J.B.; Dolan, C.P.; Motherwell, J.M.; Potter, B.K.; Dearth, C.L.; Goldman, S.M. Effect of Targeted Cytokine Inhibition on Progression of Post-Traumatic Osteoarthritis Following Intra-Articular Fracture. Int. J. Mol. Sci. 2023, 24, 13606. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, X.; Zhou, Z.; Zhu, W.; Chen, X.; He, Y.; He, N.; Han, X.; Zhou, D.; Duan, X.; et al. A naringin-derived bioink enhances the shape fidelity of 3D bioprinting and efficiency of cartilage defect repair. J. Mater. Chem. B 2022, 10, 7030–7044. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Zhang, Y.; Dai, K.; Wei, Y. 3D-bioprinted gradient-structured scaffold generates anisotropic cartilage with vascularization by pore-size-dependent activation of HIF1alpha/FAK signaling axis. Nanomedicine 2021, 37, 102426. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Zhai, Z.; Dai, K. 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics 2019, 9, 6949–6961. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, T.; Gao, C.; Cao, F.; Li, H.; Liao, Z.; Fu, L.; Li, P.; Chen, W.; Sun, Z.; et al. 3D-Bioprinted Difunctional Scaffold for In Situ Cartilage Regeneration Based on Aptamer-Directed Cell Recruitment and Growth Factor-Enhanced Cell Chondrogenesis. ACS Appl. Mater. Interfaces 2021, 13, 23369–23383. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111388. [Google Scholar] [CrossRef]
- Ye, C.; Chen, J.; Qu, Y.; Liu, H.; Yan, J.; Lu, Y.; Yang, Z.; Wang, F.; Li, P. Naringin and bone marrow mesenchymal stem cells repair articular cartilage defects in rabbit knees through the transforming growth factor-beta superfamily signaling pathway. Exp. Ther. Med. 2020, 20, 59. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef]
- Chae, S.; Choi, Y.J.; Cho, D.W. Mechanically and biologically promoted cell-laden constructs generated using tissue-specific bioinks for tendon/ligament tissue engineering applications. Biofabrication 2022, 14, 025013. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Guo, X.; Zhao, Y.; Ahmed, M.M.S.; Qi, H.; Chen, X. Effect of Tensile Frequency on the Osteogenic Differentiation of Periodontal Ligament Stem Cells. Int. J. Gen. Med. 2022, 15, 5957–5971. [Google Scholar] [CrossRef]
- Hasan, O.; Atif, M.; Jessar, M.M.; Hashmi, P. Application of 3D printing in orthopaedic surgery. A new affordable horizon for cost-conscious care. J. Pak. Med. Assoc. 2019, 69 (Suppl. S1), S46–S50. [Google Scholar]
- Zamborsky, R.; Kilian, M.; Jacko, P.; Bernadic, M.; Hudak, R. Perspectives of 3D printing technology in orthopaedic surgery. Bratisl. Lek. Listy 2019, 120, 498–504. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef]
- Yang, T.; Tan, T.; Yang, J.; Pan, J.; Hu, C.; Li, J.; Zou, Y. The impact of using three-dimensional printed liver models for patient education. J. Int. Med. Res. 2018, 46, 1570–1578. [Google Scholar] [CrossRef]
- Rodriguez Colon, R.; Nayak, V.V.; Parente, P.E.L.; Leucht, P.; Tovar, N.; Lin, C.C.; Rezzadeh, K.; Hacquebord, J.H.; Coelho, P.G.; Witek, L. The presence of 3D printing in orthopedics: A clinical and material review. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2023, 41, 601–613. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S. How the Skin Thickness and Thermal Contact Resistance Influence Thermal Tactile Perception. Micromachines 2019, 10, 87. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ji, Z.; Yan, W.; Zhao, H.; Huang, W.; Liu, H. Application of Bioprinting in Ophthalmology. Int. J. Bioprint 2022, 8, 552. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Lopez, M.J.; Sevensma, K.E. Anatomy, Head and Neck, Eye Cornea. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zhang, B.; Xue, Q.; Li, J.; Ma, L.; Yao, Y.; Ye, H.; Cui, Z.; Yang, H. 3D bioprinting for artificial cornea: Challenges and perspectives. Med. Eng. Phys. 2019, 71, 68–78. [Google Scholar] [CrossRef]
- Fuest, M.; Yam, G.H.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.-F.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2019, 8, 438–449. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Kumar, H.; Mashayekhan, S.; Baradaran-Rafii, A.; Kim, K. Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent. Ann. Biomed. Eng. 2020, 48, 1955–1970. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef]
- Puistola, P.; Miettinen, S.; Skottman, H.; Mörö, A. Novel strategy for multi-material 3D bioprinting of human stem cell based corneal stroma with heterogenous design. Mater. Today Bio 2024, 24, 100924. [Google Scholar] [CrossRef]
- Kutlehria, S.; Dinh, T.C.; Bagde, A.; Patel, N.; Gebeyehu, A.; Singh, M. High-throughput 3D bioprinting of corneal stromal equivalents. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2981–2994. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Loganathan, S.; Valapa, R.B. 3D bioprinted photo crosslinkable GelMA/methylcellulose hydrogel mimicking native corneal model with enhanced in vitro cytocompatibility and sustained keratocyte phenotype for stromal regeneration. Int. J. Biol. Macromol. 2024, 264, 130472. [Google Scholar] [CrossRef]
- Kim, J.; Kong, J.S.; Kim, H.; Han, W.; Won, J.Y.; Cho, D.W. Maturation and Protection Effect of Retinal Tissue-Derived Bioink for 3D Cell Printing Technology. Pharmaceutics 2021, 13, 934. [Google Scholar] [CrossRef]
- Masaeli, E.; Forster, V.; Picaud, S.; Karamali, F.; Nasr-Esfahani, M.H.; Marquette, C. Tissue engineering of retina through high resolution 3-dimensional inkjet bioprinting. Biofabrication 2020, 12, 025006. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.Y.; Kong, J.S.; Lee, H.; Won, J.Y.; Cho, D.W. Development of 3D Printed Bruch’s Membrane-Mimetic Substance for the Maturation of Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 1095. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, M.; Li, X.Y.; Huang, S.; Han, D.; Chang, L.; Ling, L.; Huo, Y.; Alzogool, M.; Yang, N.; et al. Development of a novel bioartificial cornea using 3D bioprinting based on electrospun micro-nanofibrous decellularized extracellular matrix. Biofabrication 2024, 16, 025039. [Google Scholar] [CrossRef]
- Balters, L.; Reichl, S. 3D bioprinting of corneal models: A review of the current state and future outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar] [CrossRef]
- Jia, S.; Bu, Y.; Lau, D.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.J. Advances in 3D bioprinting technology for functional corneal reconstruction and regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1065460. [Google Scholar] [CrossRef]
- Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical insights into bioinks for 3D printing. Chem. Soc. Rev. 2019, 48, 4049–4086. [Google Scholar] [CrossRef]
- Colaco, M.; Igel, D.A.; Atala, A. The potential of 3D printing in urological research and patient care. Nat. Rev. Urol. 2018, 15, 213–221. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.N.; Kim, J.; Jang, J.; Kim, H.K.; Cho, D.W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 2041731418823382. [Google Scholar] [CrossRef]
- Xiang, Y.; Miller, K.; Guan, J.; Kiratitanaporn, W.; Tang, M.; Chen, S. 3D bioprinting of complex tissues in vitro: State-of-the-art and future perspectives. Arch. Toxicol. 2022, 96, 691–710. [Google Scholar] [CrossRef]
- He, B.; Wang, J.; Xie, M.; Xu, M.; Zhang, Y.; Hao, H.; Xing, X.; Lu, W.; Han, Q.; Liu, W. 3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration. Bioact. Mater. 2022, 17, 234–247. [Google Scholar] [CrossRef]
- Mörö, A.; Samanta, S.; Honkamäki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation. Biofabrication 2022, 15, 015020. [Google Scholar] [CrossRef]
- Gronroos, P.; Moro, A.; Puistola, P.; Hopia, K.; Huuskonen, M.; Viheriala, T.; Ilmarinen, T.; Skottman, H. Bioprinting of human pluripotent stem cell derived corneal endothelial cells with hydrazone crosslinked hyaluronic acid bioink. Stem Cell Res. Ther. 2024, 15, 81. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Wu, K.Y.; Tabari, A.; Mazerolle, É.; Tran, S.D. Towards Precision Ophthalmology: The Role of 3D Printing and Bioprinting in Oculoplastic Surgery, Retinal, Corneal, and Glaucoma Treatment. Biomimetics 2024, 9, 145. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-Del-Burgo, L.; Pedraz, J.L. Current Insights Into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308. [Google Scholar] [CrossRef]
- Song, M.J.; Quinn, R.; Nguyen, E.; Hampton, C.; Sharma, R.; Park, T.S.; Koster, C.; Voss, T.; Tristan, C.; Weber, C.; et al. Bioprinted 3D outer retina barrier uncovers RPE-dependent choroidal phenotype in advanced macular degeneration. Nat. Methods 2023, 20, 149–161. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Zhang, X.-W.; Mi, C.-H.; Qi, X.-Y.; Zhou, J.; Wei, D.-X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater. Med. 2023, 4, 59–68. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Al-Atawi, S. Three-dimensional bioprinting in ophthalmic care. Int. J. Ophthalmol. 2023, 16, 1702–1711. [Google Scholar] [CrossRef]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS ONE 2016, 11, e0158674. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef]
- Fakhoury, Y.; Ellabban, A.; Attia, U.; Sallam, A.; Elsherbiny, S. Three-dimensional printing in ophthalmology and eye care: Current applications and future developments. Ther. Adv. Ophthalmol. 2022, 14, 25158414221106682. [Google Scholar] [CrossRef]
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative bioprinting: Repairing tissues and organs in a surgical setting. Trends Biotechnol. 2020, 38, 594–605. [Google Scholar] [CrossRef]
- Samandari, M.; Mostafavi, A.; Quint, J.; Memić, A.; Tamayol, A. In situ bioprinting: Intraoperative implementation of regenerative medicine. Trends Biotechnol. 2022, 40, 1229–1247. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Liu, Y.; Li, M.; Li, D.; Jin, Z. The trend towards in vivo bioprinting. Int. J. Bioprint. 2015, 1, 15–26. [Google Scholar] [CrossRef]
- Slavin, B.V.; Ehlen, Q.T.; Costello, J.P., II; Nayak, V.V.; Bonfante, E.A.; Benalcázar Jalkh, E.B.; Runyan, C.M.; Witek, L.; Coelho, P.G. 3D Printing Applications for Craniomaxillofacial Reconstruction: A Sweeping Review. ACS Biomater. Sci. Eng. 2023, 9, 6586–6609. [Google Scholar] [CrossRef]
- Nuutila, K.; Samandari, M.; Endo, Y.; Zhang, Y.; Quint, J.; Schmidt, T.A.; Tamayol, A.; Sinha, I. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact. Mater. 2022, 8, 296–308. [Google Scholar] [CrossRef]
- MacAdam, A.; Chaudry, E.; McTiernan, C.D.; Cortes, D.; Suuronen, E.J.; Alarcon, E.I. Development of in situ bioprinting: A mini review. Front. Bioeng. Biotechnol. 2022, 10, 940896. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Tigli Aydın, R.S.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Gudapati, H.; Godzik, K.P.; Heo, D.N.; Kang, Y.; Rizk, E.; Ravnic, D.J.; Wee, H.; Pepley, D.F.; Ozbolat, V.; et al. Intra-Operative Bioprinting of Hard, Soft, and Hard/Soft Composite Tissues for Craniomaxillofacial Reconstruction. Adv. Funct. Mater. 2021, 31, 2010858. [Google Scholar] [CrossRef]
- Consultation, T. Proposed Regulatory Scheme for Personalised Medical Devices, Including 3D-Printed Devices; Administration DoHTG: Woden, Australia, 2019. [Google Scholar]
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print me an organ? Ethical and regulatory issues emerging from 3D bioprinting in medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Pullen, L.C. Non-transplantable organs and tissues: A golden opportunity. Am. J. Transplant. 2022, 22, 2127–2128. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, T.; Choong, P.F.; Wallace, G.G.; O’Connell, C.D. The regulatory challenge of 3D bioprinting. Regen. Med. 2023, 18, 659–674. [Google Scholar] [CrossRef]
- Li, P. 3D bioprinting: Regulation, innovation, and patents. In 3D Bioprinting for Reconstructive Surgery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 217–231. [Google Scholar]
- Li, P.H. 3D bioprinting technologies: Patents, innovation and access. Law. Innov. Technol. 2014, 6, 282–304. [Google Scholar] [CrossRef]

| Intended Area of Application | Model | Biomaterial | Incorporated Cell Types | 3D-Printer Used | Time In Vitro/In Vivo | Variables Assessed | Main Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Skin | Rat | Biodegradable polyurethane (PU) and gelatin hydrogel | Fibroblasts, keratinocytes, and endothelial progenitor cells (EPCs) | Planar-/curvilinear extrusion-based | 28 days | Wound closure, re-epithelialization, collagen production, and neovascularization |
| [14] |
| Skin | Mouse | Composite microfragmented adipose extracellular matrix (mFAECM), gelatin methacryloyl (GelMA), and hyaluronic acid methacryloyl (HAMA) | human umbilical vein endothelial cells (HUVECs), fibroblasts, keratinocytes | Extrusion-based | 14 days | wound closure rate, collagen deposition, and neovascularization | Cell-laden group exhibited the following:
| [15] |
| Skin | Mouse | Alginate hydrogel loaded with nitric oxide (NO) donor | Adipose-derived mesenchymal stem cells (ADSCs) | Extrusion-based | 7 and 14 days | Angiogenesis, wound closure rates, epithelialization, and collagen deposition | Cell-laden NO scaffolds significantly enhanced burn wound healing with the following:
| [16] |
| Skin | Mouse | Silk fibroin/gelatin hydrogel loaded with methylene blue nanoparticles | N/A | Extrusion-based | 1, 3, 7, 10, 14 days | Mechanical strength, biocompatibility, antimicrobial activity, and wound healing effectiveness | Hydrogel demonstrated significant wound closure efficiency and infection management relative to control | [17] |
| Nasal cartilage | In vitro | GelMA and polycaprolactone (PCL) | Chondrocytes | Extrusion-based | 50 days | Cell viability, genotoxicity from ultra-violet light exposure, mechanical properties, and ECM |
| [18] |
| Ear cartilage and skin | Immunocompromised rodent | EarSkin: type I collagen hydrogel; EarCartilage: hyaluronan transglutaminase (HATG) based bioink | EarSkin: dermal microvascular endothelial cells and fibroblasts; EarCartilage: auricular chondrocytes | Extrusion-based | 28 days | Integration of constructs with host tissue, vascularization, pigmentation, and mechanical stability | Good integration and function, showing effective vascularization and stable mechanical properties | [19] |
| Trachea | Sheep | PCL | Mesenchymal stem cells (MSCs) | Extrusion-based | 21, 42, and 84 days | Integration of material, growth of respiratory epithelium, post-operative recovery, and complications | Two animals showed complete integration with growth of respiratory epithelium; however, two others had poor post-operative recovery and one developed a wound abscess. The study noted the stiffness of the PCL as a limitation | [20] |
| Trachea | Mice and rabbit | Alternating photocrosslinkable cartilage-specific bioink and vascularized fibrous tissue-specific bioink rings | Chondrocytes and fibroblasts | Extrusion-based | N/A | Integration of material, cellular viability, mechanical properties, tissue-specific regeneration, and epithelialization | Construct successfully mimicked native trachea tissue architecture, showing the following:
| [21] |
| Microvascular tissue | In vitro | Gelatin methacryloyl-sodium alginate hydrogel with fugitive inks for microchannel creation | Endothelial cells and pericytes | Novel extrusion-based printing with dynamic mid-extrusion control | N/A | Cell viability, channel perfusability, hydrogel sheet integrity, and microvascular network formation |
| [22] |
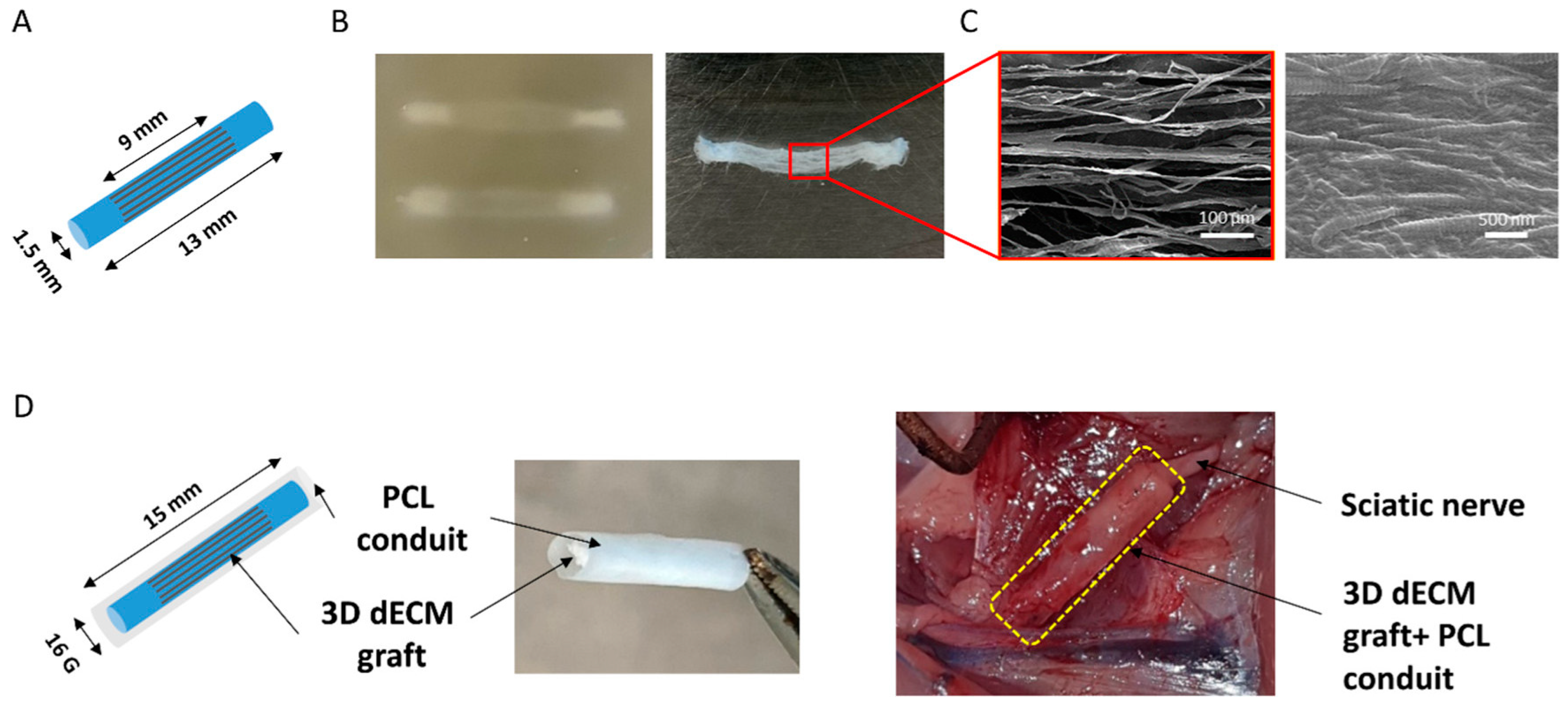
| Peripheral nerve | Rat | Decellularized extracellular matrix (dECM) with PCL conduit | N/A | Extrusion-based | N/A | Regeneration efficacy including number of regenerated axons and muscle weight ratio | The 3D-printed construct showed results comparable to autologous nerve grafts and superior to porcine decellularized nerve grafts | [23] |
| Intended Area of Application | Model | Biomaterial | Incorporated Cell Types | 3D Printing Technique | Time In Vitro/In Vivo | Variables Assessed | Significant Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Cartilage | In vitro | Chondroitin sulfate (CS) and dermatan sulfate (DS) nanocellulose–alginate | Murine MSCs differentiated into chondrocytes | Extrusion-based | 21 days | Compression Young’s modulus; in vitro cytotoxicity; swelling behavior and degradation of scaffold; reverse-transcription polymerase chain reaction (RT-PCR) expression of Collagen (COL)1, COL2, and SRY-Box Transcription Factor 9 (SOX9) |
| [76] |
| Cartilage | In vitro | Pentanoate-modified, solubilized, devitalized cartilage hydrogel (PSDVC) | Porcine cartilage and human bone marrow-derived MSCs | Mechanical Dispense | 8 days | Hydrogel yield stress, stiffness, swelling behavior, and stress relaxation, and crosslinking time; cell viability |
| [77] |
| Bone | In vitro | Alginate–calcium chloride (CaCl2), alginate–sulphate (CaSO4), alginate–gelatin, and alginate–nanocellulose | Bone marrow-derived MSCs | Extrusion-based | 7 days | Material viscosity, cell viability and morphology, anatomical accuracy | Physical properties, stromal cell viability, spreading, and osteogenic potential are all dependent on bioink type. | [78] |
| Bone | In vitro | Nanocellulose–alginate with hydroxyapatite (HA) and graphene oxide (GO) | Murine MSCs | Extrusion-based | 21 days | Bioink viscosity and elastic modulus; cytotoxicity analysis; scaffold swelling, mechanical properties, and degradation; cell viability, metabolic activity, and erythropoietin (EPO) secretion; osteogenic differentiation and mineralization |
| [79] |
| Cartilage | In vitro | Methacrylated porcine cartilage ECM-based hydrogel | Bone marrow MSCs | Extrusion-based | 21 days | Bioink viscosity and shear rate, cell viability, histological and immunohistochemical analysis, mechanical compression testing |
| [80] |
| Cartilage | In vitro | Alginate and waterborne polyurethane | Murine chondrogenic cell line | Extrusion-based | 28 days | Bioink viscosity; scaffold mechanical strength, elasticity and moistening; glycosaminoglycan and Deoxyribonucleic acid (DNA) quantification | Higher alginate content resulted in the following:
| [81] |
| Bone | In vitro | Alginate and gelatin | Human MSCs | Extrusion-based | 42 days | Geometrical and mechanical analysis of scaffold, cell viability assay, fluid dynamic simulations, micro-CT monitoring, histological analysis | Providing mechanical stimulation (shear stress) to 3D-printed scaffolds induced the following:
| [82] |
| Bone | In vitro | Gelatin and oligo (poly-(ethylene glycol) fumarate) (OPF) | Pre-osteoblast cells | Extrusion-based | 7 days | Bioink crosslinkability and printability, electron microscopy, cytotoxicity, cell viability, bone/nerve cell proliferation |
| [83] |
| Cartilage | In vitro | Extracellular matrix-functionalized alginate | Bone marrow MSC | Extrusion-based | 21 or 42 days | Bioink rheological properties and viscosity; chondrogenesis proliferation, cell viability assay, DNA/glycosaminoglycan/collagen assays, histological analysis, RT-PCR; scaffold mechanical analysis |
| [84] |
| Cartilage/Bone | Mice, Rat | Fibrinogen, type A gelatin, hyaluronic acid, and glycerol | Bone marrow-derived MSCs | Fused deposition modeling | 12 weeks | Histological and immunohistochemical analysis, micro-CT analysis |
| [85] |
| Bone | Rat | Superparamagnetic iron oxide nanoparticles (SPION) | Mice embryonic fibroblasts and bone osteoblast-like cells | Mechanical Dispense | 2, 8, and 12 weeks | Compression testing, micro-CT analysis, antibacterial activity assays, histological analysis |
| [86] |
| Cartilage | Rat | Gelatin methacrylate, hyaluronic acid methacrylate, and chondroitin sulfate methacrylate | Synovium-derived MSCs (SMSCs) | Extrusion-based | 4 and 12 weeks | Bioink preparation: scaffold morphology, swelling, degradation, mechanical strength, printability analysis; in vitro: biocompatibility, chondrogenic differentiation, and Ribonucleic acid (RNA) sequencing; in vivo: histological, microCT, and gait analysis |
| [87] |
| Bone/Cartilage | Rabbit | Anisotropic bicellular living hydrogels (ABLHs) | Articular cartilage progenitor cells (ACPCs) and bone MSCs (BMSCs) | Extrusion-based | 6 and 12 weeks | Cell proliferation rate, cell viability, confocal microscopy, gene expression via RT-PCR, histologic and immunohistochemical analysis. |
| [88] |
| Cartilage | Rat | Fibrin–gelatin–hyaluronic hydrogel | Human umbilical vein endothelial cells (HUVECs) and bone marrow-derived MSCs (BMSCs) | Stereolithography | 4 and 8 weeks | Micro-CT, histological analysis | BMSCs can be differentiated into hypertrophic cartilage microtissues, which were successfully mineralized in vivo and enhanced by pre-vascularization. | [89] |
| Bone | Mice | Gelatin, fibrinogen, hyaluronic acid, glycerol, Pluronic F-127, thrombin, PCL, and bone morphogenetic protein 4 (BMP-4) | Bone marrow-derived MSCs and endothelial progenitor cells (EPCs) | Digital light processing | 2 and 4 weeks | Scaffold visualization, porosity, and mechanical analysis and micro-CT; cell viability assay, osteogenic differentiation, osteoblast activity, angiogenesis differentiation; histological analysis and immunohistochemistry |
| [90] |
| Bone | Human | Polypropylene fumarate, free radical polymerized polyethylene glycol-polycaprolactone (PEG-PCL-PEG), and Pluronic PF 127 | NA | Stereolithography | NA | Scaffold morphology, matrix strength, and matrix resilience; drug release kinetics |
| [91] |
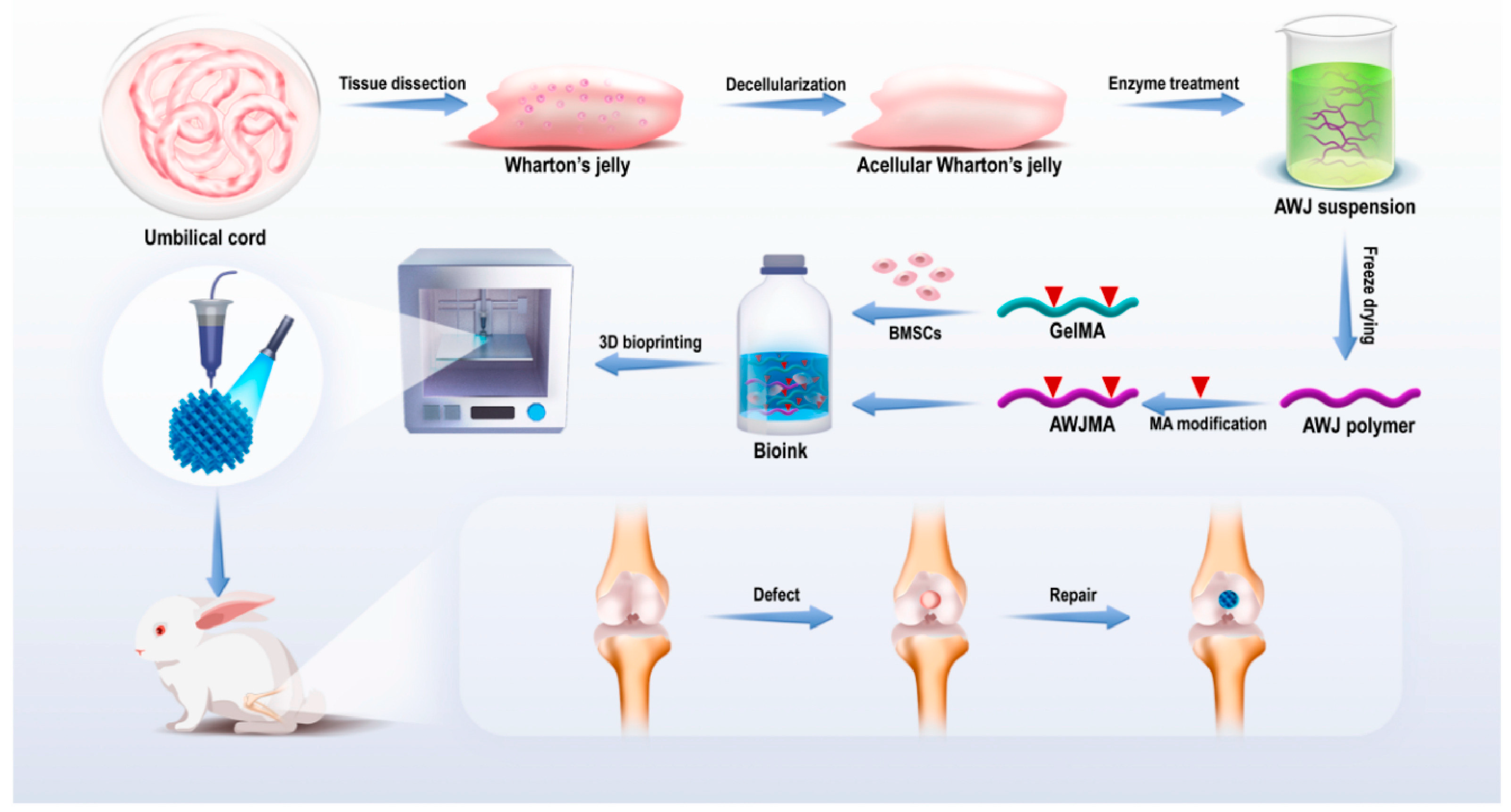
| Cartilage | Rabbit | Acellular Wharton’s jelly and gelatin methacrylate | Bone marrow-derived MSCs | Extrusion-based | 6 and 12 weeks | Scaffold morphology, proteomic analysis, mechanical analysis, swelling, porosity, and rheological analysis; Collagenase degradation, cell viability, chondrogenesis; in vivo morphology and histological analysis |
| [92] |
| Bone | Rabbit | Fibrin-based hydrogel with gelatin methacrylate and nano hydroxyapatite-coated PCL | Bone marrow-derived MSCs and human umbilical vein endothelial cells (HUVECs) | Extrusion-based | 12 weeks | In vitro: cell viability assay, micro vessel assessment; in vivo: vascular micro-CT, colony-forming unit analysis, and histological analysis | Successful development of pre-vascularized tissues leading to accelerated angiogenesis and early bone formation in a critically sized femoral defect. | [93] |
| Bone | Mice | PCL with decellularized bone ECM | Bone marrow MSCs | Extrusion-based | 2 and 8 weeks | Mechanical analysis; cell viability assay, DNA quantification, calcium deposition, ECM characterization, immunofluorescence analysis, micro-CT imaging, histochemical analysis | Adding decellularized bone matrix to PCL scaffolds enhanced mechanical properties and osteogenesis while promoting the following:
| [94] |
| Intended Area of Application | Model | Biomaterial | Incorporated Cell Types | 3D Printing Technique | Time In Vitro/In Vivo | Variables Assessed | Significant Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| Cornea | In vitro | Sodium alginate, gelatin Type B, and Type I bovine collagen hydrogel matrix | Human corneal keratocyte (HCK) cells | Extrusion-based | 14 days | Cell viability, cellular morphology, and scaffold structural integrity |
| [182] |
| Cornea | In vitro | Gelatin methacryloyl/methylcellulose (GelMA/MC) hydrogels | Goat stromal cells | Pneumatic extrusion-based 3D bioprinter | 14 days | Cell viability and proliferation, hydrogel mechanical property, degradation rates, and optical transparency |
| [183] |
| Cornea | In vitro | Hyaluronic acid (HA) -based bioink | Human adipose tissue -derived stem cells (hASCs) | Extrusion-based | 7 days | scaffold mechanical properties and transparency, cell viability and proliferation, cell morphology and tissue formation, and ex vivo integration on porcine cornea |
| [181] |
| Retina | Laser induced neovascularization mouse model; induced retinal degeneration mouse model | Retinal decellularized extracellular matrix | Human Muller cells | Extrusion-based | 7 days | Bioink properties, cell viability and differentiation, and protective effects in reducing vascular abnormalities and retinal protection |
| [184] |
| Retina | Rabbit | Gelatin methacrylate (GelMa) solution | Retinal pigment epithelial (RPE) cells | Inkjet bioprinting | 3 days | Cell viability and proliferation, printability and mechanical stability of bioinks, post-implantation integration, and functionality |
| [185] |
| Retina | Rat | Bruchs membrane extracellular matrix | RPE cells | Extrusion-based | 21 days | Bioink properties, cell viability and proliferation, barrier function, phagocytosis ability, polarized secretion, and implantation integration |
| [186] |
| Cornea | Rabbit | Decellularized extracellular matrix | Primary human and rabbit corneal fibroblasts | Digital light processing (DLP) 3D bioprinting | 14 days | Hydrogel properties, cell viability and proliferation, surgical outcomes, corneal healing | A bioprinted cornea was studied in vivo using an electrospun micro-nanofibrous decellularized extracellular matrix. The cornea demonstrated the following:
| [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirsky, N.A.; Ehlen, Q.T.; Greenfield, J.A.; Antonietti, M.; Slavin, B.V.; Nayak, V.V.; Pelaez, D.; Tse, D.T.; Witek, L.; Daunert, S.; et al. Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine. Bioengineering 2024, 11, 777. https://doi.org/10.3390/bioengineering11080777
Mirsky NA, Ehlen QT, Greenfield JA, Antonietti M, Slavin BV, Nayak VV, Pelaez D, Tse DT, Witek L, Daunert S, et al. Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine. Bioengineering. 2024; 11(8):777. https://doi.org/10.3390/bioengineering11080777
Chicago/Turabian StyleMirsky, Nicholas A., Quinn T. Ehlen, Jason A. Greenfield, Michael Antonietti, Blaire V. Slavin, Vasudev Vivekanand Nayak, Daniel Pelaez, David T. Tse, Lukasz Witek, Sylvia Daunert, and et al. 2024. "Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine" Bioengineering 11, no. 8: 777. https://doi.org/10.3390/bioengineering11080777
APA StyleMirsky, N. A., Ehlen, Q. T., Greenfield, J. A., Antonietti, M., Slavin, B. V., Nayak, V. V., Pelaez, D., Tse, D. T., Witek, L., Daunert, S., & Coelho, P. G. (2024). Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine. Bioengineering, 11(8), 777. https://doi.org/10.3390/bioengineering11080777









