Abstract
A screw-fixed superstructure is predominantly selected for implant prostheses because of the concern regarding developing peri-implantitis, although its infection route remains unclear. Focusing on microleakage from access holes, the present study clinically investigated the bacterial flora in access holes with different sealing materials. We examined 38 sites in 19 patients with two adjacent screw-fixed superstructures. Composite resin was used in the control group, and zinc-containing glass ionomer cement was used in the test group. Bacteria were collected from the access holes 28 days after superstructure placement and were subjected to DNA hybridization analysis. The same patient comparisons of the bacterial counts showed a significant decrease in 14 bacterial species for the red, yellow, and purple complexes in the test group (p < 0.05). In addition, the same patient comparisons of the bacterial ratios showed a significant decrease in six bacterial species for the orange, green, yellow, and purple complexes in the test group (p < 0.05). Furthermore, the same patient comparisons of the implant positivity rates showed a significant decrease in the six bacterial species for the orange, yellow, and purple complexes in the test group. The results of this study indicate that zinc-containing glass ionomer cement is effective as a sealing material for access holes.
1. Introduction
Currently, dental implant treatment is selected for single, partial, and full jaw defect prostheses and is a highly predictive treatment with a high long-term survival rate. However, this high survival rate is influenced by various factors, including operator-dependent factors, patient-dependent factors, and implant-component factors [1,2,3]. With the recent increase in the number of implant-treatment patients, there has also been an increase in the incidence rate of peri-implant diseases, such as peri-implant mucositis and peri-implantitis, during the maintenance period [4,5].
Healthy peri-implant tissues are essential for the long-term maintenance and stability of implants. Peri-implant mucositis is the inflammation of the surrounding tissues without peri-implant bone loss. Peri-implantitis is an inflammation of the surrounding tissues accompanied by the loss of the peri-implant alveolar bone, which has been reported to be caused by bacterial infection in the oral cavity. Thus, screw-retained prostheses are currently selected for implant prostheses because they pose a lower risk of developing peri-implantitis caused by residual cement and offer easier management and recovery in cases of surrounding inflammation.
Regarding bacterial flora, some studies have reported differences between periodontitis and peri-implantitis, while others have reported otherwise; there have been a range of reports on the differences in the bacterial layers [6,7,8]. In particular, differences in periodontal-disease bacterial flora in the red and orange complexes have been indicated; however, it is unclear where this bacterial invasion occurs [9]. Furthermore, periodontal-disease bacterial flora in the red and purple complexes have been suggested to differ between healthy implants and peri-implantitis, although it remains unclear where individual bacteria invade.
Among the components of the dental implant system, the implant–abutment interface (IAI) and screw access hole (SAH) have been reported as sites of bacterial microleakage. However, previous studies have focused only on microleakage in the IAI [10,11], with few reports of bacterial invasion through access holes. Kofron et al. reported that the main disadvantage of the two-piece implant system lies in the presence of micro-gaps along the IAI, even though the abutment is fixed to the implant body by an abutment screw, and that a micro-gap, sized 10 and 135 µm, may cause biological and mechanical complications [4]. Considering the average size of bacteria (width: 0.2–1.5 μm, length: 1–10 μm) [12,13] and the aforementioned size of a micro-gap, it is clear that the space between an abutment and an implant function as a reservoir for bacteria. Consequently, bacteria are transported into and out of the implant body through the IAI, owing to the micro-movement of the abutment. As reported by Jervøe-Storm et al. on the contamination inside an implant following the removal of the abutment of a cement-retained prosthesis [14], infection of the peri-implant tissues can be attributed not only to bacterial invasion from the peri-implant groove but also to bacterial microleakage from the junction owing to the micro-gap and micro-movements of superstructures.
Regarding the bacterial flora with different SAH-sealing methods, do Nascimento et al. applied different temporary sealing methods for single, screw-fixed superstructures [15] and reported that a combination of polytetrafluoroethylene (PTFE) tape and light-polymerized resin resulted in the lowest mean bacterial count, whereas a combination of cotton pellet and instant polymerization resin resulted in the highest mean bacterial count. This indicates that the bacterial count inside an implant body varies greatly depending on the method used to seal the access hole. Furthermore, an in vitro study conducted by Park et al. showed that microleakage occurred only from access holes and not from the IAI [16], suggesting that microleakage from access holes is involved in the development of peri-implantitis. Therefore, the present study focused on the bacterial flora in access holes of screw-fixed superstructures and examined the differences in bacterial flora when two types of sealing materials were used to connect crowns in the same patients.
2. Materials and Methods
This study was approved by the Institutional Review Board of the Showa University Dental Hospital (approval no. DH2020-09; approval date, 28 July 2020). This study was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent to participate in this study. Informed consent was obtained from all participants involved in the study.
2.1. Participant Selection
All patients underwent placement surgery of two adjacent implants at the Department of Implant Dentistry at Showa University Dental Hospital, and those participating in the study were randomly selected from patients aged between 20 and 80 years who wore the final superstructures. A bone-level implant (Straumann) was used as the implant system for all patients, and connected zirconia superstructures of two adjacent teeth with screw-retained abutments were examined.
Exclusion criteria were as follows: Fully edentulous patients; patients with periodontal disease or diabetes mellitus; patients receiving radiation therapy or orthodontic treatment; patients who were pregnant or breastfeeding; patients who had undergone bone grafting at the time of implant placement; patients with bruxism; patients requiring prophylactic antibiotics or who were under steroid medication; patients who had marginal bone loss at the time of superstructure placement; patients wearing a prosthetic device without an intervening abutment (Table 1). These were modified with reference to the criteria of Zhang et al. [17].

Table 1.
Inclusion and exclusion criteria.
2.1.1. Superstructure
After the placement of the abutment, the titanium base and connecting crown (Zirconia, GeoMedi Co., Ltd., Fukuoka, Japan) were bonded using resin cement (3M, Saint Paul MN, USA) on the verification model. In addition, they were immersed in an H2O2 solution before being placed in the oral cavity.
2.1.2. Placement of the Final Superstructures and Sealing Materials (Figure 1)
The twisted PTFE was pressure-welded to the lower part of the access hole of the final superstructure. For the upper part, (1) a dental composite resin (CR: SHOFU INC, Kyoto, Japan) (control group) or (2) zinc-containing glass ionomer cement (GI: GC Corporation, Tokyo, Japan; (test group) was placed in either the medial or distal access hole of the connected superstructures of the two adjacent teeth in a randomized manner and sealed.
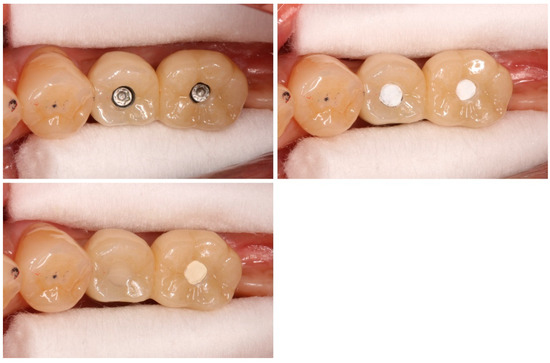
Figure 1.
Final superstructures. The final superstructures are placed in the oral cavity, and the twisted PTFE is pressure-welded to the lower part of the access hole. The upper part is sealed after placing (left) CR (control group) or (right) GI (test group) in a randomized manner.
2.1.3. Zinc-containing Glass Ionomer (GI) Cement
The GI cement released F, Zn, and Ca ions from the filling site. Zn ions suppress bacterial acid production [18], decalcification [19], and degradation of MMP-derived collagen [20]. F and Ca ions are known to suppress decalcification and promote recalcification; when combined with Zn ions, they are expected to exert multiple effects. In addition, an antibacterial test has shown that GI cement suppresses the growth of S. mutans, S. sobrinus, and other bacteria [21], and a biofilm formation test on material surfaces demonstrated that it exhibits effective anti-biofilm properties by reducing bacterial adhesion [22].
2.2. Sampling
The PTFE tape in the access holes was collected 28 days after placement of the superstructures and subjected to DNA hybridization analysis.
2.3. DNA Hybridization
As the first step of quantitative detection, we measured the total amount of 16S rRNA using the standard calibration curve plotted by Yazawa et al. [23]. Next, we determined the number of each bacterial species using a species-specific probe SI corrected with the hybridization affinity ratio.
Data from the Ribosomal RNA Database version 5.5 (Ann Arbor, MI, USA) were used to determine the number of copies of 16SrRNA. Without appropriate information, the median value for the genus was used. To calculate the total number of bacteria in the samples, 16S rRNA copy numbers relative to genomic DNA were assumed to be 4.5, which was calculated based on a weighted average reported in a study wherein the predominant and prevalent bacterial species in the saliva of orally healthy participants were determined using pyrosequencing [24]. The bacterial counts were calculated by multiplying Avogadro’s constant with the molecular weight of the genome (i.e., the molecular weight of 16S rRNA was divided by the number of 16S rRNA copies).
2.4. Items for Investigation
For the 28 bacterial species and 34 items shown in Figure 2, we examined the (i) bacterial count, (ii) bacterial ratio, and (iii) implant positivity rate, which were compared between sealing materials and between the same individuals.
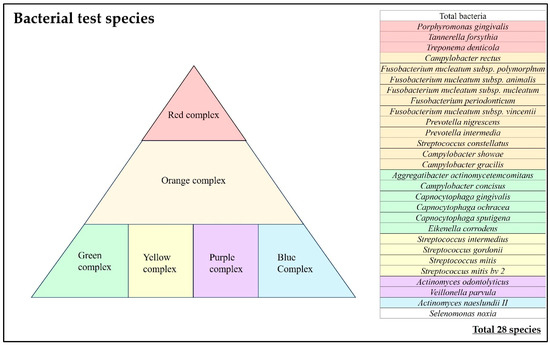
Figure 2.
Bacteria test species.
2.5. Date Analysis
Data were analyzed using statistical software (IBM SPSS Statics 29; IBM, Tokyo, Japan). The Wilcoxon signed-rank test was used to evaluate and compare the sealing materials, and the Mann–Whitney U test was used to evaluate and compare data between individuals. Statistical significance was set at p < 0.05.
3. Results
We examined superstructures (access holes, at 38 sites in 19 patients (Table 2).

Table 2.
Patient data.
3.1. Differences between Sealing Materials
3.1.1. Bacterial Count (Table 3)
In the test group, a significant decrease in bacterial count was observed in total bacteria, one species of the red complex (T. denticola (p = 0.019 < 0.05)), total red complex, two species of the orange complex (F. nucleatum subsp. vincentii (p = 0.034, <0.05), and C. gracilis (p = 0.044, <0.05)), total orange complex, one species of the green complex (C. concisus (p = 0.017, <0.05)), total green complex, one species of the yellow complex (S. gordonii (p = 0.0059, <0.01)), total yellow complex, one species of the purple complex (V. parvula (p = 0.029, <0.05)), and total purple complex. In the test group, 12 of 34 items and 6 out of 28 bacterial species showed a significant decrease in the bacterial count.
In particular, a marked decrease in the bacterial count was observed in the total bacteria, S. gordonii, and the total purple complex.

Table 3.
Differences in bacteria count between sealing materials.
Table 3.
Differences in bacteria count between sealing materials.
| Control | Test | |
|---|---|---|
| Total Bacteria | 13.0 × 106 | 4.3 × 106 ** |
| Porphyromonas gingivalis | 57,920 | 107,953 |
| Tannerella forsythia | 134,959 | 61,406 |
| Treponema denticola | 193,136 | 46,575 * |
| Red Complex | 386,014 | 215,934 * |
| Campylobacter rectus | 151,445 | 36,014 |
| Fusobacterium nucleatum subsp. polymorphum | 76,099 | 43,432 |
| Fusobacterium nucleatum subsp. animalis | 434,247 | 157,379 |
| Fusobacterium nucleatum subsp. nucleatum | 112,125 | 88,747 |
| Fusobacterium periodonticum | 114,794 | 48,227 |
| Fusobacterium nucleatum subsp. vincentii | 42,976 | 18,606 * |
| Prevotella nigrescens | 110,071 | 40,947 |
| Prevotella intermedia | 70,909 | 123,354 |
| Streptococcus constellatus | 25,113 | 37,062 |
| Campylobacter showae | 17,134 | 3481 |
| Campylobacter gracilis | 40,177 | 3371 * |
| Orange Complex | 1,195,088 | 600,620 * |
| Aggregatibacter actinomycetemcomitans | 462 | 66 |
| Campylobacter concisus | 28,056 | 3503 * |
| Capnocytophaga gingivalis | 5081 | 1624 |
| Capnocytophaga ochracea | 28,994 | 1410 |
| Capnocytophaga sputigena | 54,692 | 6540 |
| Eikenella corrodens | 3004 | 4051 |
| Green Complex | 120,290 | 17,194 * |
| Streptococcus intermedius | 6942 | 189 |
| Streptococcus gordonii | 104,047 | 17,282 ** |
| Streptococcus mitis | 22,251 | 23,349 |
| Streptococcus mitis bv 2 | 19,752 | 20,367 |
| Yellow Complex | 152,992 | 61,186 * |
| Actinomyces odontolyticus | 1707 | 1529 |
| Veillonella parvula | 110,860 | 46,849 * |
| Purple Complex | 112,567 | 48,377 ** |
| Actinomyces naeslundii II | 77,696 | 44,997 |
| Selenomonas noxia | 22,013 | 2144 |
* p < 0.05, ** p < 0.01.
3.1.2. Bacterial Ratio (Table 4)
In the test group, a significant decrease in the bacterial ratio was observed in C. gracilis (p = 0.05) of the orange complex, C. concisus (p = 0.00, <0.05) of the green complex, and the total green complex. In the test group, 3 of 32 items and 2 of 28 bacterial species showed a significant decrease in the bacterial ratio.

Table 4.
Differences in bacterial ratio between sealing materials.
Table 4.
Differences in bacterial ratio between sealing materials.
| Control | Test | |
|---|---|---|
| Total Bacteria | ||
| Porphyromonas gingivalis | 0.24 | 0.23 |
| Tannerella forsythia | 0.50 | 0.00 |
| Treponema denticola | 0.64 | 0.00 |
| Red Complex | 4.36 | 0.23 |
| Campylobacter rectus | 0.48 | 0.00 |
| Fusobacterium nucleatum subsp. polymorphum | 0.38 | 0.19 |
| Fusobacterium nucleatum subsp. animalis | 1.46 | 0.00 |
| Fusobacterium nucleatum subsp. nucleatum | 0.35 | 0.00 |
| Fusobacterium periodonticum | 0.49 | 0.27 |
| Fusobacterium nucleatum subsp. vincentii | 0.18 | 0.01 |
| Prevotella nigrescens | 0.40 | 0.02 |
| Prevotella intermedia | 0.18 | 0.00 |
| Streptococcus constellatus | 0.11 | 0.00 |
| Campylobacter showae | 0.08 | 0.00 |
| Campylobacter gracilis | 0.24 | 0.05 * |
| Orange Complex | 4.36 | 0.54 |
| Aggregatibacter actinomycetemcomitans | 0.00 | 0.00 |
| Campylobacter concisus | 0.24 | 0.00 * |
| Capnocytophaga gingivalis | 0.03 | 0.01 |
| Capnocytophaga ochracea | 0.18 | 0.00 |
| Capnocytophaga sputigena | 0.25 | 0.43 |
| Eikenella corrodens | 0.02 | 0.00 |
| Green Complex | 0.72 | 0.44 * |
| Streptococcus intermedius | 0.05 | 1.51 |
| Streptococcus gordonii | 1.01 | 0.55 |
| Streptococcus mitis | 0.20 | 0.00 |
| Streptococcus mitis bv 2 | 0.19 | 1.34 * |
| Yellow Complex | 1.45 | 3.40 |
| Actinomyces odontolyticus | 0.02 | 2.91 * |
| Veillonella parvula | 2.32 | 0.16 |
| Purple Complex | 2.34 | 3.07 |
| Actinomyces naeslundii II | 0.93 | 2.31 |
| Selenomonas noxia | 0.09 | 1.00 |
* p < 0.05.
3.1.3. Implant Positivity Rate (Table 5)
In the test group, a significant decrease in implant positivity rate was observed in four species of the orange complex (F. nucleatum subsp. animalis, F. periodonticum, F. nucleatum subsp. vincentii, and C. gracilis), total orange complex, one species of the green complex (C. concisus), and total green complex.
In the test group, 7 of 34 items and 5 of 28 bacterial species showed a significant decrease in the implant positivity rate. In particular, a marked decrease in the implant positivity rate was observed for the total orange and green complexes.

Table 5.
Differences in implant positivity rate between sealing materials.
Table 5.
Differences in implant positivity rate between sealing materials.
| Control | Test | |
|---|---|---|
| Total Bacteria | ||
| Porphyromonas gingivalis | 26.3% | 15.8% |
| Tannerella forsythia | 78.9% | 78.9% |
| Treponema denticola | 84.2% | 63.2% |
| Red Complex | 63.2% | 52.6% |
| Campylobacter rectus | 26.3% | 26.3% |
| Fusobacterium nucleatum subsp. polymorphum | 42.1% | 15.8% |
| Fusobacterium nucleatum subsp. animalis | 47.4% | 15.8% * |
| Fusobacterium nucleatum subsp. nucleatum | 57.9% | 31.6% |
| Fusobacterium periodonticum | 47.4% | 15.8% * |
| Fusobacterium nucleatum subsp. vincentii | 52.6% | 15.8% * |
| Prevotella nigrescens | 31.6% | 21.1% |
| Prevotella intermedia | 26.3% | 15.8% |
| Streptococcus constellatus | 42.1% | 36.8% |
| Campylobacter showae | 31.6% | 10.5% |
| Campylobacter gracilis | 42.1% | 10.5% * |
| Orange Complex | 40.7% | 19.6% ** |
| Aggregatibacter actinomycetemcomitans | 10.5% | 5.3% |
| Campylobacter concisus | 57.9% | 21.1% * |
| Capnocytophaga gingivalis | 36.8% | 15.8% |
| Capnocytophaga ochracea | 31.6% | 10.5% |
| Capnocytophaga sputigena | 36.8% | 21.1% |
| Eikenella corrodens | 21.1% | 10.5% |
| Green Complex | 32.5% | 14.0% ** |
| Streptococcus intermedius | 42.1% | 15.8% |
| Streptococcus gordonii | 89.5% | 78.9% |
| Streptococcus mitis | 89.5% | 84.2% |
| Streptococcus mitis bv 2 | 94.7% | 89.5% |
| Yellow Complex | 78.9% | 67.1% * |
| Actinomyces odontolyticus | 89.5% | 89.5% |
| Veillonella parvula | 73.7% | 47.4% * |
| Purple Complex | 81.6% | 68.4% |
| Actinomyces naeslundii II | 89.5% | 89.5% |
| Selenomonas noxia | 36.8% | 15.8% |
* p < 0.05, ** p < 0.01.
3.2. Differences between Patients
3.2.1. Bacterial Count (Table 6)
In the test group, a significant decrease in bacterial count was observed in total bacteria, one species of the red complex (T. denticola), total red complex, eight species of the orange complex (F. nucleatum subsp. polymorphum, F. nucleatum subsp. animals, F. nucleatum subsp. nucleatum, F. periodonticum, F. nucleatum subsp. vincentii, P. nigrescens, C. showae, and C. gracilis), total orange complex, two species of the green complex (C. concisus and Capnocytophaga ochracea), total green complex, two species of the yellow complex (S. intermedius and S. gordonii), total yellow complex, two species of the purple complex (A. odontolyticus and V. parvula), total purple complex, and two species of the blue complex (A. naeslundii II and Selenomonas noxia).
In the same patient comparisons, the test group showed a significant decrease in bacterial counts in 23 out of 34 items and 17 out of 28 bacterial species.

Table 6.
Differences in the same individuals.
Table 6.
Differences in the same individuals.
| Bacterial Count | Bacterial Ratio | |
|---|---|---|
| Total Bacteria | 0.000091 ** | |
| Porphyromonas gingivalis | 0.68 | 0.344 |
| Tannerella forsythia | 0.068 | 0.159 |
| Treponema denticola | 0.003 ** | 0.398 |
| Red Complex | 0.029 * | 0.621 |
| Campylobacter rectus | 0.368 | 0.368 |
| Fusobacterium nucleatum subsp. polymorphum | 0.0058 ** | 0.288 |
| Fusobacterium nucleatum subsp. animalis | 0.011 * | 0.043 * |
| Fusobacterium nucleatum subsp. nucleatum | 0.025 * | 0.628 |
| Fusobacterium periodonticum | 0.0038 ** | 0.26 |
| Fusobacterium nucleatum subsp. vincentii | 0.0026 ** | 0.194 |
| Prevotella nigrescens | 0.046 * | 0.252 |
| Prevotella intermedia | 0.681 | 0.344 |
| Streptococcus constellatus | 0.297 | 0.054 |
| Campylobacter showae | 0.014 * | 0.014 * |
| Campylobacter gracilis | 0.0058 ** | 0.0086 ** |
| Orange Complex | 0.025 * | 0.943 |
| Aggregatibacter actinomycetemcomitans | 0.328 | 0.328 |
| Campylobacter concisus | 0.017 * | 0.123 |
| Capnocytophaga gingivalis | 0.104 | 0.338 |
| Capnocytophaga ochracea | 0.015 * | 0.014 * |
| Capnocytophaga sputigena | 0.034 | 0.2 |
| Eikenella corrodens | 0.68 | 0.344 |
| Green Complex | 0.018 * | 0.127 |
| Streptococcus intermedius | 0.0086 ** | 0.288 |
| Streptococcus gordonii | 0.0005 ** | 0.128 |
| Streptococcus mitis | 0.293 | 0.0049 ** |
| Streptococcus mitis bv 2 | 0.357 | 0.0033 ** |
| Yellow Complex | 0.0069 ** | 0.188 |
| Actinomyces odontolyticus | 0.043 * | 0.0078 ** |
| Veillonella parvula | 0.00061 ** | 0.018 * |
| Purple Complex | 0.000091 ** | 0.063 |
| Actinomyces naeslundii II | 0.043 ** | 0.099 |
| Selenomonas noxia | 0.0086 ** | 0.018 * |
* p < 0.05, ** p < 0.01.
3.2.2. Bacterial Ratio (Table 6)
In the test group, a significant decrease in bacterial ratio was observed in three species of the orange complex (F. nucleatum subsp. animalis, C. showae, and C. gracilis), two species of the yellow complex (Capnocytophaga ochracea and S. mitis), one species of the green complex (Capnocytophaga ochracea), one species of the purple complex (Veillonella parvula), and Selenomonas noxia.
In the same patient comparisons, the test group showed a significant decrease in the bacterial ratio in 8 of 28 bacterial species.
3.2.3. Implant Positivity Rate (Figure 3)
The implant positivity rate was 51.3% in the control group and 34.2% in the test group, thereby showing a significant decrease in the test group (p = 0.0019, <0.005).
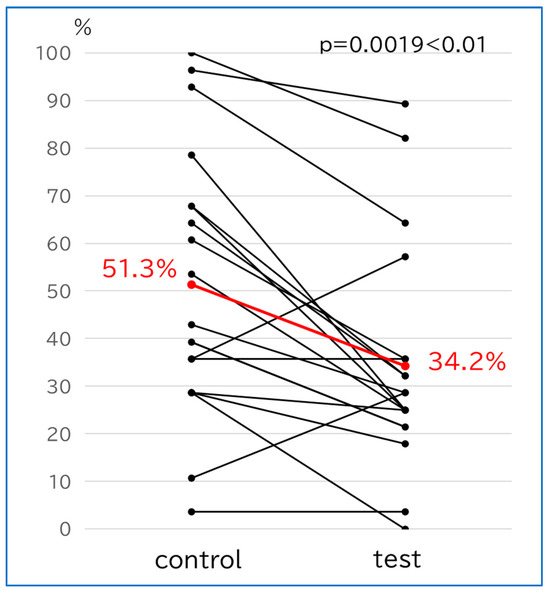
Figure 3.
Differences in implant positivity rate in the same individual patients. The red lines indicate the respective averages.
4. Discussion
In bacteriological research on peri-implantitis, oral bacteria of the “red” and “orange” complexes are believed to be closely associated with periodontal disease and peri-implantitis. Furthermore, there have been many recent reports on the differences in bacterial flora between periodontitis and peri-implantitis and between healthy implants and peri-implantitis [6,7,8]. In addition, a review published in 2021 stated that the bacterial flora for peri-implantitis differs from that of periodontitis and that a comparison of peri-implant health showed a similar trend in the involvement of many types of bacteria and differences in bacterial species [9]. For example, the bacterial flora common to periodontitis and peri-implantitis includes P. gingivalis in the red complex, Fusobacterium spp. in the orange complex, and Streptococcus spp. in the yellow complex, whereas bacterial flora unique to peri-implantitis include T. denticola in the red complex and P. nigrescens in the orange complex. In addition, the bacterial floras common to healthy implants and peri-implantitis include Fusobacterium spp., Campylobacter gracilis, and Streptococcus spp. of the orange complex, while the bacterial flora unique to peri-implantitis include T. denticola, P. gingivalis, and T. forsythia of the red complex and Actinomyces of the purple complex.
Regarding the bacterial positivity rate, Cortelli et al. reported that peri-implantitis had a higher implant positivity rate for T. forsythia, T. denticola, and P. intermedia than periodontitis [25]. In contrast, Zhuang et al. reported no obvious difference in the implant positivity rates for T. denticola and P. intermedia [17]. Canullo et al. reported that compared to healthy implants, the bacterial flora in the internal connection of the implant after crown/abutment removal in peri-implantitis showed a greatly different implant positivity rate for T. denticola of the red complex, P. intermedia of the orange complex, and E. corrodens and C. albicans of the green complex [26].
There have been various reports on the bacterial flora in peri-implantitis, with different types of bacteria examined and bacterial analysis methods used (DNA probe analysis and PCR method). A review by Pérez-Chaparro et al. showed evidence for the association of peri-implantitis with P. gingivalis, T. forsythia, and T. denticola of the red complex, as well as the association of peri-implantitis with P. intermedia of the orange complex [27]. Furthermore, a systematic review by Lafaurie et al. reported that the red complex bacteria were detected at a slightly high frequency in the bacterial flora of peri-implantitis, that the orange complex bacteria, such as P. intermedia and P. nigrescens, were more commonly associated with peri-implantitis, that there was a low association of the red complex bacteria, and that uncultivable anaerobic Gram-positive bacteria, Gram-negative bacteria, and oral resident bacteria, such as Staphylococcus aureus, were also identified [28]. Since the present prospective study was conducted only on healthy implants, we could not examine the suppressive effect on the surrounding inflammation. However, sealing with glass ionomer cement, used in this study, was suggested to be highly effective in preventing peri-implantitis, as it suppressed subgingival bacterial flora, including the total red and orange complexes.
Regarding bacterial invasion from access holes into the implant body, the bone-level implant (two-piece implant system) used in this study had a superstructure and an abutment mechanically connected at or below the bone margin, thereby containing multiple routes for bacterial invasion, such as IAI and SAH. Moreover, sealing materials used in the access hole of the screw-retain superstructure are prone to intraoral contamination. Although various materials have been studied as sealing materials for SAH in screw-retained superstructures [16,29,30,31,32,33], few studies have focused on the capacity of materials to prevent or minimize microbial/bacterial leakage from SAH. Cavalcanti et al. compared gutta-percha (GP) and PTFE as sealing materials for the lower part of access holes, reporting that GP was significantly more effective than PTFE [32]. In contrast, Alshehri et al. reported that PTFE was significantly more effective than GP [33]. Furthermore, the insertion and removal of PTFE was clinically easy, although twisted PTFE had no sealing effect, even when compressed, owing to the lack of chemical bonds. Although GP can be easily compressed and chemically bonded, their insertion is difficult, and their removal is time-consuming. Thus, there are advantages and disadvantages to sealing materials for the lower part of access holes. The results of this study suggest that F, Zn, and Ca ions released from the site filled with GI cement may have acted in an anti-bacterial manner. In addition, we believe that GI cement achieved superior bacterial suppression compared to CR in this study because it can even be applied to sites where moisture-proofing is difficult, it does not require a bonding material, and it exhibits no polymerization shrinkage. Furthermore, our results suggest that reducing the bacterial count and implant positivity rate would suppress the development of peri-implantitis. In addition, future studies will examine differences in bacterial flora according to age.
5. Limitations
This study made comparisons not only between sealing materials but also between the same individuals, as each patient has different oral bacterial flora. However, because this study was conducted on healthy implants, we did not evaluate the association with the bacterial flora of peri-implantitis, the difference from the bacterial flora of the peri-implant gingival groove, and the difference in bacterial flora in patients with cement-retained prosthesis. In addition, because a superstructure is placed in the oral cavity for a long period of time, it is necessary to conduct long-term observational studies, including examination of attrition and abrasion, and further studies are needed in the future.
6. Conclusions
We compared the sealing materials for the upper part of the access holes of screw-retained superstructures. Our results showed that GI cement reduced the bacterial count in access holes and suppressed the implant positivity rate when compared with other materials and in the same patients. These results suggest that GI cement is a useful sealing material for access holes.
Author Contributions
K.Y.: Data curation, conceptualization, methodology, validation, investigation, writing-original draft, and visualization. M.M.: Conceptualization; Methodology, Formal analysis; Investigation; Writing—review and editing; Supervision. K.I.: Resources; Software. T.U.: Validation; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Showa University Dental Hospital (approval no. DH2020—9 and 28 July 2020 of approval). This study was conducted in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
All participants provided written informed consent to participate in this study. Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A Systematic Review of the Survival and Complication Rates of Implant-Supported Fixed Dental Prostheses (FDPs) after a Mean Observation Period of at Least 5 Years. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; dos Santos, D.M.; Santiago, J.F.; Moreno, A.; Pellizzer, E.P. Longevity of Dental Implants in Type IV Bone: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef]
- Jimbo, R.; Albrektsson, T. Long-Term Clinical Success of Minimally and Moderately Rough Oral Implants: A Review of 71 Studies with 5 Years or More of Follow-Up. Implant. Dent. 2015, 24, 62–69. [Google Scholar] [CrossRef]
- Kofron, M.D.; Carstens, M.; Fu, C.; Wen, H.B. In Vitro Assessment of Connection Strength and Stability of Internal Implant-Abutment Connections. Clin. Biomech. 2019, 65, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rismanchian, M.; Hatami, M.; Badrian, H.; Khalighinejad, N.; Goroohi, H. Evaluation of Microgap Size and Microbial Leakage in the Connection Area of 4 Abutments with Straumann (ITI) Implant. J. Oral Implantol. 2012, 38, 677–685. [Google Scholar] [CrossRef]
- Apatzidou, D.; Lappin, D.F.; Hamilton, G.; Papadopoulos, C.A.; Konstantinidis, A.; Riggio, M.P. Microbiome of Peri-Implantitis Affected and Healthy Dental Sites in Patients with a History of Chronic Periodontitis. Arch. Oral Biol. 2017, 83, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hämmerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the Microbiome of Healthy and Diseased Peri-Implant Sites Using Illumina Sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef]
- Quirynen, M.; van Steenberghe, D. Bacterial colonization of the internal part of two-stage implants. An in vivo study. Clin. Oral Implants Res. 1993, 4, 158–161. [Google Scholar] [CrossRef]
- Sasada, Y.; Cochran, D.L. Implant-Abutment Connections: A Review of Biologic Consequences and Peri-implantitis Implications. Int. J. Oral Maxillofac. Implants 2017, 32, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.D.; Pita, M.S.; Santos Ede, S.; Monesi, N.; Pedrazzi, V.; Albuquerque Junior, R.F.; Ribeiro, R.F. Microbiome of titanium and zirconia dental implants abutments. Dent. Mater. 2016, 32, 93–101. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, E.; Giannì, A.B.; Mancini, G.E.; Cura, F.; Carinci, F. Implant-abutment leaking of replace conical connection nobel biocare® implant system. An in vitro study of the microbiological penetration from external environment to implant-abutment space. Oral Implantol. 2016, 9, 76–82. [Google Scholar] [CrossRef]
- Jervøe-Storm, P.M.; Jepsen, S.; Jöhren, P.; Mericske-Stern, R.; Enkling, N. Internal Bacterial Colonization of Implants: Association with Peri-Implant Bone Loss. Clin. Oral Implants Res. 2015, 26, 957–963. [Google Scholar] [CrossRef]
- do Nascimento, C.; Pita, M.S.; Calefi, P.L.; de Oliveira Silva, T.S.; Dos Santos, J.B.; Pedrazzi, V. Different Sealing Materials Preventing the Microbial Leakage into the Screw-Retained Implant Restorations: An In Vitro Analysis by DNA Checkerboard Hybridization. Clin. Oral Implants Res. 2017, 28, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Park, S.D.; Lee, Y.; Kim, Y.L.; Yu, S.H.; Bae, J.M.; Cho, H.W. Microleakage of Different Sealing Materials in Access Holes of Internal Connection Implant. Systems. J. Prosthet. Dent. 2012, 108, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.F.; Watt, R.M.; Mattheos, N.; Si, M.S.; Lai, H.C.; Lang, N.P. Periodontal and Peri-Implant Microbiota in Patients with Healthy and Inflamed Periodontal and Peri-Implant Tissues. Clin. Oral Implants Res. 2016, 27, 13–21. [Google Scholar] [CrossRef]
- He, G.; Pearce, E.I.; Sissons, C.H. Inhibitory Effect of ZnCl2 on Glycolysis in Human Oral Microbes. Arch. Oral Biol. 2002, 2, 117–129. [Google Scholar] [CrossRef]
- Mohammed, N.R.; Mneimne, M.; Hill, R.G.; Al-Jawad, M.; Lynch, R.J.; Anderson, P. Physical Chemical Effects of Zinc on In Vitro Enamel Demineralization. J. Dent. 2014, 42, 1096–1104. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Zinc-Inhibited MMP Mediated Collagen Degradation After Different Dentine Demineralization Procedures. Caries Res. 2012, 46, 201–207. [Google Scholar] [CrossRef]
- Liu, Y.; Kohno, T.; Tsuboi, R.; Thongthai, P.; Fan, D.; Sakai, H.; Kitagawa, H.; Imazato, S. Antibacterial Effects and Physical Properties of a Glass Ionomer Cement Containing BioUnion Filler with Acidity-Induced Ability to Release Zinc Ion. Dent. Mater. J. 2021, 40, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a Novel Glass Ionomer Cement Containing Fluoro-Zinc-Silicate Fillers on Biofilm Formation and Dentin Ion Incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, A.; Kamitani, S.; Togawa, N. Method for Absolute Quantification of Microbial Communities by Using Both Microarrays and Competitive PCR. J. Microbiol. Methods 2019, 165, 105718. [Google Scholar] [CrossRef] [PubMed]
- Moritani, K.; Takeshita, T.; Shibata, Y.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Acetaldehyde Production by Major Oral Microbes. Oral Dis. 2015, 21, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Cortelli, S.C.; Cortelli, J.R.; Romeiro, R.L.; Costa, F.O.; Aquino, D.R.; Orzechowski, P.R.; Araújo, V.C.; Duarte, P.M. Frequency of Periodontal Pathogens in Equivalent Peri-Implant and Periodontal Clinical Statuses. Arch. Oral Biol. 2013, 58, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Peñarrocha-Oltra, D.; Covani, U.; Rossetti, P.H. Microbiologic and Clinical Findings of Implants in Healthy Condition and with Peri-Implantitis. Int. J. Oral Maxillofac. Implants 2015, 30, 834–842. [Google Scholar] [CrossRef]
- Pérez-Chaparro, P.J.; Duarte, P.M.; Shibli, J.A.; Montenegro, S.; Lacerda Heluy, S.; Figueiredo, L.C.; Faveri, M.; Feres, M. The Current Weight of Evidence of the Microbiologic Profile Associated with Peri-Implantitis: A Systematic Review. J. Periodontol. 2016, 87, 1295–1304. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, S.; Lyu, X.; Feng, H.; Liu, M.; Wen, C. Evaluation of Sealing Efficacy and Removal Convenience of Sealing Materials for Implant Abutment Screw Access Holes. BMC Oral Health 2022, 22, 362. [Google Scholar] [CrossRef]
- Moráguez, O.D.; Belser, U.C. The Use of Polytetrafluoroethylene Tape for the Management of Screw Access Channels in Implant-Supported Prostheses. J. Prosthet. Dent. 2010, 103, 189–191. [Google Scholar] [CrossRef]
- Cakan, U.; Gultekin, P.; Guncu, M.B.; Canay, S. Effect of Screw Access Channel Filling Materials on Uniaxial Retentive Force of Cement-Retained Implant Restorations. Aust. Dent. J. 2014, 59, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.G.; Fonseca, F.T.; Zago, C.D.; Brito Junior, R.B.; França, F.M. Efficacy of Gutta-Percha and Polytetrafluoroethylene Tape to Microbiologically Seal the Screw Access Channel of Different Prosthetic Implant Abutments. Clin. Implant. Dent. Relat. Res. 2016, 18, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.; Albaqiah, H. Antimicrobial Efficacy of Materials Used for Sealing the Implant Abutment Screw Hole: An In Vitro Evaluation. Implant. Dent. 2017, 26, 911–914. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).