Manufacturing, Processing, and Characterization of Self-Expanding Metallic Stents: A Comprehensive Review
Abstract
1. Introduction
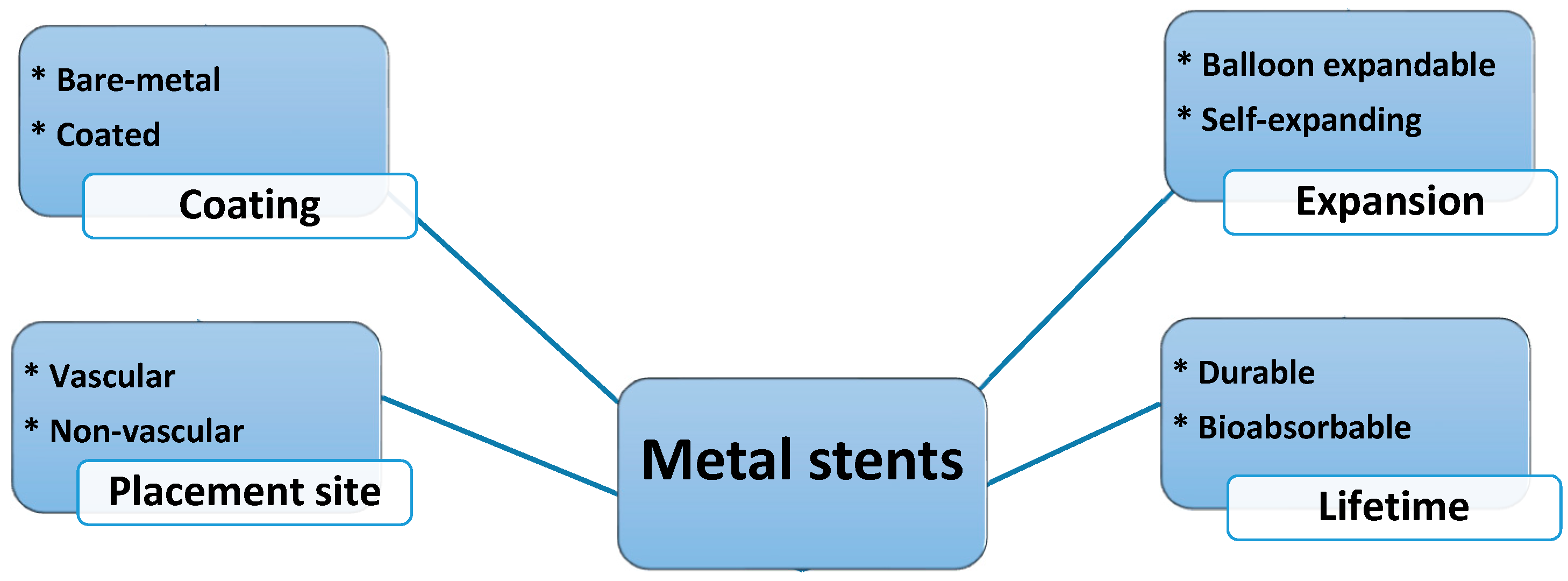
2. Metallic Stents
2.1. Materials for Stents
2.2. Classification of Stents
3. Manufacturing Processes
3.1. Conventional Manufacturing
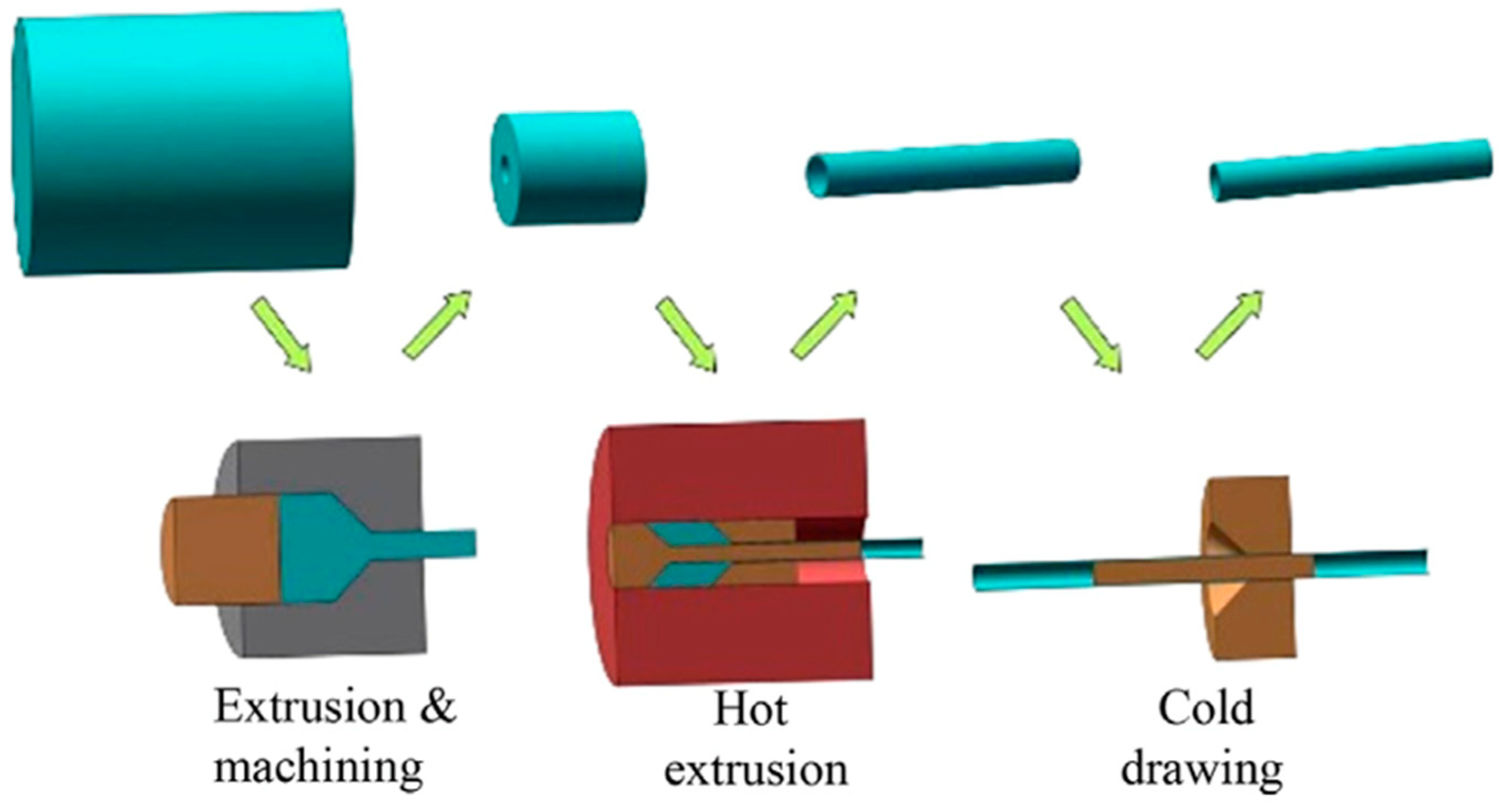
3.1.1. Primary Manufacturing Processes (Mother Tube/Sheet Fabrication)
Casting
Powder Metallurgy (PM)
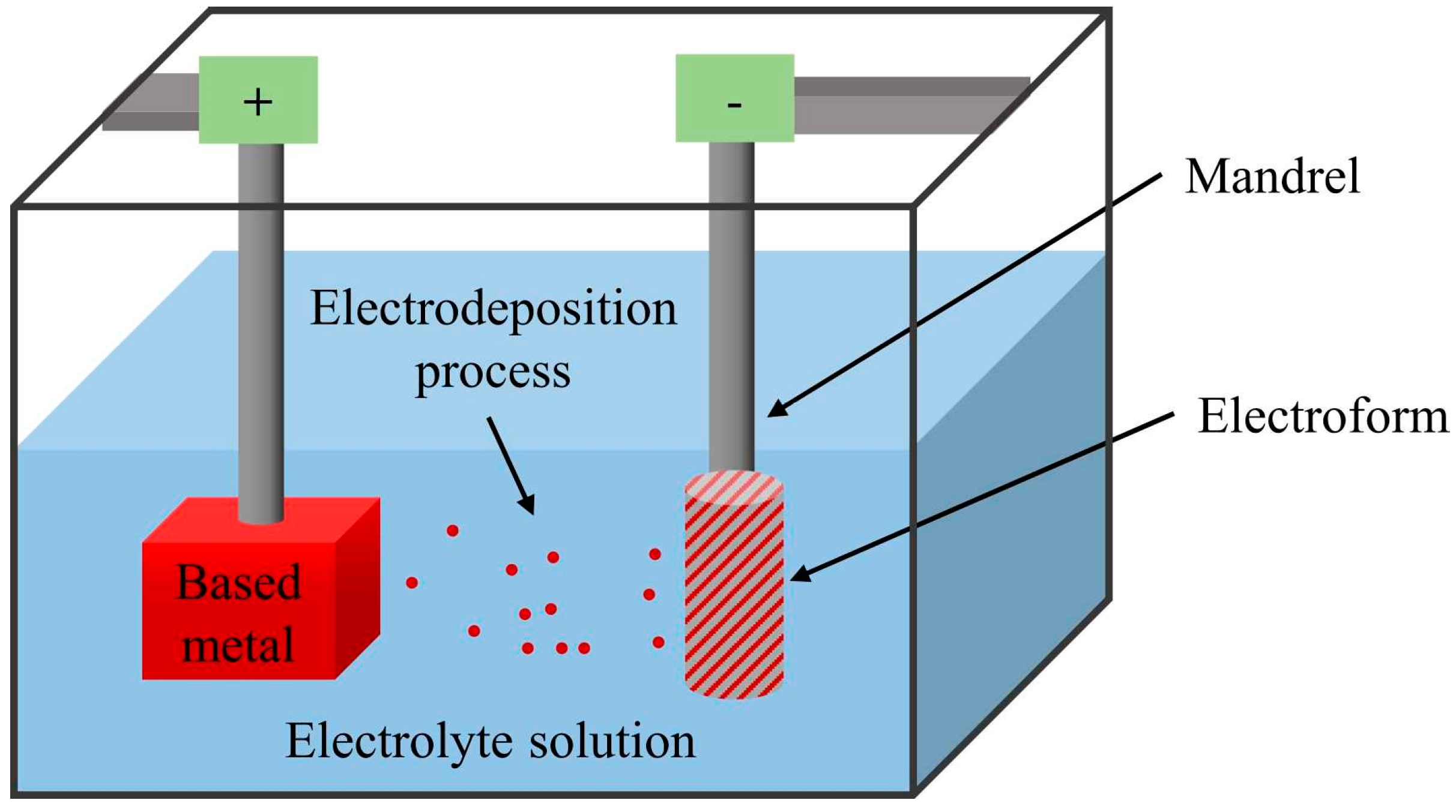
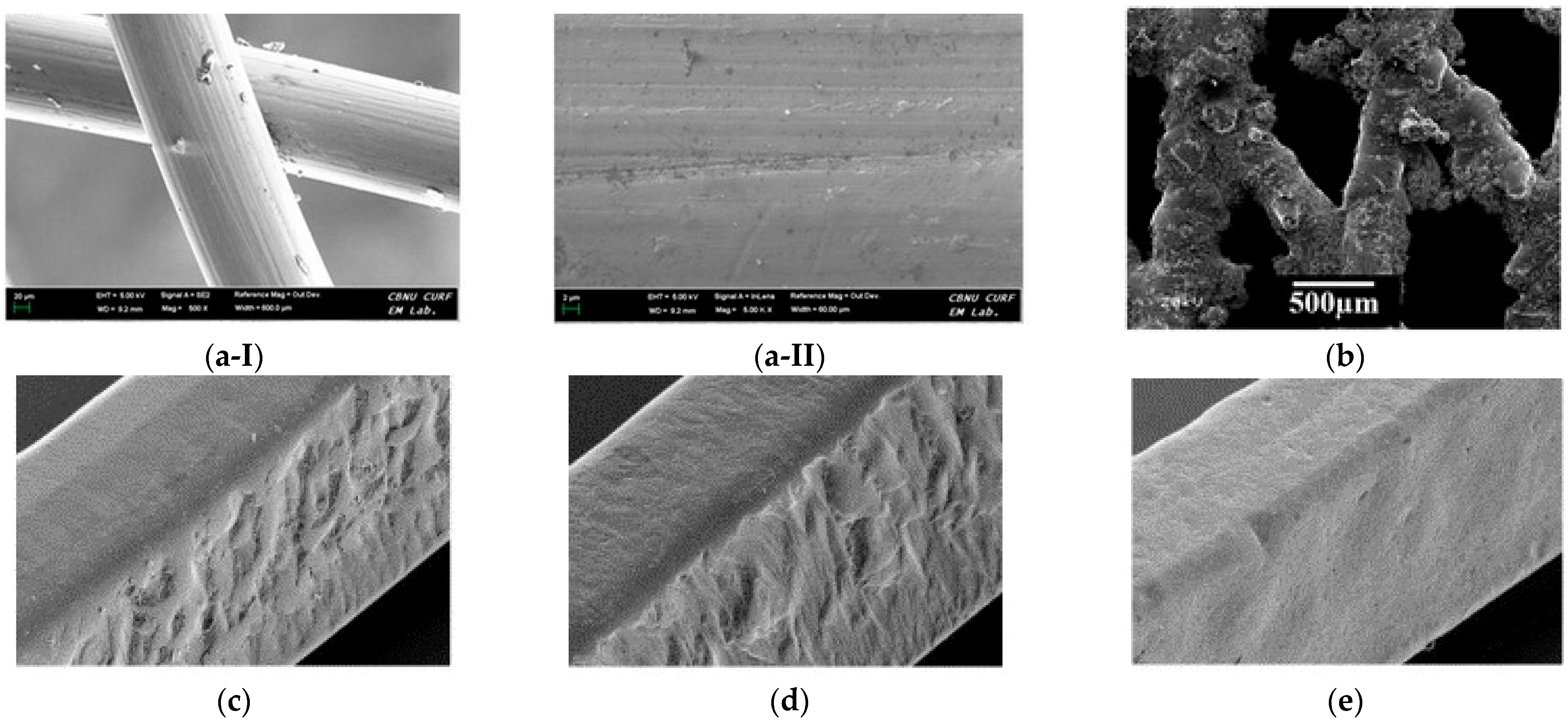
Electroforming
3.1.2. Secondary (Complementary) Manufacturing Processes
Cutting Methods
Laser Welding
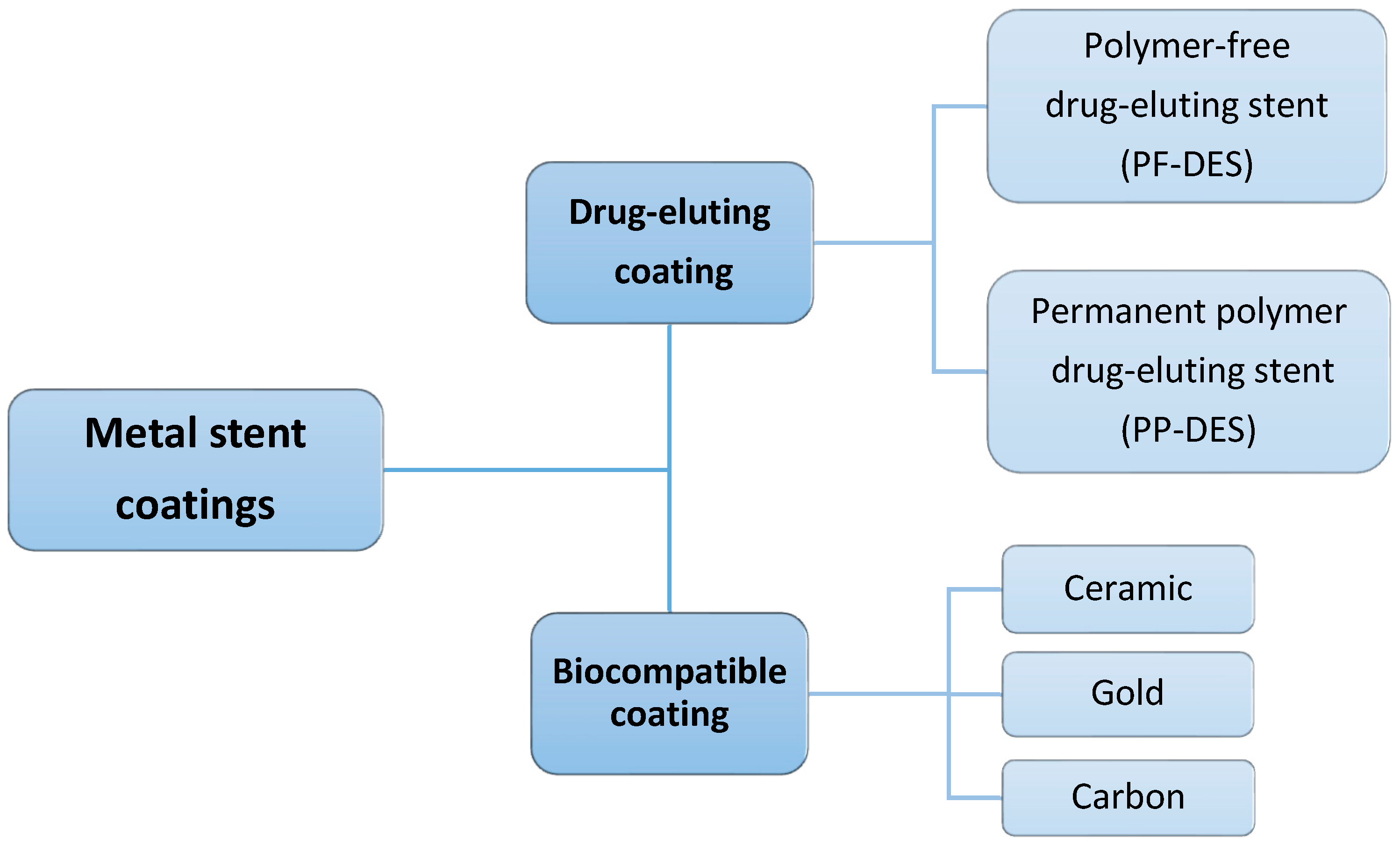
Coating
3.2. Novel Manufacturing
3.2.1. Weaving
Knitting
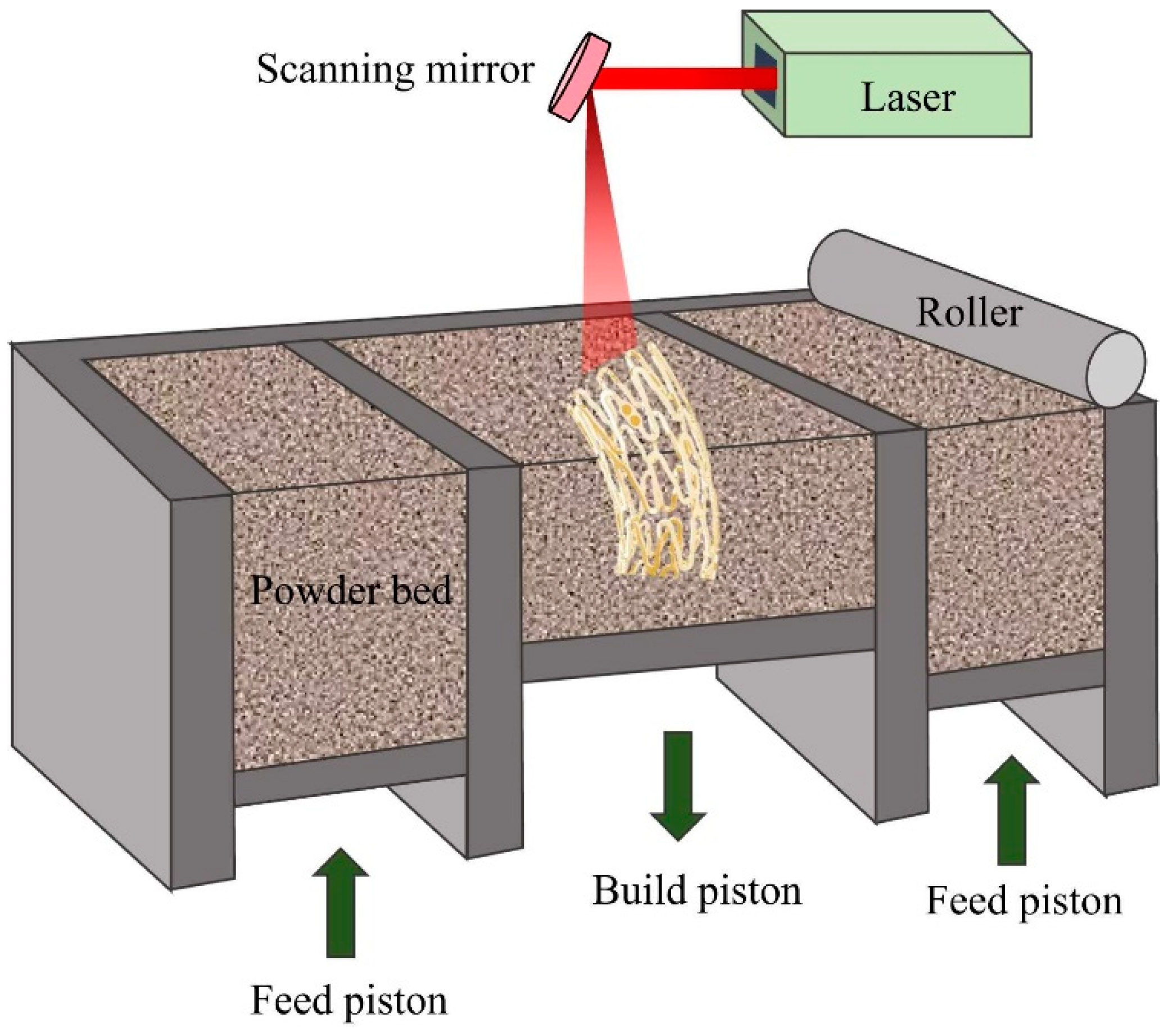
3.2.2. Additive Manufacturing
3.3. Post-Processing Techniques
3.3.1. Laser Snipping (Cutting)
3.3.2. Surface Modification
Polishing
3.3.3. Heat Treatment
4. Biomimetic Design
4.1. Stent Basic Design
4.1.1. Coiled Stents
4.1.2. Slotted Tube Stents
4.1.3. Tubular Mesh or Woven Stents
Fiber-Based (Fibrous) Stent
Braided Stent
Knitted Stent
4.1.4. Covered Stents
4.2. Design of Patient-Specific Stents
4.2.1. Stent Strut Design
4.2.2. Cell Design
Closed-Cell
Open-Cell
Helical Patterns
4.2.3. The Effect of Geometry on the Final Properties of Stents and Hemodynamic Factors
5. Characteristics
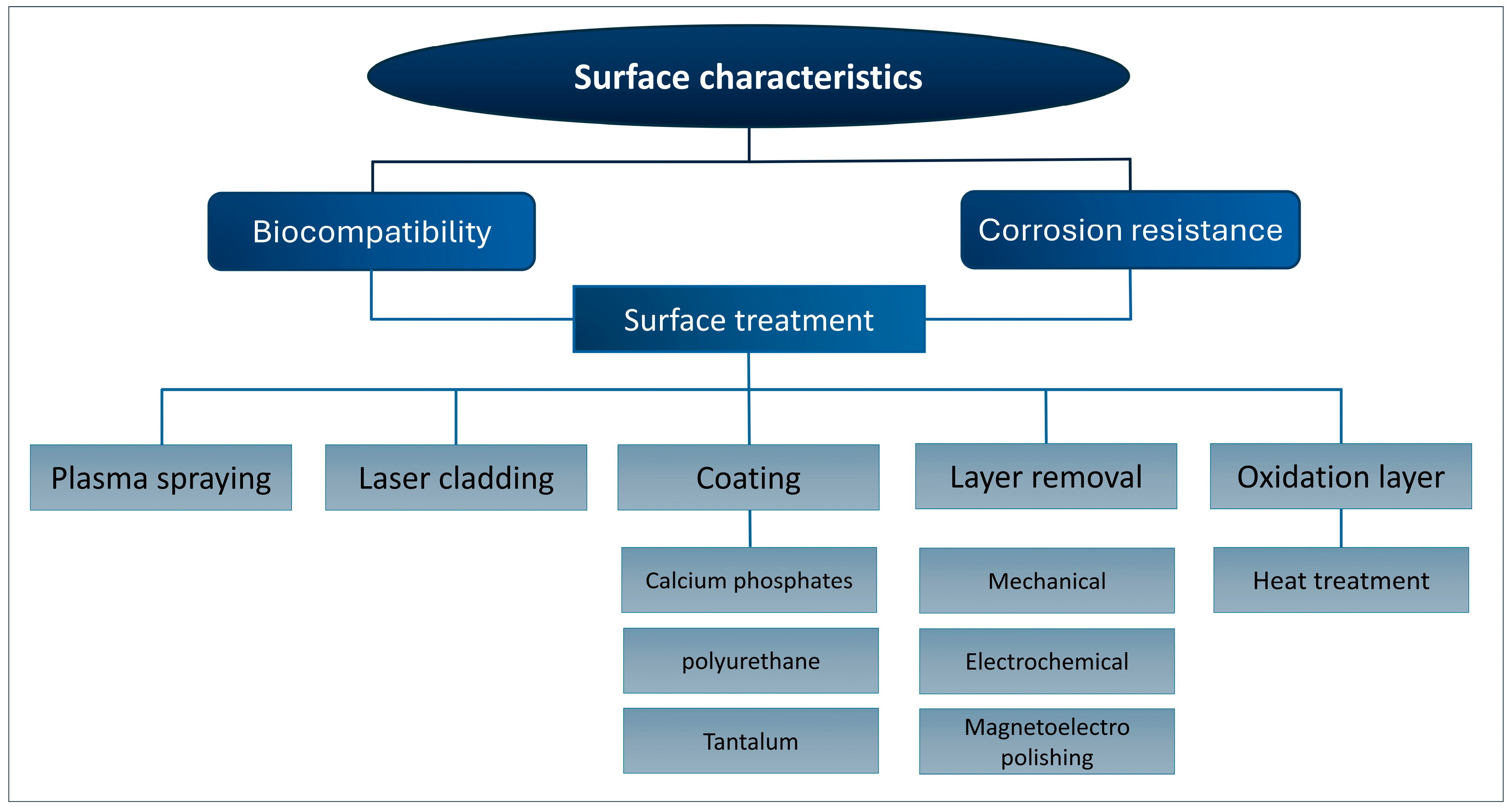
5.1. Surface Characteristics
5.2. Corrosion Properties
5.3. Biocompatibility
5.4. Wear Properties: Friction between Stent and Artery
5.5. Fatigue and Durability
5.6. Shape Memory and Superelasticity
6. Biocompatibility and Hemodynamics of Stents
7. Performance
7.1. Patency Rate
7.2. FDA Approvals
8. Modeling/Simulation Studies
8.1. Mathematical Modeling of Stents
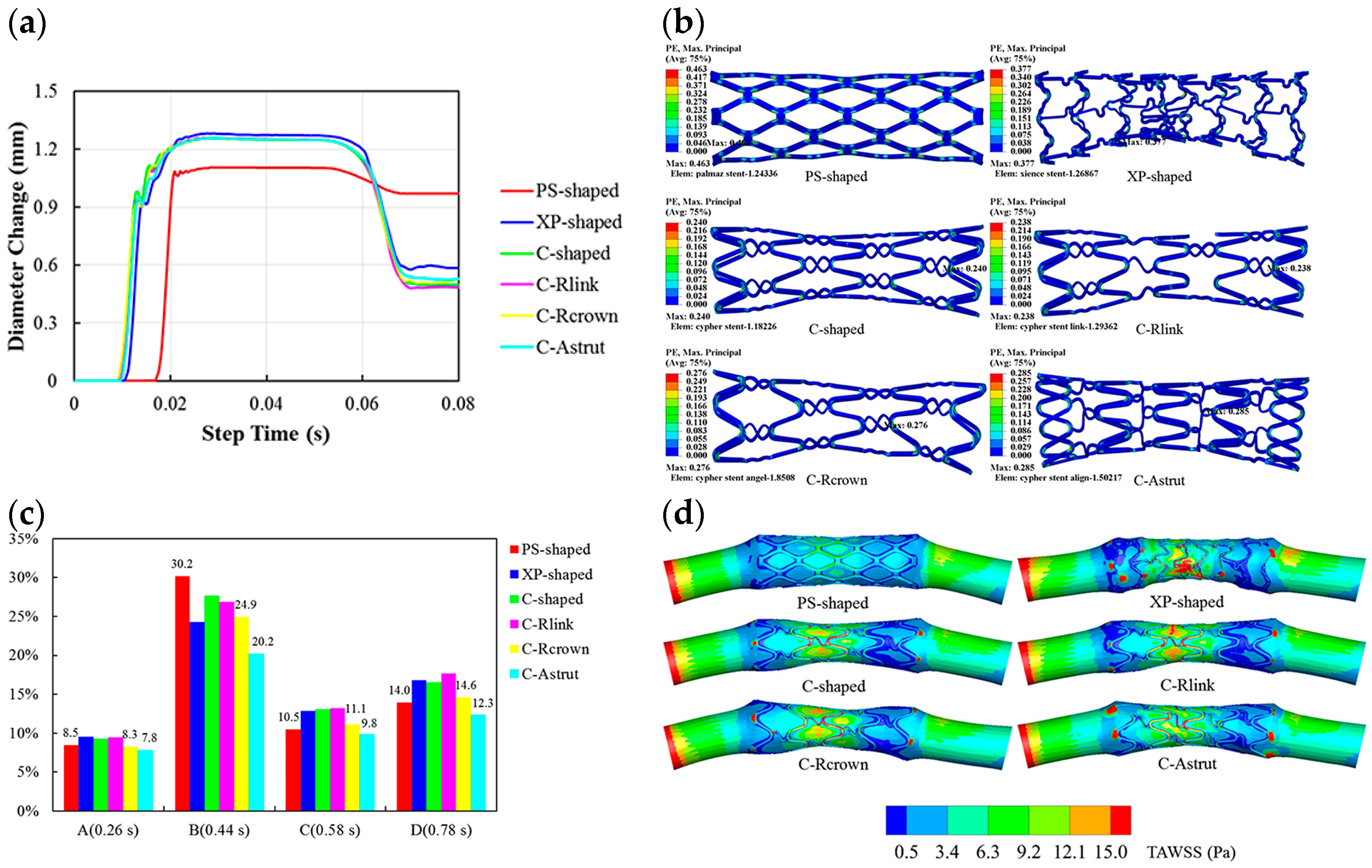
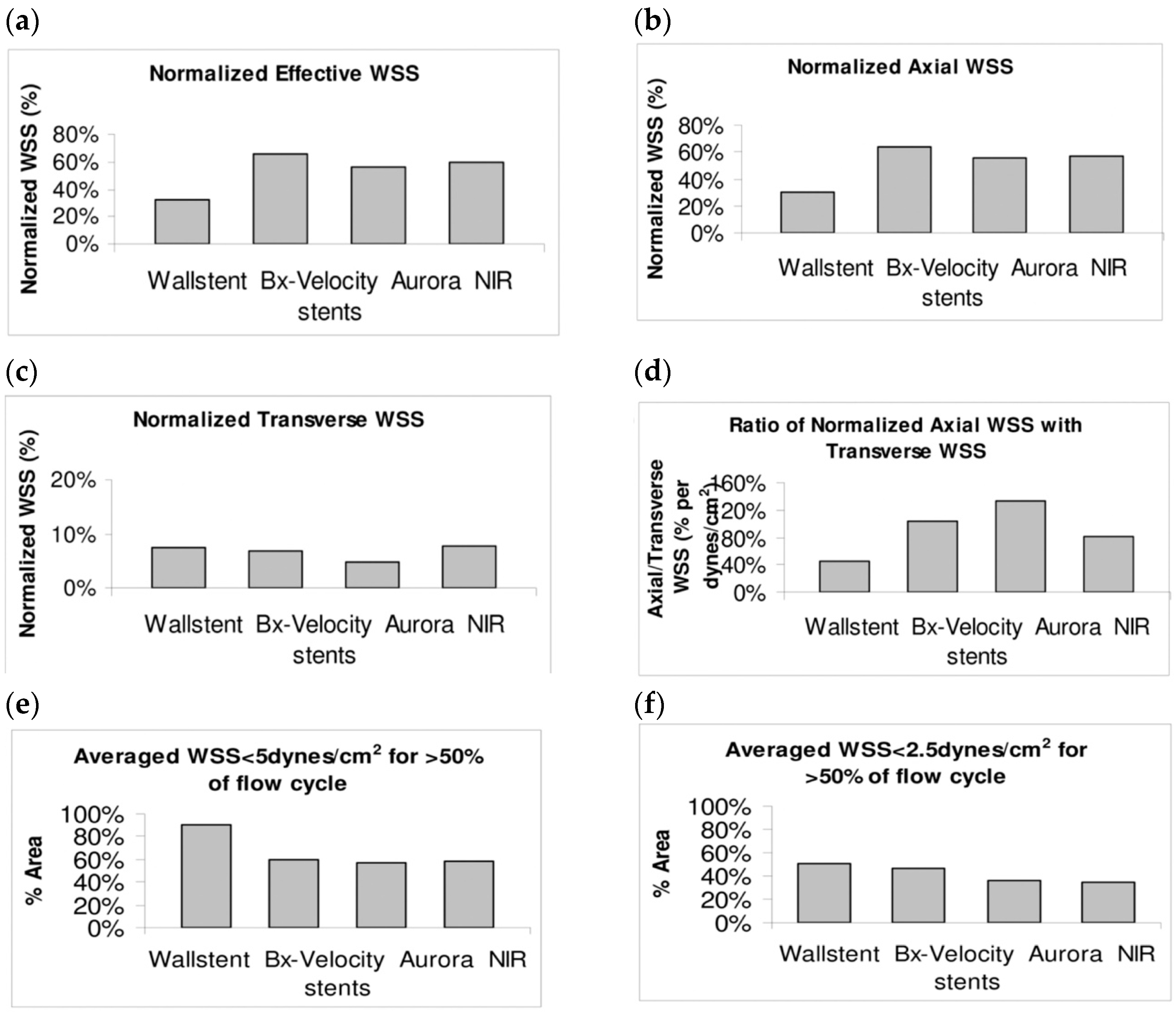
8.2. Simulated Stents Undergone Finite Element Modeling (FEM) and Computational Fluid Dynamic (CFD)
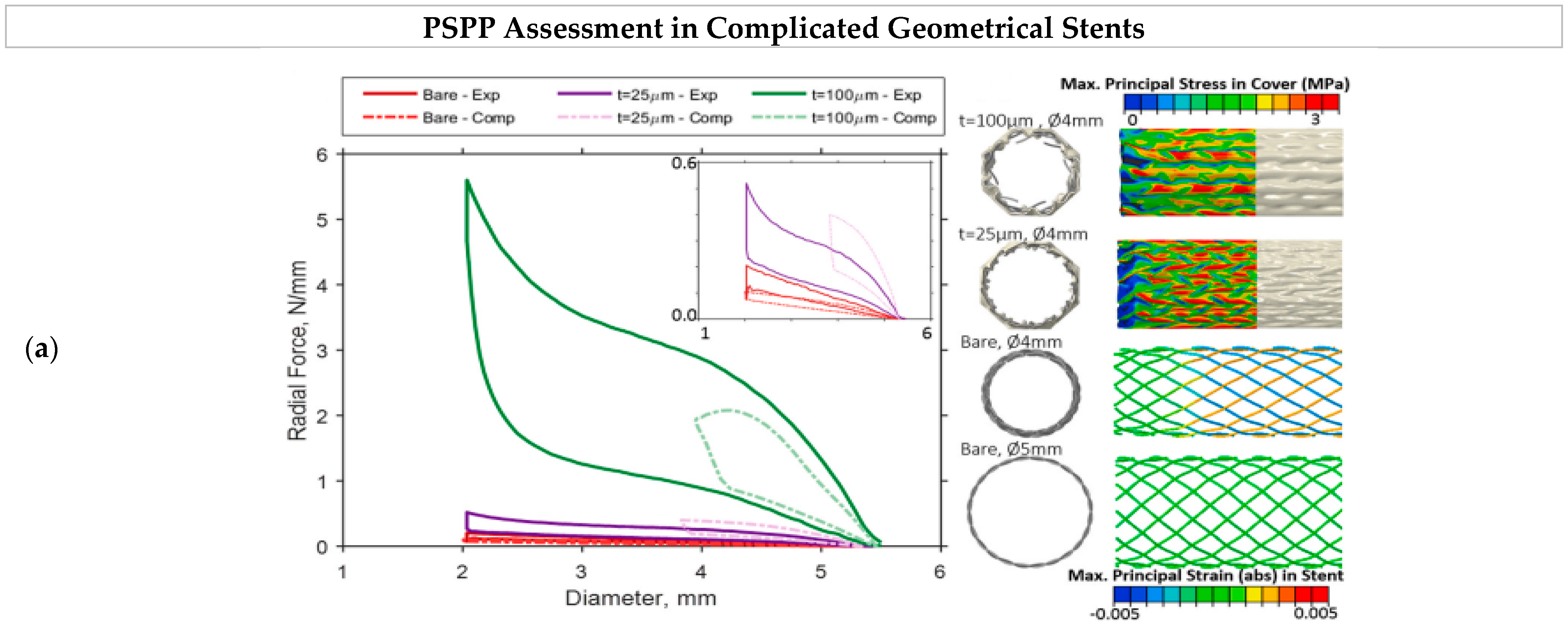
8.3. Stents’ Process–Structure–Properties–Performance
8.4. Machine Learning Complications in StentNet
9. Testing Methodologies for the Evaluation of Stents
9.1. In Vivo and In Vitro Testing Methodologies
9.2. Common In Vitro Tests
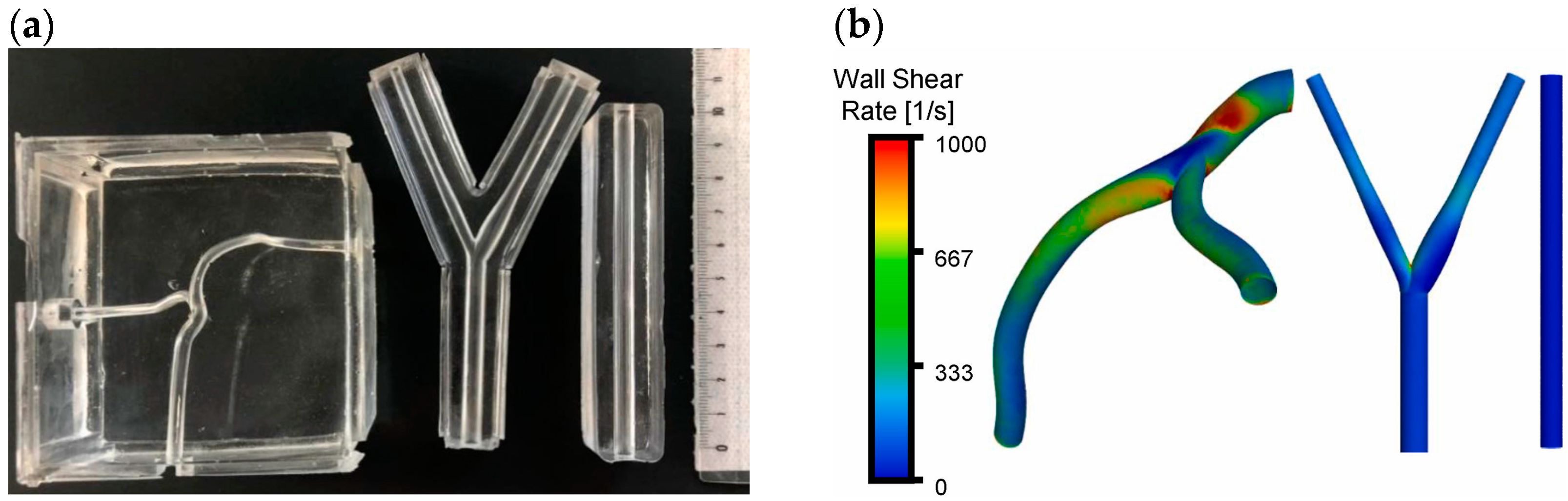
9.3. Hemodynamic Assessments
9.4. Testing for Thrombogenic Response
9.5. Endothelialization Evaluation
10. Challenges and Approaches
10.1. Manufacturing Challenges
10.2. Stent Failure
10.3. Microbial Biofilm Formation
11. Outlook and Perspectives
11.1. Current Statistics and Outlook
11.2. Market Data for Stents
12. Extended Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emre, A.; Sertkaya, M.; Akbulut, S.; Erbil, O.; Yurttutan, N.; Kale, İ.T.; Bülbüloğlu, E. Self-expandable metallic stent application for the management of upper gastrointestinal tract disease. Turk. J. Surg. 2018, 34, 101. [Google Scholar] [CrossRef] [PubMed]
- Dutau, H.; Musani, A.I.; Plojoux, J.; Laroumagne, S.; Astoul, P. The use of self-expandable metallic stents in the airways in the adult population. Expert Rev. Respir. Med. 2014, 8, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, F.; Azadi, M.; Biglari, M.; Bodaghi, M.; Khaleghian, A. Evaluation of coronary stents: A review of types, materials, processing techniques, design, and problems. Heliyon 2023, 9, e13575. [Google Scholar] [CrossRef]
- Cheung, D.Y.; Lee, Y.K.; Yang, C.H. Status and literature review of self-expandable metallic stents for malignant colorectal obstruction. Clin. Endosc. 2014, 47, 65–73. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, H.-Y.; Shin, J.H. Malignant gastric outlet obstructions: Treatment with self-expandable metallic stents. Gut Liver 2010, 4 (Suppl. S1), S32. [Google Scholar] [CrossRef]
- Lepsenyi, M.; Santen, S.; Syk, I.; Nielsen, J.; Nemeth, A.; Toth, E.; Thorlacius, H. Self-expanding metal stents in malignant colonic obstruction: Experiences from Sweden. BMC Res. Notes 2011, 4, 274. [Google Scholar] [CrossRef]
- Yoshida, S.; Isayama, H.; Koike, K. Palliative self-expandable metallic stent placement for colorectal obstruction caused by an extracolonic malignancy. Gastrointest. Interv. 2014, 3, 75–79. [Google Scholar] [CrossRef][Green Version]
- Atukorale, Y.N.; Church, J.L.; Hoggan, B.L.; Lambert, R.S.; Gurgacz, S.L.; Goodall, S.; Maddern, G.J. Self-expanding metallic stents for the management of emergency malignant large bowel obstruction: A systematic review. J. Gastrointest. Surg. 2016, 20, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.; Pinho, R.; Leite, M.; Proença, L.; Silva, J.; Ponte, A.; Rodrigues, J.; Maciel-Barbosa, J.; Carvalho, J. Reevaluation of self-expanding metal stents as a bridge to surgery for acute left-sided malignant colonic obstruction: Six years experience. GE Port. J. Gastroenterol. 2016, 23, 76–83. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Sun, P.; Yu, Q.; Wang, K.; Chang, W.; Song, Z.; Zheng, Q. Covered versus uncovered self-expandable metal stents for managing malignant distal biliary obstruction: A meta-analysis. PLoS ONE 2016, 11, e0149066. [Google Scholar] [CrossRef]
- Pan, Y.-M.; Pan, J.; Guo, L.-K.; Qiu, M.; Zhang, J.-J. Covered versus uncovered self-expandable metallic stents for palliation of malignant gastric outlet obstruction: A systematic review and meta-analysis. BMC Gastroenterol. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.-Y.; Li, J.Z.; Peng, W.P.; Zhang, M.M.; Lao, B.; Hong, J.M.; Li, L.B. Covered and uncovered self-expandable metallic stents in the treatment of malignant biliary obstruction. Iran. Red Crescent Med. J. 2020, 22, 7. [Google Scholar]
- Santos, M.P.C.; Palmela, C.; Ferreira, R.; Barjas, E.; Santos, A.A.; Maio, R.; Cravo, M. Self-expandable metal stents for colorectal cancer: From guidelines to clinical practice. GE Port. J. Gastroenterol. 2016, 23, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pu, C.; Fisher, R.K.; Mountain, D.J.; Gao, Y.; Liaw, P.K.; Zhang, W.; He, W. A Zr-based bulk metallic glass for future stent applications: Materials properties, finite element modeling, and in vitro human vascular cell response. Acta Biomater. 2015, 25, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Jafary-Zadeh, M.; Cui, F. Deployment of a bulk metallic glass-based self-expandable stent in a patient-specific descending aorta. ACS Biomater. Sci. Eng. 2016, 2, 1951–1958. [Google Scholar] [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Jung, Y.; Kim, D.-H.; Kim, S.H. Current status and future direction of metallic and polymeric materials for advanced vascular stents. Prog. Mater. Sci. 2022, 126, 100922. [Google Scholar] [CrossRef]
- Shishehbor, M.H.; Jaff, M.R. Percutaneous therapies for peripheral artery disease. Circulation 2016, 134, 2008–2027. [Google Scholar] [CrossRef]
- Payne, M.M. Charles Theodore Dotter: The father of intervention. Tex. Heart Inst. J. 2001, 28, 28. [Google Scholar]
- Wang, L.; Jiao, L.; Pang, S.; Yan, P.; Wang, X.; Qiu, T. The development of design and manufacture techniques for bioresorbable coronary artery stents. Micromachines 2021, 12, 990. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, J.H.; Yoon, C.J. History of self-expandable metal and self-expandable plastic stent development. Self-Expand. Stents Gastrointest. Tract. 2013, 35–49. [Google Scholar]
- Jiang, W.; Zhao, W.; Zhou, T.; Wang, L.; Qiu, T. A review on manufacturing and post-processing technology of vascular stents. Micromachines 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Luangsukrerk, T. Mechanical Property and Problems of the Self-expandable Metal Stent in Pancreaticobiliary Cancer. Korean Soc. Gastrointest. Cancer 2022, 10, 92–98. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, E.; Pereira, P.; Baron, T.-H.; Macedo, G. Self-expandable metal stents are a valid option in long-term survivors of advanced esophageal cancer. Rev. Española De Enfermedades Dig. 2018, 110, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pinto, E.; Pereira, P.; Lopes, S.; Ribeiro, A.; Moutinho-Ribeiro, P.; Peixoto, A.; Macedo, G. Outcome of endoscopic self-expandable metal stents in acute malignant colorectal obstruction at a tertiary center. Rev. Española De Enfermedades Dig. 2015, 107, 534–538. [Google Scholar] [CrossRef][Green Version]
- Mariette, C.; Gronnier, C.; Duhamel, A.; Mabrut, J.Y.; Bail, J.P.; Carrere, N.; Lefevre, J.H.; Meunier, B.; Collet, D.; Piessen, G.; et al. Self-expanding covered metallic stent as a bridge to surgery in esophageal cancer: Impact on oncologic outcomes. J. Am. Coll. Surg. 2015, 220, 287–296. [Google Scholar] [CrossRef]
- Yim, H.B. Self-expanding metallic stents and self-expanding plastic stents in the palliation of malignant oesophageal dysphagia. Ann Palliat Med 2014, 3, 41–46. [Google Scholar]
- Kang, Y. A review of self-expanding esophageal stents for the palliation therapy of inoperable esophageal malignancies. BioMed Res. Int. 2019, 2019, 9265017. [Google Scholar] [CrossRef] [PubMed]
- Self-Expanding Stents Market Size &Trends. Available online: https://www.grandviewresearch.com/industry-analysis/self-expanding-stents-market-report (accessed on 31 July 2024).
- Gardner, T.B.; Spangler, C.C.; Byanova, K.L.; Ripple, G.H.; Rockacy, M.J.; Levenick, J.M.; Smith, K.D.; Colacchio, T.A.; Barth, R.J.; Zaki, B.I.; et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest. Endosc. 2016, 84, 460–466. [Google Scholar] [CrossRef]
- Korei, N.; Solouk, A.; Nazarpak, M.H.; Nouri, A. A review on design characteristics and fabrication methods of metallic cardiovascular stents. Mater. Today Commun. 2022, 31, 103467. [Google Scholar] [CrossRef]
- Montaño-Machado, V.; Sikora-Jasinska, M.; Bortolan, C.C.; Chevallier, P.; Mantovani, D. Medical devices: Coronary stents. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; pp. 386–398. [Google Scholar]
- Kondoh, K.; Umeda, J.; Soba, R.; Tanabe, Y. Advanced TiNi shape memory alloy stents fabricated by a powder metallurgy route. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 583–590. [Google Scholar]
- Wiesent, L.; Spear, A.; Nonn, A. Computational analysis of the effects of geometric irregularities on the interaction of an additively manufactured 316L stainless steel stent and a coronary artery. J. Mech. Behav. Biomed. Mater. 2022, 125, 104878. [Google Scholar] [CrossRef]
- Tyagi, P.; Goulet, T.; Riso, C.; Stephenson, R.; Chuenprateep, N.; Schlitzer, J.; Benton, C.; Garcia-Moreno, F. Reducing the roughness of internal surface of an additive manufacturing produced 316 steel component by chempolishing and electropolishing. Addit. Manuf. 2019, 25, 32–38. [Google Scholar] [CrossRef]
- Langi, E.; Zhao, L.G.; Jamshidi, P.; Attallah, M.; Silberschmidt, V.V.; Willcock, H.; Vogt, F. A comparative study of microstructures and nanomechanical properties of additively manufactured and commercial metallic stents. Mater. Today Commun. 2022, 31, 103372. [Google Scholar] [CrossRef]
- Omar, M.A.; Baharudin, B.; Sulaiman, S.; Ismail, M.; Omar, M.A. Evaluation of chemical composition, heat treatment, mechanical properties and electro chemical polishing for additively manufactured stent using ASTM F75 cobalt based superalloy (CoCrMo) by selective laser melting (SLM) technology. Adv. Mater. Process. Technol. 2022, 8, 1635–1654. [Google Scholar] [CrossRef]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef]
- Syarif, J.; Pratesa, Y.; Prasetyo, Y.; Harjanto, S. Ball Milling Effect on Corrosion and Biocompatibility Behavior of FeMnC Alloys Produced by Powder Metallurgy in Simulated Body Fluids Environment. Metals 2021, 11, 1597. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef]
- Kandala, B.S.P.K.; Zhang, G.; LCorriveau, C.; Paquin, M.; Chagnon, M.; Begun, D.; Shanov, V. Preliminary study on modelling, fabrication by photo-chemical etching and in vivo testing of biodegradable magnesium AZ31 stents. Bioact. Mater. 2021, 6, 1663–1675. [Google Scholar] [CrossRef]
- Moeri, L.; Lichtenberg, M.; Gnanapiragasam, S.; Barco, S.; Sebastian, T. Braided or laser-cut self-expanding nitinol stents for the common femoral vein in patients with post-thrombotic syndrome. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 760–769. [Google Scholar] [CrossRef]
- Stoeckel, D.; Bonsignore, C.; Duda, S. A survey of stent designs. Minim. Invasive Ther. Allied Technol. 2002, 11, 137–147. [Google Scholar] [CrossRef]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-expanding Nitinol stents for the treatment of vascular disease. In Shape Memory Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2009; pp. 237–256. [Google Scholar]
- Luo, Y.; Jiang, Y.; Zhang, P.; Wang, X.; Ke, H.; Zhang, P. A novel ni-free Zr-based bulk metallic glass with high glass forming ability, corrosion resistance and thermal stability. Chin. J. Mech. Eng. 2020, 33, 1–7. [Google Scholar] [CrossRef]
- Nelson, D.B. Expandable metal stents: Physical properties and tissue responses. Tech. Gastrointest. Endosc. 2001, 3, 70–74. [Google Scholar] [CrossRef]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-expanding nitinol stents: Material and design considerations. Eur. Radiol. 2004, 14, 292–301. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Blencowe, A.; Albrecht, H.; Song, Y.; Garg, S. Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J. Control. Release 2019, 308, 209–231. [Google Scholar] [CrossRef]
- Škrlová, K.; Malachová, K.; Muñoz-Bonilla, A.; Měřinská, D.; Rybková, Z.; Fernández-García, M.; Plachá, D. Biocompatible polymer materials with antimicrobial properties for preparation of stents. Nanomaterials 2019, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Babapulle, M.N.; Eisenberg, M.J. Coated stents for the prevention of restenosis: Part I. Circulation 2002, 106, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, H.; Sawitowski, T.; Wnendt, S.; Fischer, A.; Dirsch, O.; Karoussos, I.A.; Erbel, R. Stent coating: A new approach in interventional cardiology. Herz 2002, 27, 518. [Google Scholar] [CrossRef]
- Yamao, K.; Kitano, M.; Chiba, Y.; Ogura, T.; Eguchi, T.; Moriyama, I.; Yamashita, Y.; Kato, H.; Kayahara, T.; Hoki, N. Endoscopic placement of covered versus uncovered self-expandable metal stents for palliation of malignant gastric outlet obstruction. Gut 2020, 70, 1244–1252. [Google Scholar] [CrossRef]
- Guerra, A.J.; Ciurana, J. Stent’s manufacturing field: Past, present, and future prospects. Angiography 2019, 73817, 41–60. [Google Scholar]
- Alam, S.T.; Ansari, A.; Urooj, S.; Aldobali, M. A review based on biodegradable and bioabsorbable stents for coronary artery disease. Procedia Comput. Sci. 2019, 152, 354–359. [Google Scholar] [CrossRef]
- Gori, T. Vascular wall reactions to coronary stents—Clinical implications for stent failure. Life 2021, 11, 63. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Zhao, Y.-N.; Zhang, Y.-Q.; Ming, T.; Li, C.-F. Equal channel angular extrusion of NiTi shape memory alloy tube. Trans. Nonferrous Met. Soc. China 2013, 23, 2021–2028. [Google Scholar] [CrossRef]
- Ge, Q.; Dellasega, D.; Demir, A.G.; Vedani, M. The processing of ultrafine-grained Mg tubes for biodegradable stents. Acta Biomater. 2013, 9, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Gansesan, S.; Karthikeyan, S. An overview on the influence of equal channel angular pressing parameters and its effect on materials: Methods and applications. Adv. Mater. Process. Technol. 2024, 10, 1814–1855. [Google Scholar] [CrossRef]
- Wang, L.; Fang, G.; Qian, L.; Leeflang, S.; Duszczyk, J.; Zhou, J. Forming of magnesium alloy microtubes in the fabrication of biodegradable stents. Prog. Nat. Sci. Mater. Int. 2014, 24, 500–506. [Google Scholar] [CrossRef]
- Guo, K.; Liu, M.; Wang, J.; Sun, Y.F.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Microstructure and texture evolution of fine-grained Mg-Zn-Y-Nd alloy micro-tubes for biodegradable vascular stents processed by hot extrusion and rapid cooling. J. Magnes. Alloys 2020, 8, 873–882. [Google Scholar] [CrossRef]
- Yoshida, K.; Furuya, H. Mandrel drawing and plug drawing of shape-memory-alloy fine tubes used in catheters and stents. J. Mater. Process. Technol. 2004, 153, 145–150. [Google Scholar] [CrossRef]
- Moravej, M.; Prima, F.; Fiset, M.; Mantovani, D. Electroformed iron as new biomaterial for degradable stents: Development process and structure–properties relationship. Acta Biomater. 2010, 6, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Purnama, A.; Mostavan, A.; Paternoster, C.; Mantovani, D. Electroforming as a new method for fabricating degradable pure iron stent. In Advances in Metallic Biomaterials: Processing and Applications; Springer: Cham, Swizterland, 2015; pp. 85–100. [Google Scholar]
- Kandala, B.S.P.K.; Zhang, G.; Hopkins, T.M.; An, X.; Pixley, S.K.; Shanov, V. In vitro and in vivo testing of zinc as a biodegradable material for stents fabricated by photo-chemical etching. Appl. Sci. 2019, 9, 4503. [Google Scholar] [CrossRef]
- Pujiyulianto, E.; Amalia, Y.; Wahyuningsih, T.; Field, F.Y.; Mirahati, R.Z.; Suyitno, S. The effect of EDM process on the microstructure of CP-titanium grade 2 and AISI 316 L in cardiovascular stent manufacturing. Key Eng. Mater. 2020, 867, 1–7. [Google Scholar] [CrossRef]
- Takahata, K. Micro-electro-discharge machining technologies for MEMS. Micro Electron. Mech. Syst. 2009, 386, 143. [Google Scholar]
- Pang, S.; Zhao, W.; Qiu, T.; Liu, W.; Jiao, L.; Wang, X. Study on surface quality and mechanical properties of micro-milling WE43 magnesium alloy cardiovascular stent. J. Manuf. Process. 2023, 101, 1080–1090. [Google Scholar] [CrossRef]
- Rosli, A.M.; Jamaludin, A.S.; Razali, M.N.M. Recent study on hard to machine material–micromilling process. EVERGREEN Jt. J. Nov. Carbon Resour. Sci. Green Asia Strategy 2021, 8, 445–453. [Google Scholar] [CrossRef]
- Yamagata, W.; Fujisawa, T.; Sasaki, T.; Ishibashi, R.; Saito, T.; Yoshida, S.; No, S.; Inoue, K.; Nakai, Y.; Sasahira, N.; et al. Evaluation of the mechanical properties of current biliary self-expandable metallic stents: Axial and radial force, and axial force zero border. Clin. Endosc. 2023, 56, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.G.; Previtali, B. Lasers in the manufacturing of cardiovascular metallic stents: Subtractive and additive processes with a digital tool. Procedia Comput. Sci. 2023, 217, 604–613. [Google Scholar] [CrossRef]
- Heo, Y.-C.; Han, D.-K.; Kim, M.T. Therapeutic effect of local photothermal heating of gold nanoparticle-coated self-expandable metallic stents for suppressing granulation tissue formation in the mouse colon. PLoS ONE 2021, 16, e0249530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, X.; Wang, Y.; Li, C.; Zhao, Y.; Li, Z.; Chen, J.; Li, J.; Ren, K.; et al. Comparison of three kinds of self-expandable metallic stents induced granulation tissue hyperplasia in the rabbit trachea. Sci. Rep. 2021, 11, 23115. [Google Scholar] [CrossRef]
- Kang, J.M.; Oh, D.; Kim, S.H.; Ryu, D.S.; Park, Y.; Won, D.S.; Kim, J.W.; Zeng, C.H.; Park, J.H.; Lee, S.S. Interwoven versus knitted self-expandable metallic stents: A comparison study of stent-induced tissue hyperplasia in the rat esophagus. Appl. Sci. 2021, 12, 346. [Google Scholar] [CrossRef]
- Aibibu, D.; Hild, M.; Cherif, C. An overview of braiding structure in medical textile: Fiber-based implants and tissue engineering. In Advances in Braiding Technology; Springer: Cham, Swizterland, 2016; pp. 171–190. [Google Scholar]
- KUeng, C.; Wen, S.-P.; Lou, C.-W.; Lin, J.-H. Stainless steel/nitinol braid coronary stents: Braiding structure stability and cut section treatment evaluation. J. Ind. Text. 2016, 45, 965–977. [Google Scholar]
- Garcia-Villen, F.; López-Zárraga, F.; Viseras, C.; Ruiz-Alonso, S.; Al-Hakim, F.; Diez-Aldama, I.; Saenz-del-Burgo, L.; Scaini, D.; Pedraz, J.L. 219Three-dimensional printing as a cutting-edge, versatile and personalizable vascular stent manufacturing procedure: Toward tailor-made medical devices. Int. J. Bioprinting 2023, 9, 2. [Google Scholar] [CrossRef]
- Chen, K.; Wan, H.; Fang, X.; Chen, H. Laser Additive Manufacturing of Anti-Tetrachiral Endovascular Stents with Negative Poisson’s Ratio and Favorable Cytocompatibility. Micromachines 2022, 13, 1135. [Google Scholar] [CrossRef]
- Gaines, P. Biomimetic stents and the benefits of swirling flow. Endovasc. Today 2015, 6, 64–68. [Google Scholar]
- Langi, E.; Bisht, A.; Silberschmidt, V.V.; Ruiz, P.D.; Vogt, F.; Mailto, L.; Masseling, L.; Zhao, L. Characterisation of additively manufactured metallic stents. Procedia Struct. Integr. 2019, 15, 41–45. [Google Scholar] [CrossRef]
- McGee, O.M.; Geraghty, S.; Hughes, C.; Jamshidi, P.; Kenny, D.P.; Attallah, M.M.; Lally, C. An investigation into patient-specific 3D printed titanium stents and the use of etching to overcome Selective Laser Melting design constraints. J. Mech. Behav. Biomed. Mater. 2022, 134, 105388. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Bordbar-Khiabani, A.; Khalil-Allafi, J.; Mozafari, M.; Visai, L. Additive manufacturing: An opportunity for the fabrication of near-net-shape NiTi implants. J. Manuf. Mater. Process. 2022, 6, 65. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Kamariah, M.S.I.N.; Muhamad, N.; Ghani SA, C.; Ahmad, F.; Mohamed, Z. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Elsisy, M.; Shayan, M.; Chen, Y.; Tillman, B.W.; Go, C.; Chun, Y. Assessment of mechanical and biocompatible performance of ultra-large nitinol endovascular devices fabricated via a low-energy laser joining process. J. Biomater. Appl. 2021, 36, 332–345. [Google Scholar] [CrossRef]
- Tareq, S.; Rahman, T.; Poudel, B.; Chung, H.; Kwon, P. Heat treatment protocol for additively manufactured nitinol shape memory alloys in biomedical applications. Mater. Sci. Eng. A 2024, 897, 146274. [Google Scholar] [CrossRef]
- Butany, J.; Carmichael, K.; Leong, S.; Collins, M. Coronary artery stents: Identification and evaluation. J. Clin. Pathol. 2005, 58, 795. [Google Scholar] [CrossRef]
- Schiavone, A.; Zhao, L.; Abdel-Wahab, A.A. Effects of material, coating, design and plaque composition on stent deployment inside a stenotic artery—Finite element simulation. Mater. Sci. Eng. C 2014, 42, 479–488. [Google Scholar] [CrossRef]
- Freitas, A.; de Araujo, M.; Zu, W.; Fangueiro, R. Development of weft-knitted and braided polypropylene stents for arterial implant. J. Text. Inst. 2010, 101, 1027–1034. [Google Scholar] [CrossRef]
- Liu, M.; Tian, Y.; Cheng, J.; Zhang, Y.; Zhao, G.; Ni, Z. Mixed-braided stent: An effective way to improve comprehensive mechanical properties of poly (L-lactic acid) self-expandable braided stent. J. Mech. Behav. Biomed. Mater. 2022, 128, 105123. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.C.; Rodrigues, A.; Figueiredo, L.; Rocha, L.A.; Tavares, J.M.R. Mechanical properties of stent–graft materials. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2012, 226, 330–341. [Google Scholar] [CrossRef]
- Singh, C.; Wang, X. A biomechanically optimized knitted stent using a bio-inspired design approach. Text. Res. J. 2016, 86, 380–392. [Google Scholar] [CrossRef]
- Lin, M.-C.; Lou, C.-W.; Lin, J.-Y.; Lin, T.A.; Chen, Y.-S.; Lin, J.-H. Biodegradable polyvinyl alcohol vascular stents: Structural model and mechanical and biological property evaluation. Mater. Sci. Eng. C 2018, 91, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Reijm, A.N.; Didden, P.; Schelling, S.J.; Siersema, P.D.; Bruno, M.J.; Spaander, M.C. Self-expandable metal stent placement for malignant esophageal strictures–changes in clinical outcomes over time. Endoscopy 2019, 51, 18–29. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural design of vascular stents: A review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Chang, F.-C.; Luo, C.-B.; Guo, W.-Y. Influence of stenting with open-cell stents vs. close-cell stents on the outcomes of patients with bilateral carotid stenosis. J. Chin. Med. Assoc. 2019, 82, 66–71. [Google Scholar] [CrossRef]
- Koźlik, M.; Harpula, J.; Chuchra, P.J.; Nowak, M.; Wojakowski, W.; Gąsior, P. Drug-Eluting Stents: Technical and Clinical Progress. Biomimetics 2023, 8, 72. [Google Scholar] [CrossRef]
- Kim, C.G.; Choi, I.J.; Lee, J.Y.; Cho, S.J.; Park, S.R.; Lee, J.H.; Ryu, K.W.; Kim, Y.W.; Park, Y.I. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: A randomized, prospective study. Gastrointest. Endosc. 2010, 72, 25–32. [Google Scholar] [CrossRef]
- Oldenburg, J.; Borowski, F.; Schmitz, K.P.; Stiehm, M.; Öner, A.Ö.; Quirin, L.; John, K.; Bruschewski, M.; Grundmann, S. MRV-validated numerical flow analysis of thrombotic potential of coronary stent designs. Curr. Dir. Biomed. Eng. 2019, 5, 77–80. [Google Scholar] [CrossRef]
- Valentim, M.X.G.; Zinani, F.S.F.; da Fonseca, C.E.; Wermuth, D.P. Systematic review on the application of computational fluid dynamics as a tool for the design of coronary artery stents. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 1–8. [Google Scholar] [CrossRef]
- Iannaccone, M.; Gatti, P.; Barbero, U.; Bassignana, A.; Gallo, D.; de Benedictis, M.; Helft, G.; Morbiducci, U.; Doronzo, B.; D’Ascenzo, F. Impact of strut thickness and number of crown and connectors on clinical outcomes on patients treated with second-generation drug eluting stent. Catheter. Cardiovasc. Interv. 2020, 96, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Condello, F.; Spaccarotella, C.; Sorrentino, S.; Indolfi, C.; Stefanini, G.G.; Polimeni, A. Stent thrombosis and restenosis with contemporary drug-eluting stents: Predictors and current evidence. J. Clin. Med. 2023, 12, 1238. [Google Scholar] [CrossRef]
- Khalil-Allafi, J.; Amin-Ahmadi, B.; Zare, M. Biocompatibility and corrosion behavior of the shape memory NiTi alloy in the physiological environments simulated with body fluids for medical applications. Mater. Sci. Eng. C 2010, 30, 1112–1117. [Google Scholar] [CrossRef]
- MElahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Putters, J.; Sukul, D.K.; De Zeeuw, G.; Bijma, A.; Besselink, P. Comparative cell culture effects of shape memory metal (Nitinol®), nickel and titanium: A biocompatibility estimation. Eur. Surg. Res. 1992, 24, 378–382. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Zhao, G.; Gao, Y.; Liu, G.; Wang, K. Study on magnetic abrasive finishing of the inner surface of Ni–Ti alloy cardiovascular stents tube. Int. J. Adv. Manuf. Technol. 2022, 118, 2299–2309. [Google Scholar] [CrossRef]
- Finazzi, V.; Demir, A.G.; Biffi, C.A.; Chiastra, C.; Migliavacca, F.; Petrini, L.; Previtali, B. Design rules for producing cardiovascular stents by selective laser melting: Geometrical constraints and opportunities. Procedia Struct. Integr. 2019, 15, 16–23. [Google Scholar] [CrossRef]
- Puneet, G.; Musaramthota, V.; Munroe, N.; Datye, A.; Dua, R.; Haider, W.; McGoron, A.; Rokicki, R. Surface modification of Ni–Ti alloys for stent application after magnetoelectropolishing. Mater. Sci. Eng. C 2015, 50, 37–44. [Google Scholar]
- Aguilar, L.E.; Tumurbaatar, B.; Ghavaminejad, A.; Park, C.H.; Kim, C.S. Functionalized non-vascular nitinol stent via electropolymerized polydopamine thin film coating loaded with bortezomib adjunct to hyperthermia therapy. Sci. Rep. 2017, 7, 9432. [Google Scholar] [CrossRef]
- Jamshidi, P.; Panwisawas, C.; Langi, E.; Cox, S.C.; Feng, J.; Zhao, L.; Attallah, M.M. Development, characterisation, and modelling of processability of nitinol stents using laser powder bed fusion. J. Alloys Compd. 2022, 909, 164681. [Google Scholar] [CrossRef]
- Tepe, G.; Schmehl, J.; Wendel, H.P.; Schaffner, S.; Heller, S.; Gianotti, M.; Claussen, C.D.; Duda, S.H. Reduced thrombogenicity of nitinol stents—in vitro evaluation of different surface modifications and coatings. Biomaterials 2006, 27, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, C.; Yang, Z.; Zhang, D. A study on strengthening the corrosion resistance of NiTi SMA by composite femtosecond and nanosecond laser-induced hierarchical micro/nanostructures. Opt. Laser Technol. 2023, 163, 109339. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Hu, Z. Effect of Ni addition on the corrosion resistance of NiTi alloy coatings on AISI 316L substrate prepared by laser cladding. Coatings 2021, 11, 1139. [Google Scholar] [CrossRef]
- Trepanier, C.; Tabrizian, M.; Yahia, L.H.; Bilodeau, L.; Piron, D.L. Effect of modification of oxide layer on NiTi stent corrosion resistance. J. Biomed. Mater. Res. 1998, 43, 433–440. [Google Scholar] [CrossRef]
- Andani, M.T.; Saedi, S.; Turabi, A.S.; Karamooz, M.R.; Haberland, C.; Karaca, H.E.; Elahinia, M. Mechanical and shape memory properties of porous Ni50. 1Ti49. 9 alloys manufactured by selective laser melting. J. Mech. Behav. Biomed. Mater. 2017, 68, 224–231. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T.; Pulletikurthi, C.; Rokosz, K.; Munroe, N. Towards a better corrosion resistance and biocompatibility improvement of nitinol medical devices. J. Mater. Eng. Perform. 2015, 24, 1634–1640. [Google Scholar] [CrossRef]
- Bae, I.; Kim, B.-H.; Kim, D.-G.; Sohn, I.-B.; Yang, S.-W. Salt heat treatment and passivation to improve the corrosion resistance of Nitinol (Ni-Ti). Materials 2021, 14, 7789. [Google Scholar] [CrossRef] [PubMed]
- Say, Y.; Aksakal, B. Enhanced corrosion properties of biological NiTi alloy by hydroxyapatite and bioglass based biocomposite coatings. J. Mater. Res. Technol. 2020, 9, 1742–1749. [Google Scholar] [CrossRef]
- Mazumder, M.M.; De, S.; Trigwell, S.; Ali, N.; Mazumder, M.K.; Mehta, J.L. Corrosion resistance of polyurethane-coated nitinol cardiovascular stents. J. Biomater. Sci. Polym. Ed. 2003, 14, 1351–1362. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Lu, Y.; Li, X.; Zhou, J.; Zadpoor, A.A.; Wang, L. Additive manufacturing of vascular stents. Acta Biomaterialia 2023, 167, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Köster, R.; Vieluf, D.; Kiehn, M.; Sommerauer, M.; Kähler, J.; Baldus, S.; Meinertz, T.; Hamm, C.W. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 2000, 356, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Chen, M.; Zhang, L.; Hu, R.; Zhu, S.; Yang, X. Improving the biocompatibility of NiTi alloy by chemical treatments: An in vitro evaluation in 3T3 human fibroblast cell. Mater. Sci. Eng. C 2008, 28, 1117–1122. [Google Scholar] [CrossRef]
- Rodrigues, O.R.; Minamoto, H.; Canzian, M.; Correia, A.T.; Jatene, F.B. Biocompatibility of a new device of self-expandable covered and non-covered tracheal stent: Comparative study in rats. Acta Cir. Bras. 2013, 28, 10–18. [Google Scholar] [CrossRef][Green Version]
- Park, C.; Kim, S.; Kim, H.-E.; Jang, T.-S. Mechanically stable tantalum coating on a nano-roughened NiTi stent for enhanced radiopacity and biocompatibility. Surf. Coat. Technol. 2016, 305, 139–145. [Google Scholar] [CrossRef]
- Ohtsu, N.; Suginishi, S.; Hirano, M. Antibacterial effect of nickel-titanium alloy owing to nickel ion release. Appl. Surf. Sci. 2017, 405, 215–219. [Google Scholar] [CrossRef]
- Safavi, M.S.; Khalil-Allafi, J.; Restivo, E.; Ghalandarzadeh, A.; Hosseini, M.; Dacarro, G.; Malavasi, L.; Milella, A.; Listorti, A.; Visai, L. Enhanced in vitro immersion behavior and antibacterial activity of NiTi orthopedic biomaterial by HAp-Nb2O5 composite deposits. Sci. Rep. 2023, 13, 16045. [Google Scholar] [CrossRef]
- Ahmed, R.A.; Fadl-allah, S.A.; El-Bagoury, N.; El-Rab, S.M.G. Improvement of corrosion resistance and antibacterial effect of NiTi orthopedic materials by chitosan and gold nanoparticles. Appl. Surf. Sci. 2014, 292, 390–399. [Google Scholar] [CrossRef]
- Kadhim, S.E.; Al-rawi, B.K.; Khalaf, M.K. Antibacterial activity of NiTi alloy with sputtered tantalum. J. Univ. Anbar Pure Sci. 2023, 17, 210–224. [Google Scholar]
- Tanœ, L.; Bauer, J.; Croneœ, W.C.; Albrechtœ, R. Biocompatibility improvement of NiTi with a functionally graded surface in Society for Experimental Mechanics. In Proceedings of the 2002 SEM Annual Conference Proceedings, Milwaukee, WI, USA, 10–12 June 2002. [Google Scholar]
- Vad, S.; Eskinazi, A.; Corbett, T.; McGloughlin, T.; Geest, J.P.V. Determination of coefficient of friction for self-expanding stent-grafts. J. Biomech. Eng. 2010, 132, 121007. [Google Scholar] [CrossRef]
- Popoola, O.; Moine, P.; Villain, J. Wear resistance of NiTi alloys after implantation of light interstitial species. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1987, 26, 532–538. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Pelton, A.; Schroeder, V.; Mitchell, M.; Gong, X.-Y.; Barney, M.; Robertson, S. Fatigue and durability of Nitinol stents. J. Mech. Behav. Biomed. Mater. 2008, 1, 153–164. [Google Scholar] [CrossRef]
- Schuessler, A.; Salgues, J.; Strobel, M.; Siekmeyer, G. Effect of surface quality and microstructure on fatigue behavior of nitinol stent components. In Proceedings of the International Conference on Shape Memory and Superelastic Technologies, Pacific Grove, CA, USA, 7–11 May December 2006. [Google Scholar]
- Smouse, H.B.; Nikanorov, A.; LaFlash, D. Biomechanical forces in the femoropopliteal arterial segment. Endovasc Today 2005, 4, 60–66. [Google Scholar]
- Stankiewicz, J.; Robertson, S.; Ritchie, R. Fatigue-crack growth properties of thin-walled superelastic austenitic Nitinol tube for endovascular stents. J. Biomed. Mater. Res. Part A 2007, 81, 685–691. [Google Scholar] [CrossRef]
- Harvey, S.M. Nitinol stent fatigue in a peripheral human artery subjected to pulsatile and articulation loading. J. Mater. Eng. Perform. 2011, 20, 697–705. [Google Scholar] [CrossRef]
- Finazzi, V.; Berti, F.; Petrini, L.; Previtali, B.; Demir, A.G. Additive manufacturing and post-processing of superelastic NiTi micro struts as building blocks for cardiovascular stents. Addit. Manuf. 2023, 70, 103561. [Google Scholar] [CrossRef]
- Guo, Y.; Klink, A.; Fu, C.; Snyder, J. Machinability and surface integrity of Nitinol shape memory alloy. CIRP Ann. 2013, 62, 83–86. [Google Scholar] [CrossRef]
- Liu, H. Improving the hemocompatibility of stents. In Hemocompatibility of Biomaterials for Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–394. [Google Scholar]
- Mitra, A.; Agrawal, D.K. In stent restenosis: Bane of the stent era. J. Clin. Pathol. 2006, 59, 232. [Google Scholar] [CrossRef]
- Huang, N.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wang, J.; Wang, G.J.; Ding, P.D.; Xi, T.F.; Leng, Y. Hemocompatibility of titanium oxide films. Biomaterials 2003, 24, 2177–2187. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Nammas, W. Titanium-nitride-oxide-coated coronary stents: Insights from the available evidence. Ann. Med. 2017, 49, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pan, C.; Zhou, S.; Li, J.; Huang, N.; Dong, L. Improving hemocompatibility and accelerating endothelialization of vascular stents by a copper-titanium film. Mater. Sci. Eng. C 2016, 69, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Barras, C.; Myers, K. Nitinol–its use in vascular surgery and other applications. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 564–569. [Google Scholar] [CrossRef]
- Chakraborty, R.; Datta, S.; Raza, M.S.; Saha, P. A comparative study of surface characterization and corrosion performance properties of laser surface modified biomedical grade nitinol. Appl. Surf. Sci. 2019, 469, 753–763. [Google Scholar] [CrossRef]
- Maleckis, K.; Anttila, E.; Aylward, P.; Poulson, W.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Nitinol stents in the femoropopliteal artery: A mechanical perspective on material, design, and performance. Ann. Biomed. Eng. 2018, 46, 684–704. [Google Scholar] [CrossRef]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef]
- Saqib, M.; Beshchasna, N.; Pelaccia, R.; Roshchupkin, A.; Yanko, I.; Husak, Y.; Kyrylenko, S.; Reggiani, B.; Cuniberti, G.; Pogorielov, M.; et al. Tailoring surface properties, biocompatibility and corrosion behavior of stainless steel by laser induced periodic surface treatment towards developing biomimetic stents. Surf. Interfaces 2022, 34, 102365. [Google Scholar] [CrossRef]
- Grossmann, S.; Bohne, E.; Stiehm, M.; Schmitz, K.-P.; Siewert, S. Method for fabrication of laser-induced periodic surface structures on vascular stents. Curr. Dir. Biomed. Eng. 2023, 9, 627–629. [Google Scholar] [CrossRef]
- Dong, J.; Pacella, M.; Liu, Y.; Zhao, L. Surface engineering and the application of laser-based processes to stents-A review of the latest development. Bioact. Mater. 2022, 10, 159–184. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Davies, P.F. Hemodynamically driven stent strut design. Ann. Biomed. Eng. 2009, 37, 1483–1494. [Google Scholar] [CrossRef]
- Jiménez, J.M.; Prasad, V.; Yu, M.D.; Kampmeyer, C.P.; Kaakour, A.H.; Wang, P.J.; Maloney, S.F.; Wright, N.; Johnston, I.; Jiang, Y.Z.; et al. Macro-and microscale variables regulate stent haemodynamics, fibrin deposition and thrombomodulin expression. J. R. Soc. Interface 2014, 11, 20131079. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Smith, A.F.; Jiménez, J.M. Stent strut streamlining and thickness reduction promote endothelialization. J. R. Soc. Interface 2021, 18, 20210023. [Google Scholar] [CrossRef] [PubMed]
- Sprague, E.A.; Luo, J.; Palmaz, J.C. Human aortic endothelial cell migration onto stent surfaces under static and flow conditions. J. Vasc. Interv. Radiol. 1997, 8, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.P.; Giannopoulos, A.A.; Wentzel, J.J.; Joner, M.; Giannoglou, G.D.; Virmani, R.; Chatzizisis, Y.S. Impact of local flow haemodynamics on atherosclerosis in coronary artery bifurcations. EuroIntervention 2015, 11 (Suppl. V), V18–V22. [Google Scholar] [CrossRef]
- Casa, L.D.; Deaton, D.H.; Ku, D.N. Role of high shear rate in thrombosis. J. Vasc. Surg. 2015, 61, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Casa, L.D.; Ku, D.N. Thrombus formation at high shear rates. Annu. Rev. Biomed. Eng. 2017, 19, 415–433. [Google Scholar] [CrossRef]
- Berry, J.L.; Manoach, E.; Mekkaoui, C.; Rolland, P.H.; Moore, J.E., Jr.; Rachev, A. Hemodynamics and wall mechanics of a compliance matching stent: In vitro and in vivo analysis. J. Vasc. Interv. Radiol. 2002, 13, 97–105. [Google Scholar] [CrossRef]
- Hoi, Y.; Zhou, Y.-Q.; Zhang, X.; Henkelman, R.M.; Steinman, D.A. Correlation between local hemodynamics and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann. Biomed. Eng. 2011, 39, 1414–1422. [Google Scholar] [CrossRef]
- Brindise, M.C.; Chiastra, C.; Burzotta, F.; Migliavacca, F.; Vlachos, P.P. Hemodynamics of stent implantation procedures in coronary bifurcations: An in vitro study. Ann. Biomed. Eng. 2017, 45, 542–553. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Cui, M.; Ren, C.; Yu, H.; Gao, W.; Li, D.; Zhao, W. The improvement of the shear stress and oscillatory shear index of coronary arteries during Enhanced External Counterpulsation in patients with coronary heart disease. PLoS ONE 2020, 15, e0230144. [Google Scholar] [CrossRef]
- Williamson, P.N.; Docherty, P.D.; Yazdi, S.G.; Khanafer, A.; Kabaliuk, N.; Jermy, M.; Geoghegan, P.H. Review of the development of hemodynamic modeling techniques to capture flow behavior in arteries affected by aneurysm, atherosclerosis, stenting. J. Biomech. Eng. 2022, 144, 040802. [Google Scholar] [CrossRef] [PubMed]
- Sabath, B.F.; Casal, R.F. Airway stenting for central airway obstruction: A review. Mediastinum 2023, 7, 18. [Google Scholar] [CrossRef]
- Tian, S.; Huang, H.; Hu, Z.; Dong, Y.; Bai, C. A narrative review of progress in airway stents. J. Thorac. Dis. 2022, 14, 1674. [Google Scholar] [CrossRef]
- Chen, B.; Lin, R.; Dai, H.; Yang, J.; Tang, K.; Li, N.; Huang, Y. One-year outcomes and predictive factors for primary patency after stent placement for treatment of central venous occlusive disease in hemodialysis patients. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063039. [Google Scholar]
- Chen, R.; Feng, R.; Jiang, S.; Chang, G.; Hu, Z.; Yao, C.; Jia, B.; Wang, S.; Wang, S. Stent patency rates and prognostic factors of endovascular intervention for iliofemoral vein occlusion in post-thrombotic syndrome. BMC Surg. 2022, 22, 269. [Google Scholar] [CrossRef]
- Rocha-Singh, K.J.; Jaff, M.R.; Crabtree, T.R.; Bloch, D.A.; Ansel, G.I.; VIVA Physicians. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter. Cardiovasc. Interv. 2007, 69, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Gibson, K.; Sapoval, M.; Dexter, D.J.; Kolluri, R.; Razavi, M.; Black, S. Pivotal study evaluating the safety and effectiveness of the abre venous self-expanding stent system in patients with symptomatic iliofemoral venous outflow obstruction. Circ. Cardiovasc. Interv. 2022, 15, e010960. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. Optimizing the design of a bioabsorbable metal stent using computer simulation methods. Biomaterials 2013, 34, 8049–8060. [Google Scholar] [CrossRef]
- Iida, O.; Takahara, M.; Soga, Y.; Nakano, M.; Yamauchi, Y.; Zen, K.; Kawasaki, D.; Nanto, S.; Yokoi, H.; et al.; ZEPHYR Investigators 1-Year results of the ZEPHYR Registry (Zilver PTX for the femoral artery and proximal popliteal artery) predictors of restenosis. JACC Cardiovasc. Interv. 2015, 8, 1105–1112. [Google Scholar] [CrossRef]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. Comparing coronary stent material performance on a common geometric platform through simulated bench testing. J. Mech. Behav. Biomed. Mater. 2012, 12, 129–138. [Google Scholar] [CrossRef]
- Nobili, M.; Sheriff, J.; Morbiducci, U.; Redaelli, A.; Bluestein, D. Platelet activation due to hemodynamic shear stresses: Damage accumulation model and comparison to in vitro measurements. ASAIO J. (Am. Soc. Artif. Intern. Organs 1992) 2008, 54, 64. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Boston Scientific’s Innova Stent System for SFA/PPA Treatment—Endovascular Today. 2015. Available online: https://evtoday.com/news/fda-approves-boston-scientifics-innova-stent-system-for-sfappa-treatment (accessed on 18 August 2015).
- Medtronic’s Abre Venous Stent Launched in Europe—Endovascular Today. 2017. Available online: https://evtoday.com/news/medtronics-abre-venous-stent-launched-in-europe (accessed on 21 December 2017).
- First and Only Peripheral Tri-axial 4-French Low-Profile Self-Expanding Stent System Receives FDA Approval. 2022. Available online: https://www.prnewswire.com/news-releases/first-and-only-peripheral-tri-axial-4-french-low-profile-self-expanding-stent-system-receives-fda-approval-301593604.html / (accessed on 26 July 2022).
- Karanasiou GSPapafaklis, M.I.; Conway, C.; Michalis, L.K.; Tzafriri, R.; Edelman, E.R.; Fotiadis, D.I. Stents: Biomechanics, biomaterials, and insights from computational modeling. Ann. Biomed. Eng. 2017, 45, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Parvizi, S.; Baghbanijavid, H.; Tan, A.T.; Nematollahi, M.; Ramazani, A.; Fang, N.X.; Elahinia, M. Computational modelling of process–structure–property–performance relationships in metal additive manufacturing: A review. Int. Mater. Rev. 2022, 67, 1–46. [Google Scholar] [CrossRef]
- Fereidoonnezhad, B.; Naghdabadi, R.; Holzapfel, G.A. Stress softening and permanent deformation in human aortas: Continuum and computational modeling with application to arterial clamping. J. Mech. Behav. Biomed. Mater. 2016, 61, 600–616. [Google Scholar] [CrossRef]
- Damanpack, A. A 3D finite-strain beam model for thermo-mechanical deformations of 2D shape memory alloys in 3D space. Finite Elem. Anal. Des. 2022, 211, 103817. [Google Scholar] [CrossRef]
- Volegov, P.S.; Knyazev, N.A.; Gerasimov, R.M.; Silberschmidt, V.V. Inelastic Deformation of Coronary Stents: Two-Level Model. Materials 2022, 15, 6948. [Google Scholar] [CrossRef]
- Weisbecker, H.; Pierce, D.M.; Regitnig, P.; Holzapfel, G.A. Layer-specific damage experiments and modeling of human thoracic and abdominal aortas with non-atherosclerotic intimal thickening. J. Mech. Behav. Biomed. Mater. 2012, 12, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, F.; Fereidoonnezhad, B.; Zakerzadeh, M.R.; Baghani, M. A computational study on vascular damage caused by shape memory alloy self-expandable and balloon-expandable stents in a stenosed artery. J. Intell. Mater. Syst. Struct. 2019, 30, 3113–3123. [Google Scholar] [CrossRef]
- Wu, W.; Gastaldi, D.; Yang, K.; Tan, L.; Petrini, L.; Migliavacca, F. Finite element analyses for design evaluation of biodegradable magnesium alloy stents in arterial vessels. Mater. Sci. Eng. B 2011, 176, 1733–1740. [Google Scholar] [CrossRef]
- Lally, C.; Dolan, F.; Prendergast, P. Cardiovascular stent design and vessel stresses: A finite element analysis. J. Biomech. 2005, 38, 1574–1581. [Google Scholar] [CrossRef]
- Zahora, J.; Bezrouk, A.; Hanus, J. Models of stents-Comparison and applications. Physiol. Res. 2007, 56, S115. [Google Scholar] [CrossRef] [PubMed]
- Nematzadeh, F.; Sadrnezhaad, S. Effects of material properties on mechanical performance of Nitinol stent designed for femoral artery: Finite element analysis. Sci. Iran. 2012, 19, 1564–1571. [Google Scholar] [CrossRef]
- Etave, F.; Finet, G.; Boivin, M.; Boyer, J.-C.; Rioufol, G.; Thollet, G. Mechanical properties of coronary stents determined by using finite element analysis. J. Biomech. 2001, 34, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, F.; Conti, M.; De Beule, M.; De Santis, G.; Verhegghe, B. Carotid artery stenting simulation: From patient-specific images to finite element analysis. Med. Eng. Phys. 2011, 33, 281–289. [Google Scholar] [CrossRef]
- Auricchio, F.; Conti, M.; Ferrara, A.; Morganti, S.; Reali, A. Patient-specific finite element analysis of carotid artery stenting: A focus on vessel modeling. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Kim, D.; Kim, D.; Lee, J.-W.; Suh, S. Bailout stent deployment during coil embolization of intracranial aneurysms. Am. J. Neuroradiol. 2009, 30, 1028–1034. [Google Scholar] [CrossRef]
- Saied, A.; Elsaid, N.; Joshi, K.; Gomaa, M.; Amer, T.; Saad, M.; Lopes, D. Factors affecting the degree of angular remodeling in stent-assisted coiling of bifurcation aneurysms. Interv. Neurol. 2020, 8, 220–230. [Google Scholar] [CrossRef]
- Cai, Y.; Meng, Z.; Jiang, Y.; Zhang, X.; Yang, X.; Wang, S. Finite element modeling and simulation of the implantation of braided stent to treat cerebral aneurysm. Med. Nov. Technol. Devices 2020, 5, 100031. [Google Scholar] [CrossRef]
- Laubrie, J.D.; Mousavi, J.S.; Avril, S. A new finite-element shell model for arterial growth and remodeling after stent implantation. Int. J. Numer. Methods Biomed. Eng. 2020, 36, e3282. [Google Scholar] [CrossRef]
- Zhang, H.; Du, T.; Chen, S.; Liu, Y.; Yang, Y.; Hou, Q.; Qiao, A. Finite Element Analysis of the Non-Uniform Degradation of Biodegradable Vascular Stents. J. Funct. Biomater. 2022, 13, 152. [Google Scholar] [CrossRef]
- Kumar, A.; Bhatnagar, N. Finite element simulation and testing of cobalt-chromium stent: A parametric study on radial strength, recoil, foreshortening, and dogboning. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Petrini, L.; Gastaldi, D.; Villa, T.; Vedani, M.; Lesma, E.; Previtali, B.; Migliavacca, F. Finite element shape optimization for biodegradable magnesium alloy stents. Ann. Biomed. Eng. 2010, 38, 2829–2840. [Google Scholar] [CrossRef]
- Donik, Ž.; Nečemer, B.; Glodež, S.; Kramberger, J. Finite element analysis of the mechanical performance of a two-layer polymer composite stent structure. Eng. Fail. Anal. 2022, 137, 106267. [Google Scholar] [CrossRef]
- Britto, J.J.J.; Venkatesh, R.; Prabhakaran, R.; Amudhan, K. Design optimization of biomedical stent under the influence of the radial pressure using FEM. Mater. Today Proc. 2021, 39, 1332–1336. [Google Scholar] [CrossRef]
- Torki, M.M.; Hassanajili, S.; Jalisi, M.M. Design optimizations of PLA stent structure by FEM and investigating its function in a simulated plaque artery. Math. Comput. Simul. 2020, 169, 103–116. [Google Scholar] [CrossRef]
- Paisal, M.S.A.; Taib, I. Computational analysis on stent geometries in carotid artery: A review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 165, 012003. [Google Scholar] [CrossRef]
- Wei, L.; Leo, H.L.; Chen, Q.; Li, Z. Structural and hemodynamic analyses of different stent structures in curved and stenotic coronary artery. Front. Bioeng. Biotechnol. 2019, 7, 366. [Google Scholar] [CrossRef]
- Migliavacca, F.; Petrini, L.; Colombo, M.; Auricchio, F.; Pietrabissa, R. Mechanical behavior of coronary stents investigated through the finite element method. J. Biomech. 2002, 35, 803–811. [Google Scholar] [CrossRef]
- Mejia, J.; Ruzzeh, B.; Mongrain, R.; Leask, R.; Bertrand, O.F. Evaluation of the effect of stent strut profile on shear stress distribution using statistical moments. Biomed. Eng. Online 2009, 8, 1–10. [Google Scholar] [CrossRef]
- Balossino, R.; Gervaso, F.; Migliavacca, F.; Dubini, G. Effects of different stent designs on local hemodynamics in stented arteries. J. Biomech. 2008, 41, 1053–1061. [Google Scholar] [CrossRef]
- Prithipaul, P.K.; Kokkolaras, M.; Pasini, D. Assessment of structural and hemodynamic performance of vascular stents modelled as periodic lattices. Med. Eng. Phys. 2018, 57, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, N.; Schoephoerster, R.T.; Moore, J.E.M., Jr. Comparison of Near-wall Hemodynamic Parameters in Stented Artery Models. J. Biomech. Eng. 2009, 131, 061006. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.G.; Vaughan, T.J. A finite element investigation on design parameters of bare and polymer-covered self-expanding wire braided stents. J. Mech. Behav. Biomed. Mater. 2021, 115, 104305. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.G.; Vaughan, T.J. An experimental evaluation of the mechanics of bare and polymer-covered self-expanding wire braided stents. J. Mech. Behav. Biomed. Mater. 2020, 103, 103549. [Google Scholar] [CrossRef] [PubMed]
- Meoli, A.; Dordoni, E.; Petrini, L.; Migliavacca, F.; Dubini, G.; Pennati, G. Computational modelling of in vitro set-ups for peripheral self-expanding Nitinol stents: The importance of stent–wall interaction in the assessment of the fatigue resistance. Cardiovasc. Eng. Technol. 2013, 4, 474–484. [Google Scholar] [CrossRef]
- Chen, C.L.; Mahjoubfar, A.; Tai, L.C.; Blaby, I.K.; Huang, A.; Niazi, K.R.; Jalali, B. Deep learning in label-free cell classification. Sci. Rep. 2016, 6, 21471. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Muhammad, K.; Wu, W.; Ullah, A.; Baik, S.W. Multi-grade brain tumor classification using deep CNN with extensive data augmentation. J. Comput. Sci. 2019, 30, 174–182. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Liu, Y.; Lu, Z.; You, J.; Yang, M.; Yao, S.; Zhao, G.; Xu, Y.; Chen, T.; et al. Clinically applicable deep learning framework for organs at risk delineation in CT images. Nat. Mach. Intell. 2019, 1, 480–491. [Google Scholar] [CrossRef]
- O’Loughlin, D.; Oliveira, B.L.; Glavin, M.; Jones, E.; O’Halloran, M. Comparing radar-based breast imaging algorithm performance with realistic patient-specific permittivity estimation. J. Imaging 2019, 5, 87. [Google Scholar] [CrossRef]
- Karray, F.; Campilho, A.; Yu, A. Image Analysis and Recognition: 16th International Conference, ICIAR 2019, Waterloo, ON, Canada, 27–29 August 2019, Proceedings, Part I; Springer: Berlin/Heilderberg, Germany, 2019. [Google Scholar]
- Xu, M.; Seenivasan, L.; Yeo, L.L.L.; Ren, H. Stent Deployment Detection Using Radio Frequency-Based Sensor and Convolutional Neural Networks. Adv. Intell. Syst. 2020, 2, 2000092. [Google Scholar] [CrossRef]
- Cornelissen AFlorescu, R.A.; Reese, S.; Behr, M.; Ranno, A.; Manjunatha, K.; Schaaps, N.; Böhm, C.; Liehn, E.A.; Zhao, L.; Nilcham, P. In-vivo assessment of vascular injury for the prediction of in-stent restenosis. Int. J. Cardiol. 2023, 388, 131151. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.B.; Passos, S.D. Vascular stent prototype: In vivo swine studies. J. Vasc. Interv. Radiol. 1997, 8, 107–111. [Google Scholar] [CrossRef]
- Chen CChen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; Edelman, E.R. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wan, H.; Liu, X.; Yu, T.; Yang, Y.; Dai, Y.; Han, Y.; Xu, K.; Yang, L.; Wang, Y.; et al. Engineering Immunomodulatory Stents Using Zinc Ion-Lysozyme Nanoparticle Platform for Vascular Remodeling. ACS Nano 2023, 17, 23498–23511. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, X.; Gao, B.; Ren, C.; Du, H.; Su, X.; Zhang, D.; Bao, T.; Qiao, Z.; Cao, Q. Endothelialization of PTFE-covered stents for aneurysms and arteriovenous fistulas created in canine carotid arteries. Sci. Rep. 2024, 14, 4803. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Z.; Bian, C.; Guo, L.; Tong, Z.; Guo, J.; Qi, L.; Cui, S.; Zhang, C.; Chen, Y.; et al. In vivo evaluation of safety and performance of a tapered nitinol venous stent with inclined proximal end in an ovine iliac venous model. Sci Rep. 2024, 14, 7669. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Oh, S.; Jin, Y.J.; Na, M.H.; Kim, M.; Kim, J.H.; Hyun, D.Y.; Cho, K.H.; Hong, Y.J.; Kim, J.H.; et al. Preliminary Investigation on Efficacy and Safety of Substance P-Coated Stent for Promoting Re-Endothelialization: A Porcine Coronary Artery Restenosis Model. Tissue Eng. Regen. Med. 2024, 21, 53–64. [Google Scholar] [CrossRef]
- Graf, T.; Kancerevycius, G.; Jonusauskas, L.; Eberle, P. Rational Design of Microfluidic Glaucoma Stent. Micromachines 2022, 13, 978. [Google Scholar] [CrossRef]
- Chichareon, P.; Katagiri, Y.; Asano, T.; Takahashi, K.; Kogame, N.; Modolo, R.; Tenekecioglu, E.; Chang, C.-C.; Tomaniak, M.; Kukreja, N.; et al. Mechanical properties and performances of contemporary drug-eluting stent: Focus on the metallic backbone. Expert Rev. Med. Devices 2019, 16, 211–228. [Google Scholar] [CrossRef]
- Conway, C. Coronary Stent Fracture: Clinical Evidence Vs. the Testing Paradigm. Cardiovasc. Eng. Technol. 2018, 9, 752–760. [Google Scholar] [CrossRef]
- Liu, R.; He, H.; Zhang, L.; Fan, Y.; Wang, J.; Wang, W. In vitro models for the experimental evaluation of mechanical thrombectomy devices in acute ischemic stroke. Interv. Neuroradiol. 2023, 29, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, M.; Sassani, F.; Gagnon, J.; Hsiang, Y.; Phani, A.S. Deformation mechanics of self-expanding venous stents: Modelling and experiments. J. Biomech. 2021, 120, 110333. [Google Scholar] [CrossRef] [PubMed]
- Tsauo, J.; Fu, Y.; Liu, Y.; Zhang, X.; Zhao, H.; Li, X. Characteristics of four commonly used self-expanding biliary stents: An in vitro study. Eur. Radiol. Exp. 2024, 8, 24. [Google Scholar] [CrossRef]
- Chytrosz, P.; Golda-Cepa, M.; Wlodarczyk, J.; Kuzdzal, J.; El Fray, M.; Kotarba, A. Characterization of Partially Covered Self-Expandable Metallic Stents for Esophageal Cancer Treatment: In Vivo Degradation. ACS Biomater. Sci. Eng. 2021, 7, 1403–1413. [Google Scholar] [CrossRef]
- Filipovic NNikolic, D.; Isailovic, V.; Milosevic, M.; Geroski, V.; Karanasiou, G.; Fawdry, M.; Flanagan, A.; Fotiadis, D.; Kojic, M. In vitro and in silico testing of partially and fully bioresorbable vascular scaffold. J. Biomech. 2021, 115, 110158. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Edelman, E.R.; Carter, A.; Chronos, N.A.; Rogers, C.; Robinson, K.A.; Waksman, R.; Machan, L.; Weinberger, J.; Wilensky, R.L.; et al. Preclinical evaluation of drug-eluting stents for peripheral applications: Recommendations from an expert consensus group. Circulation 2004, 110, 2498–2505. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, D.; Lee, B.; Roy, A.; Yao, R.; Chen, S.; Dong, Z.; Heineman, W.R.; Kumta, P.N. Effect of Lithium and Aluminum on the Mechanical Properties, In Vivo and In Vitro Degradation, and Toxicity of Multiphase Ultrahigh Ductility Mg-Li-Al-Zn Quaternary Alloys for Vascular Stent Application. ACS Biomater. Sci. Eng. 2020, 6, 1950–1964. [Google Scholar] [CrossRef] [PubMed]
- Schurmann KLahann, J.; Niggemann, P.; Klosterhalfen, B.; Meyer, J.; Kulisch, A.; Klee, D.; Gunther, R.W.; Vorwerk, D. Biologic response to polymer-coated stents: In vitro analysis and results in an iliac artery sheep model. Radiology 2004, 230, 151–162. [Google Scholar] [CrossRef]
- Mohan, C.C.; Chennazhi, K.P.; Menon, D. In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications. Acta Biomater. 2013, 9, 9568–9577. [Google Scholar] [CrossRef]
- Wacker, M.; Betke, U.; Borucki, K.; Hülsmann, J.; Awad, G.; Varghese, S.; Scherner, M.; Hansen, M.; Wippermann, J.; Veluswamy, P. An In Vitro Hemodynamic Loop Model to Investigate the Hemocytocompatibility and Host Cell Activation of Vascular Medical Devices. J. Vis. Exp. 2020, 162, e61570. [Google Scholar] [CrossRef]
- Santin, M.; Mikhalovska, L.; Lloyd, A.W.; Mikhalovsky, S.; Sigfrid, L.; Denyer, S.P.; Field, S.; Teer, D. In vitro host response assessment of biomaterials for cardiovascular stent manufacture. J. Mater. Sci. Mater. Med. 2004, 15, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Han, Y.; Sun, M.; Tao, J.; Yan, C.; Kang, J.; Li, S. Nanoporous CREG-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. PLoS ONE 2013, 8, e60735. [Google Scholar] [CrossRef]
- Kendall, J.; Serracino-Inglott, F.; Banks, C.; Jamshidi, P.; Attallah, M.; Feng, J. In-vitro Study of Effect of the Design of the Stent on the Arterial Waveforms. Procedia Struct. Integr. 2019, 15, 33–40. [Google Scholar] [CrossRef]
- Wang, J.; Smith, C.E.; Sankar, J.; Yun, Y.; Huang, N. Absorbable magnesium-based stent: Physiological factors to consider for in vitro degradation assessments. Regen. Biomater. 2015, 2, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.D. Velocity Field Measurement Using Particle Image Velocimetry (PIV). In Application of Thermo-Fluidic Measurement Techniques; Kim, T., Lu, T.J., Song, S.J., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 125–166. [Google Scholar]
- Charonko, J.; Karri, S.; Schmieg, J.; Prabhu, S.; Vlachos, P. In vitro, time-resolved PIV comparison of the effect of stent design on wall shear stress. Ann. Biomed. Eng. 2009, 37, 1310–1321. [Google Scholar] [CrossRef]
- Anzai, H.; Watanabe, T.; Han, X.; Putra, N.K.; Wang, Z.; Kobayashi, H.; Ohta, M. Endothelial cell distributions and migration under conditions of flow shear stress around a stent wire. Technol. Health Care 2020, 28, 345–354. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Edelman, E.R. Low background, pulsatile, in vitro flow circuit for modeling coronary implant thrombosis. J. Biomech. Eng. 2002, 124, 662–668. [Google Scholar] [CrossRef]
- Kern, A.Y.; Kreinin, Y.; Charle, L.; Epshrein, M.; Korin, N.; Mangin, P.H. A macrofluidic model to investigate the intrinsic thrombogenicity of clinically used stents and develop less thrombogenic stents. Heliyon 2024, 10, e26550. [Google Scholar] [CrossRef]
- Sanchez, P.F.; Brey, E.M.; Briceno, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Marei, I.; Ahmetaj-Shala, B.; Triggle, C.R. Biofunctionalization of cardiovascular stents to induce endothelialization: Implications for in-stent thrombosis in diabetes. Front. Pharmacol. 2022, 13, 982185. [Google Scholar] [CrossRef] [PubMed]
- Raikar, A.S.; Priya, S.; Bhilegaonkar, S.P.; Somnache, S.N.; Kalaskar, D.M. Surface Engineering of Bioactive Coatings for Improved Stent Hemocompatibility: A Comprehensive Review. Materials 2023, 16, 6940. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, P.; Hoylaerts, M.F. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin. Belg. 2006, 61, 213–219. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W. Endothelium--role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef]
- Ong, A.T.; Aoki, J.; Kutryk, M.J.; Serruys, P.W. How to accelerate the endothelialization of stents. Arch. Mal. Coeur Vaiss 2005, 98, 123–126. [Google Scholar] [PubMed]
- Cornelissen, A.; Vogt, F.J. The effects of stenting on coronary endothelium from a molecular biological view: Time for improvement? J. Cell Mol. Med. 2019, 23, 39–46. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Champmartin, S.; Shirinbayan, M.; Bakir, F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers 2022, 14, 2751. [Google Scholar] [CrossRef]
- Touroo, J.S.; Dale, J.R.; Williams, S.K. Bioengineering human blood vessel mimics for medical device testing using serum-free conditions and scaffold variations. Tissue Eng. Part C Methods 2013, 19, 307–315. [Google Scholar] [CrossRef]
- Punchard, M.A. O’Cearbhaill, E.D., Mackle, J.N., McHugh, P.E., Smith, T.J., Stenson-Cox, C. and Barron, V.; Evaluation of human endothelial cells post stent deployment in a cardiovascular simulator in vitro. Ann. Biomed. Eng. 2009, 37, 1322–1330. [Google Scholar] [CrossRef]
- Phan, T.; Jones, J.E.; Chen, M.; Strawn, T.L.; Khoukaz, H.B.; Ji, Y.; Kumar, A.; Bowles, D.K.; Fay, W.P.; Yu, Q. In vitro biological responses of plasma nanocoatings for coronary stent applications. J. Biomed. Mater. Res. A 2023, 111, 1768–1780. [Google Scholar] [CrossRef]
- Cardinal, K.O.; Williams, S.K. Assessment of the intimal response to a protein-modified stent in a tissue-engineered blood vessel mimic. Tissue Eng. Part A 2009, 15, 3869–3876. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, K.O.; Bonnema, G.T.; Hofer, H.; Barton, J.K.; Williams, S.K. Tissue-engineered vascular grafts as in vitro blood vessel mimics for the evaluation of endothelialization of intravascular devices. Tissue Eng. 2006, 12, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Herting, S.; DiBartolomeo, A.; Pipes, T.; Kunz, S.; Temnyk, K.; Truty, J.; Ur, S.; Cardinal, K.O.H. Human Umbilical Versus Coronary Cell Sources for Tissue-Engineered Blood Vessel Mimics. Appl. Vitr. Toxicol. 2016, 2, 175–182. [Google Scholar] [CrossRef]
- Chavez, R.D.; Walls, S.L.; Cardinal, K.O. Tissue-engineered blood vessel mimics in complex geometries for intravascular device testing. PLoS ONE 2019, 14, e0217709. [Google Scholar] [CrossRef]
- Tanyeri, M.; Tay, S. Viable cell culture in PDMS-based microfluidic devices. Methods Cell Biol 2018, 148, 3–33. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Weber, J.; Weber, M.; Feile, A.; Schlensak, C.; Avci-Adali, M. Development of an In Vitro Blood Vessel Model Using Autologous Endothelial Cells Generated from Footprint-Free hiPSCs to Analyze Interactions of the Endothelium with Blood Cell Components and Vascular Implants. Cells 2023, 12, 1217. [Google Scholar] [CrossRef]
- Dorn, F.; Niedermeyer, F.; Balasso, A.; Liepsch, D.; Liebig, T. The effect of stents on intra-aneurysmal hemodynamics: In vitro evaluation of a pulsatile sidewall aneurysm using laser Doppler anemometry. Neuroradiology 2011, 53, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef]
- Caro, C.; Fitz-Gerald, J.; Schroter, R. Arterial wall shear and distribution of early atheroma in man. Nature 1969, 223, 1159–1161. [Google Scholar] [CrossRef]
- Scheinert, D.; Scheinert, S.; Sax, J.; Piorkowski, C.; Bräunlich, S.; Ulrich, M.; Biamino, G.; Schmidt, A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 2005, 45, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Guagliumi, G.; Virmani, R.; Valsecchi, O.; Camrud, A.R.; Scuri, P.M.; Armati, B.; Jones, R.; Kotevski, V.; Manazzale, V.; Camrud, L.L.; et al. Optimal implantation technique for self-expanding nitinol stents: Less acute traumatic expansion and slow growth over time result in a larger final coronary lumen. In Circulation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; Volume 100, p. 293. [Google Scholar]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, Y.; Kobayashi, S.; Tsuchiya, H.; Shibuya, T.; Aoki, M.; Sumiya, J.; Ooyama, S.; Ishizawa, T.; Makino, N.; Ueno, Y.; et al. A fully covered self-expandable metallic stent coated with poly (2-methoxyethyl acrylate) and its derivative: In vitro evaluation of early-stage biliary sludge formation inhibition. Mater. Sci. Eng. C 2021, 120, 111386. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Jang, B.S.; Jung, M.K.; Pack, C.G.; Choi, J.-H.; Park, D.H. Fabrication of a silver particle-integrated silicone polymer-covered metal stent against sludge and biofilm formation and stent-induced tissue inflammation. Sci. Rep. 2016, 6, 35446. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzhan, G.; Olaifa, K.; Kitching, M.; Cahill, P.A.; Pham, T.T.; Ajunwa, O.M.; Marsili, E. Biochemical and electrochemical characterization of biofilms formed on everolimus-eluting coronary stents. Enzym. Microb. Technol. 2023, 163, 110156. [Google Scholar] [CrossRef]
- Medical Stents: Behind the Manufacturing Process 2021. 2023. Available online: https://www.newequipment.com/learning-center/article/21154754/laser-processing-systems-for-medical-stent-manufacturing (accessed on 11 February 2021).
- Stents Market Size & Share|Industry Statistics—2028 2022. 2023. Available online: https://www.gminsights.com/industry-analysis/stent-market (accessed on 30 March 2024).
- Coronary Stents Market Size, Share & Growth Report, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/coronary-stents-industry (accessed on 31 July 2024).
- Self-Expanding/Nitinol. Available online: https://medicalmaterials.com/stents/self-expanding-nitinol/ (accessed on 31 July 2024).
- Iqbal, J.; Gunn, J.; Serruys, P.W. Coronary stents: Historical development, current status and future directions. Br. Med. Bull. 2013, 106, 1. [Google Scholar] [CrossRef]
- Amg International—The Stent Company n.d. Available online: http://www.thestentcompany.com/products.html (accessed on 31 July 2024).
- Coronary Stents—Boston Scientific n.d. Available online: https://www.bostonscientific.com/en-US/products/stents--coronary.html (accessed on 31 July 2024).
- Commonwealth Scientific and Industrial Research Organisation, Australian Government—CSIRO n.d. Available online: https://www.csiro.au/en/ (accessed on 31 July 2024).
- InvoShield—Nano Therapeutics Pvt. Ltd. n.d. Available online: https://www.nano-therapeutics.net/product/invoshield/ (accessed on 31 July 2024).
- Vascular Implants & Devices—Stent Manufacturing n.d. Available online: https://nnoble.com/applications/medical-devices-implants/vascular/ (accessed on 31 July 2024).
- Optimed Self Expanding Stents. Available online: https://www.optimed.com/#products (accessed on 31 July 2024).

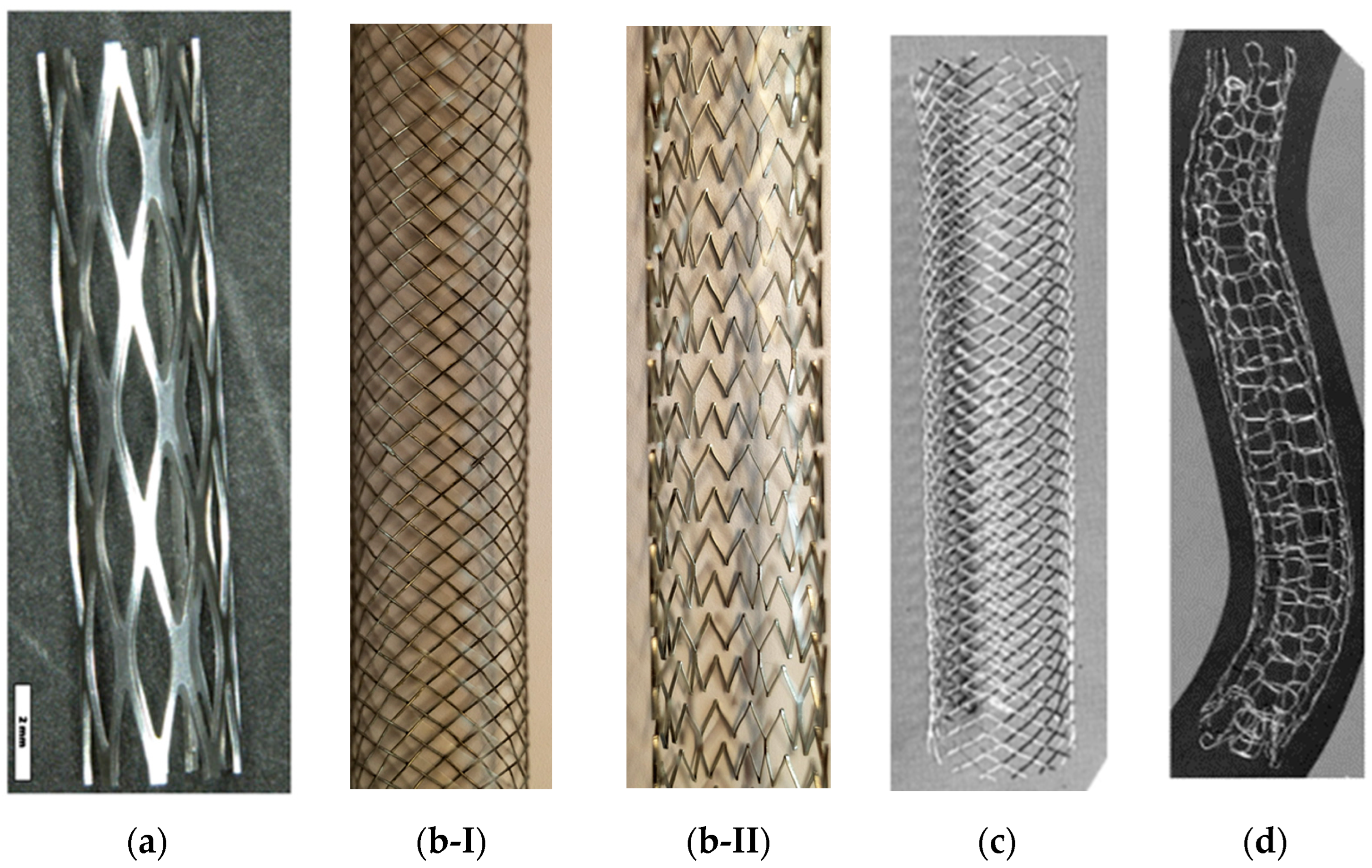









| Stent Material | Advantages | Disadvantages |
|---|---|---|
| 316L SS | Widely used material due to its good mechanical properties and corrosion resistance | Non-MRI compatibility, poor visible fluoroscopic behavior, and allergic reactions in the body |
| NiTi (nitinol) | High corrosion resistance, Shape memory alloy (showing superelasticity, shape memory effect, and damping) | Ni release causing allergic reactions, nitinol stents do not present adequate radiopacity, crevice corrosion, and pitting |
| Co-Cr alloys | Radiopaque and MRI-compatible, high corrosion and wear resistance, superior radial strength and toughness, and suitable castability | Their plasticity and workability are inferior to those of stainless steel |
| Mg alloys | Biocompatible with good mechanical performance | The degradation products of Mg stents are toxic |
| Pt-Ir alloys | Excellent radiopacity, high corrosion resistance, and reduced thrombosis and neointimal proliferation | Lack of sufficient mechanical properties |
| Ta | Excellent radiopacity, MRI compatibility, and corrosion resistance in the human body |
| Methods | Materials | Advantages | Disadvantages | |||
|---|---|---|---|---|---|---|
| Conventional Methods | Primary Manufacturing | Casting | Mg alloys | Appropriate for simple shapes, restricted to materials with high ductility | ||
| Conventional PM | CS | Stainless steel (SS316L), nitinol, Fe-Mn alloys | ||||
| SPS | Nitinol, Fe-Ag, Fe-Au alloys | |||||
| MIM | Fe | High production efficiency, good surface quality, high consistency | Difficult to processing | |||
| Braiding | Stainless steel, Ta, Co-Cr alloys, Ni-Ti alloys | Low-cost, simple, and versatile continuous fabrication method with no material loss; producing stents with superior properties and no HAZ | High axial rigidity and length variations in the produced stents, poor radial stiffness, stent shortening problems in neighboring tough tissues, Limited to simple structure | |||
| Knitting | Low-cost, simple, and versatile continuous fabrication method with no material loss; producing stents with superior properties and no HAZ | Low shortening ratio and compression resistance; biomechanical limitations; mismatch between their longitudinal flexibility and radial compliance with the artery | ||||
| Electroforming | SS316L, Fe, Fe-Mn, Fe-Zn | Low-cost, precise, and reproducible method, manufacturing products with complex shape and large size | Peeling of deposits from cathode, Limited materials so far | |||
| Secondary Manufacturing | Photochemical etching | Stainless steel, nitinol, Co-Cr alloys | Low-cost, simple, rapid, and flexible method for stent manufacturing with no residual stress and burrs | Inappropriate for manufacturing 3D complex samples, non-uniform coating creation on the stent surface | ||
| Micro-electro-discharge machining (μEDM) | Stainless steel, Mg alloys, Ti alloys | Producing stents with high surface quality and dimensional accuracy, and burr/dross-free | Limited to specific materials | |||
| Laser cutting | SS316L, Co-Cr, Fe-Mn alloys | Low cost, High fabrication speed/precision/quality | HAZ | |||
| Micro-milling | Pure Mg, Mg alloy | High process efficiency and accuracy | Burrs | |||
| Welding | Stainless steel, nitinol | Low cost, No HAZ | Formation of brittle phases | |||
| 3D printing | Selective laser melting (SLM) | NiTi alloys, Co-Cr alloys, Zn | Low-cost and fast fabrication, capable of producing stents with complex structures, improved geometrical accuracy, superior mechanical properties, high density and roughness, broad materials selection | Low strength of products, poor accuracy | ||
| Electroforming | SS316L, Fe, Fe-Mn, Fe-Zn | Low-cost and precise manufacturing method | Limited materials so far | |||
| Methods | Name | Manufacturer | Material | Stent Form | Geometry |
|---|---|---|---|---|---|
| Photochemical etching | Endotex | Nitinol | Sheet | ------ | |
| aSpire | Vascular Architects | Nitinol | Sheet | Coil | |
| Braiding | Wallstent | BSC | Co-Cr alloy | Wire | Braided |
| Knitting | ZA | Cook | Nitinol | Wire | Knitted |
| Coiling | Symphony | BSC | Nitinol | Wire | Welded coil |
| Esophacoil | InStent | Nitinol | Ribbon | Coil | |
| IntraCoil | IntraTherapeutics | Nitinol | Wire | Coil |
| Design | Material | Stent Form | Application | Advantages | Disadvantage | |
|---|---|---|---|---|---|---|
| Coiled | Metallic wire | Balloon-expandable | Successful in nonvascular: prostate and urethral | High flexibility | Limited radial strength, low expansion capability, significant elastic recoil, and a heightened risk of restenosis | |
| Not Successful in vascular | Large size | |||||
| Slotted tube | Metal tubes, followed by laser cut | Main available stent in the market | Impressive radial strength | Limited flexibility and deliverability | ||
| Tubular mesh (woven) | Wires | One or more wire strands, self-expanding and balloon-expandable (mainly SEMS) | Urological, gastrointestinal, and airway applications | Extensive coverage and minimal expansion, robust mechanical support to the arteries | ||
| Fiber-based | Fibers | Production by knitting and braiding | Easy to modify to enhance their biocompatibility, exceptional mechanical properties | |||
| Braided | Fiber, wire | Limited flexibility and a tendency for the edges to fray | ||||
| Knitted | Wrap knit | Natural flexibility due to their interconnected looped design, easily removable in the form of a wire | ||||
| Weft knit | ||||||
| Covered | Fully or partially covered SEMS | For esophageal strictures | Prevent excessive tissue growth around the wire meshes | Risk of granulation tissue forming at the exposed ends of the stent and tissue ingrowth through the disrupted covering | ||
| Closed-cell | Greater radial strength, more resistant to the growth of tumors or excessive tissue growth inward, longer patency | Less flexible | ||||
| Open-cell | Periodic connections from peak to peak, from peak to valley, and from mid-step to mid-step | Longitudinal flexibility, more pliable, reduced surface area, neointimal reaction, and arterial injury, improved access to side branches, enhanced conformability, shortening ratio of zero | Lower radial strength, higher plaque prolapse | |||
| Helical pattern | Flexibility, few or no internal connection points | Lacking longitudinal support, possible irregular cell sizes after deployment | ||||
| Company Name | Material | Manufacturing Process | Cost [274] |
|---|---|---|---|
| Abbott Laboratories (USA) | CoCr [275] | - | USD 100 |
| amg International GmbH (Germany) | CoCr, NiTi [276] | - | - |
| Bard Angiomed [46] | NiTi | Laser cut tube | - |
| Boston Scientific (USA) [277] | PtCr stainless steel [275] | - | USD 75–1400 |
| Biotronik (Germany) | NiTi | - | USD 450 |
| Cook Medical (USA) [46] | NiTi | Knitted wire | USD 100 |
| CSIRO (Australia) | NiTi [278] | 3D printing | - |
| Medtronic (USA) | CoCr [275] | - | USD 150–1700 |
| Medicorp Inc. (USA) [46] | NiTi | Braided wire | - |
| Nano Therapeutics Pvt. Ltd. (India) | CoCr [279] | - | - |
| Norman Noble, Inc. (USA) | NiTi [280] | Laser cutting | - |
| Optimed Medizinische Instrumente GmbH (Germany) [281] | NiTi | Braided | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanaei, S.; Hashemi, M.; Solouk, A.; Asghari Ilani, M.; Amili, O.; Hefzy, M.S.; Tang, Y.; Elahinia, M. Manufacturing, Processing, and Characterization of Self-Expanding Metallic Stents: A Comprehensive Review. Bioengineering 2024, 11, 983. https://doi.org/10.3390/bioengineering11100983
Vanaei S, Hashemi M, Solouk A, Asghari Ilani M, Amili O, Hefzy MS, Tang Y, Elahinia M. Manufacturing, Processing, and Characterization of Self-Expanding Metallic Stents: A Comprehensive Review. Bioengineering. 2024; 11(10):983. https://doi.org/10.3390/bioengineering11100983
Chicago/Turabian StyleVanaei, Saeedeh, Mahdi Hashemi, Atefeh Solouk, Mohsen Asghari Ilani, Omid Amili, Mohamed Samir Hefzy, Yuan Tang, and Mohammad Elahinia. 2024. "Manufacturing, Processing, and Characterization of Self-Expanding Metallic Stents: A Comprehensive Review" Bioengineering 11, no. 10: 983. https://doi.org/10.3390/bioengineering11100983
APA StyleVanaei, S., Hashemi, M., Solouk, A., Asghari Ilani, M., Amili, O., Hefzy, M. S., Tang, Y., & Elahinia, M. (2024). Manufacturing, Processing, and Characterization of Self-Expanding Metallic Stents: A Comprehensive Review. Bioengineering, 11(10), 983. https://doi.org/10.3390/bioengineering11100983











