The Assessability of Approximal Secondary Caries of Non-Invasive 3D-Printed Veneers Depending on the Restoration Thickness—An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
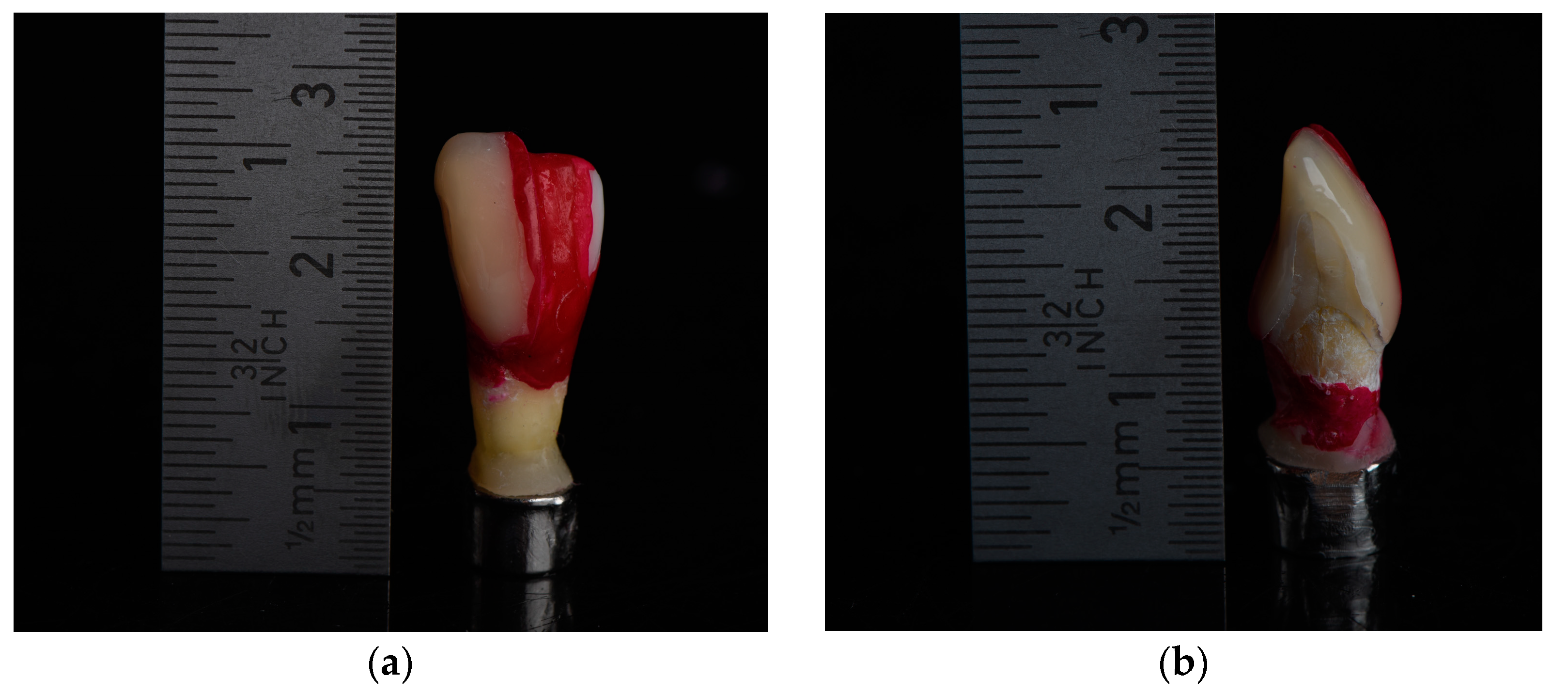
2.1. Specimen Preparation
2.2. Artificial Carious Lesions
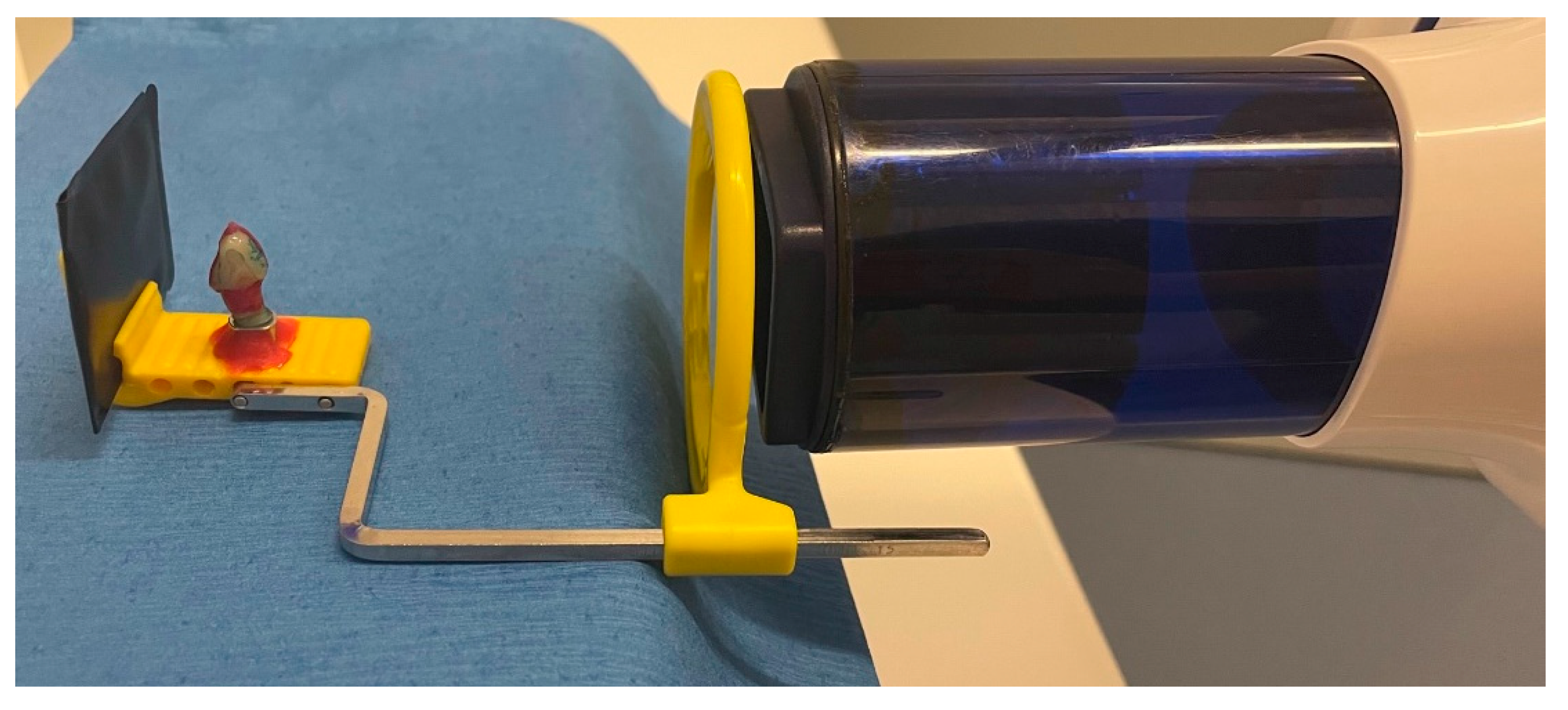
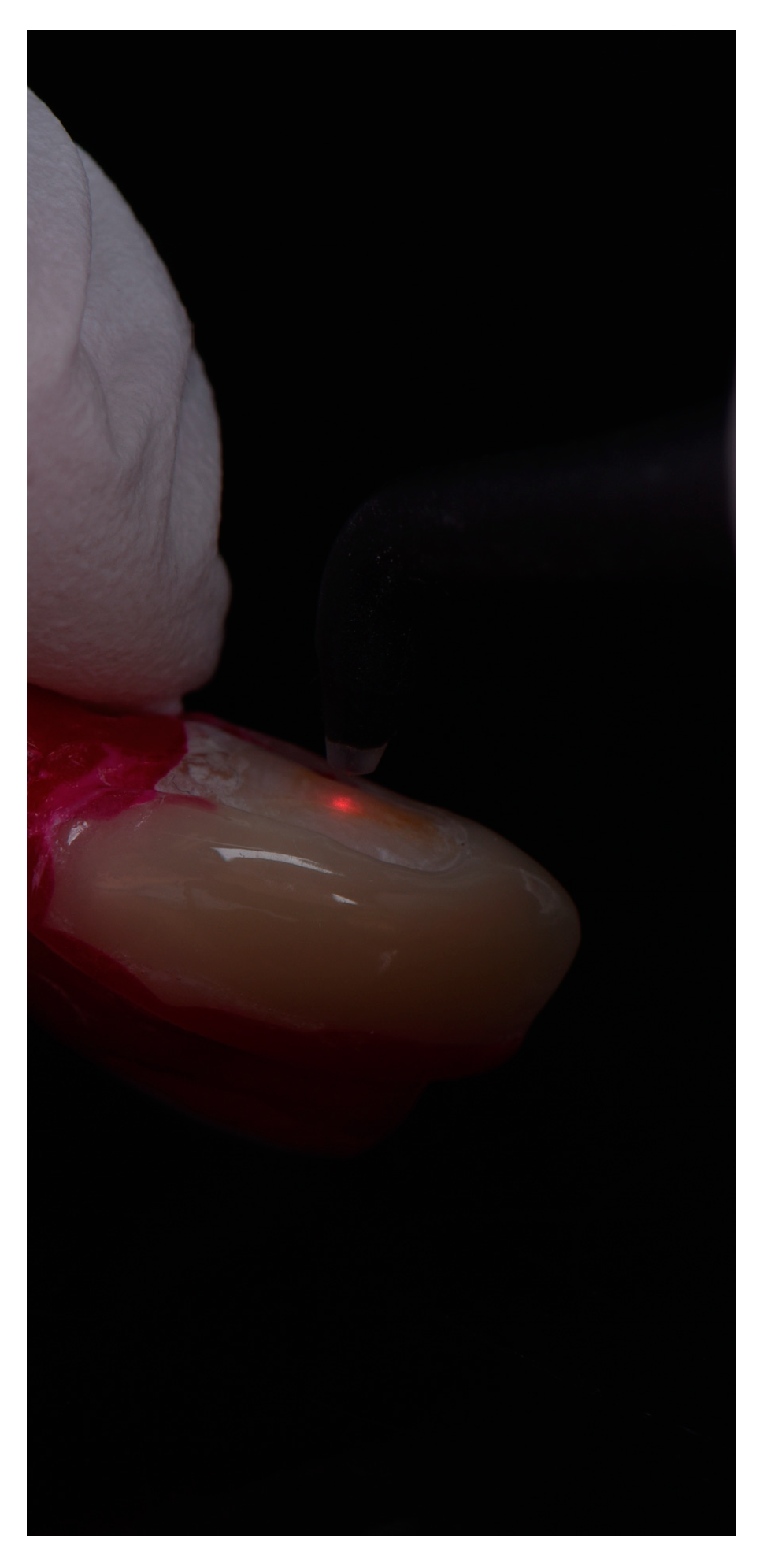
2.3. Radiological and Fluorescence Examination
2.4. Data Analyses
2.5. Statistical Analyses
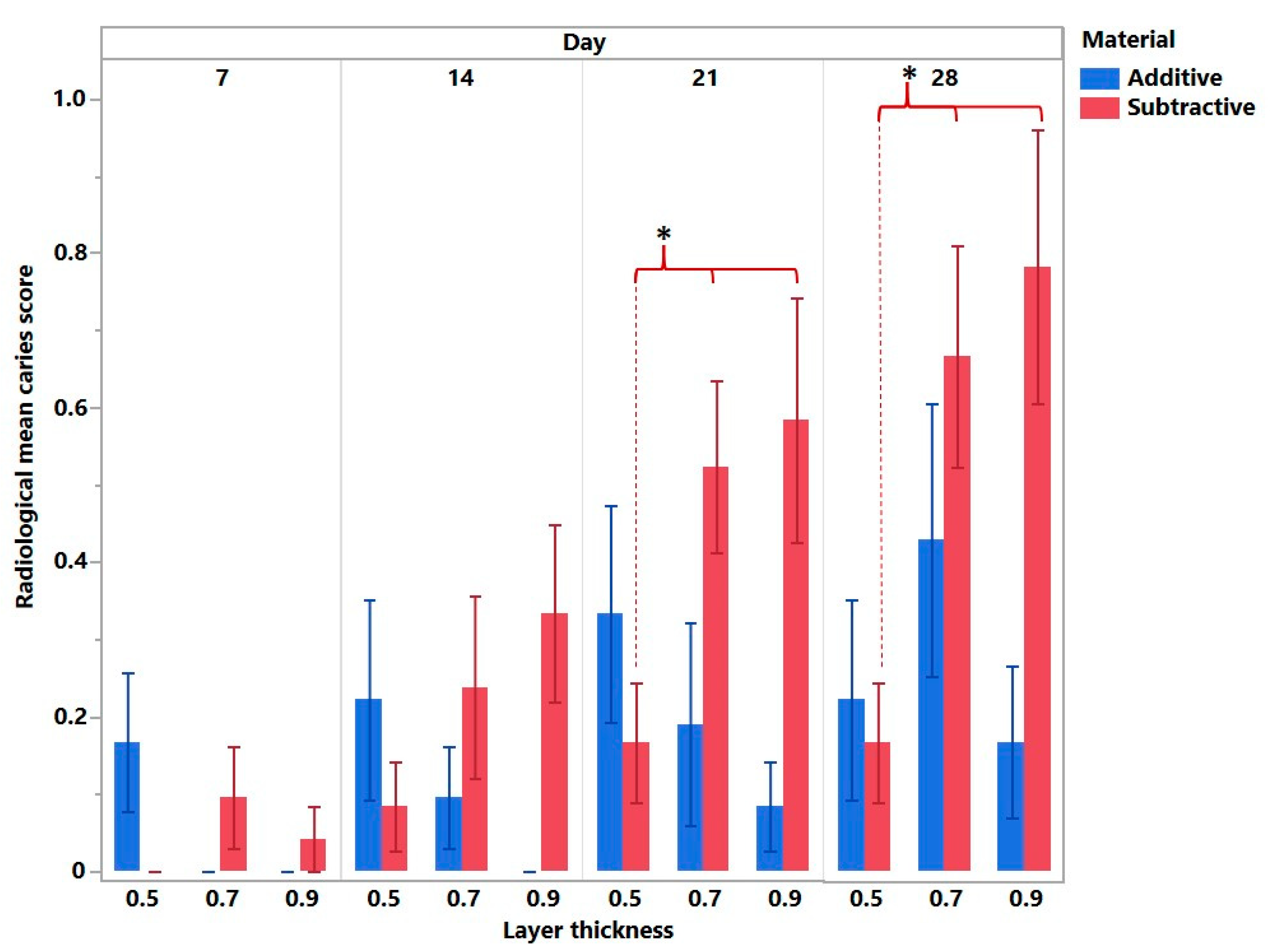
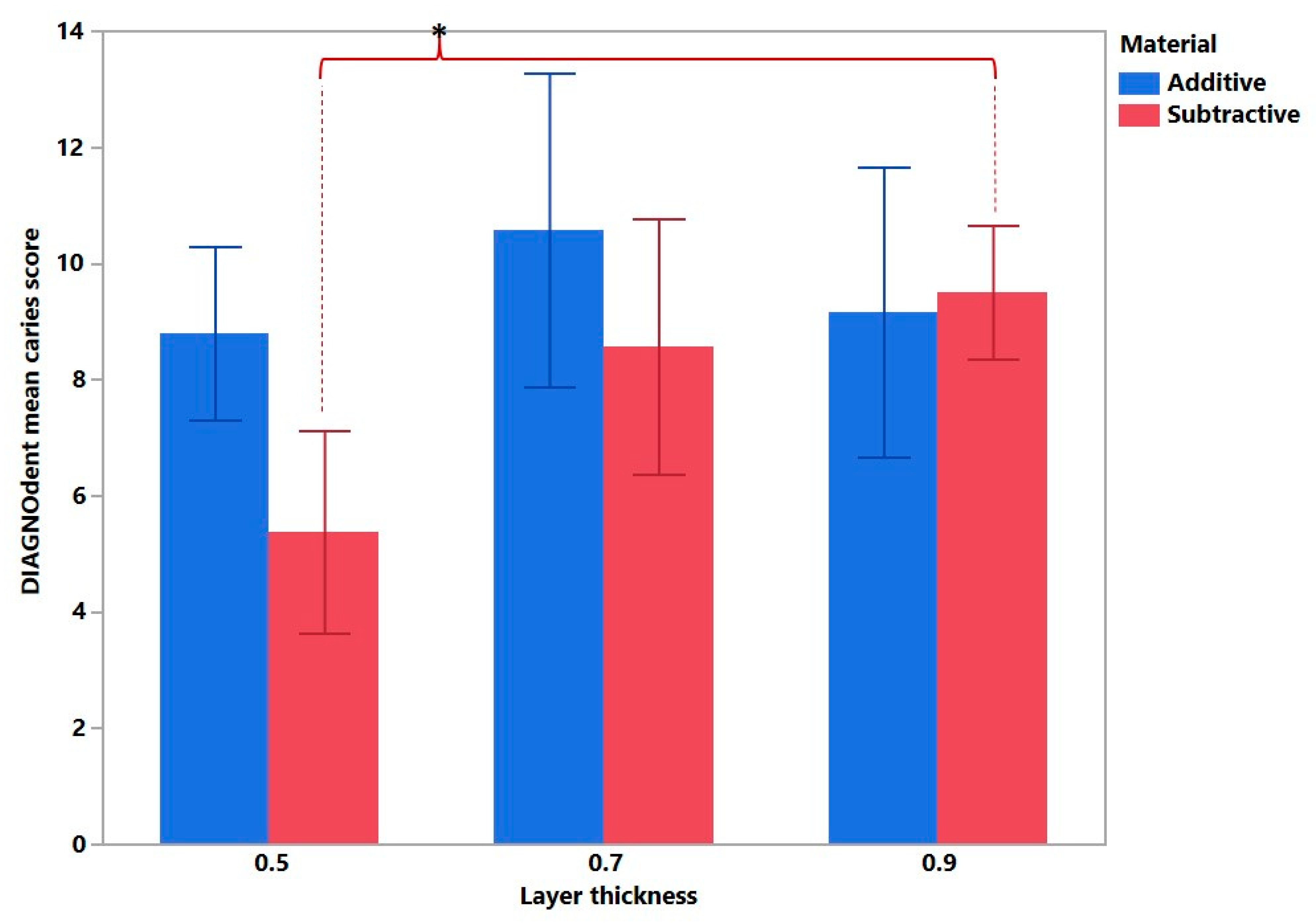
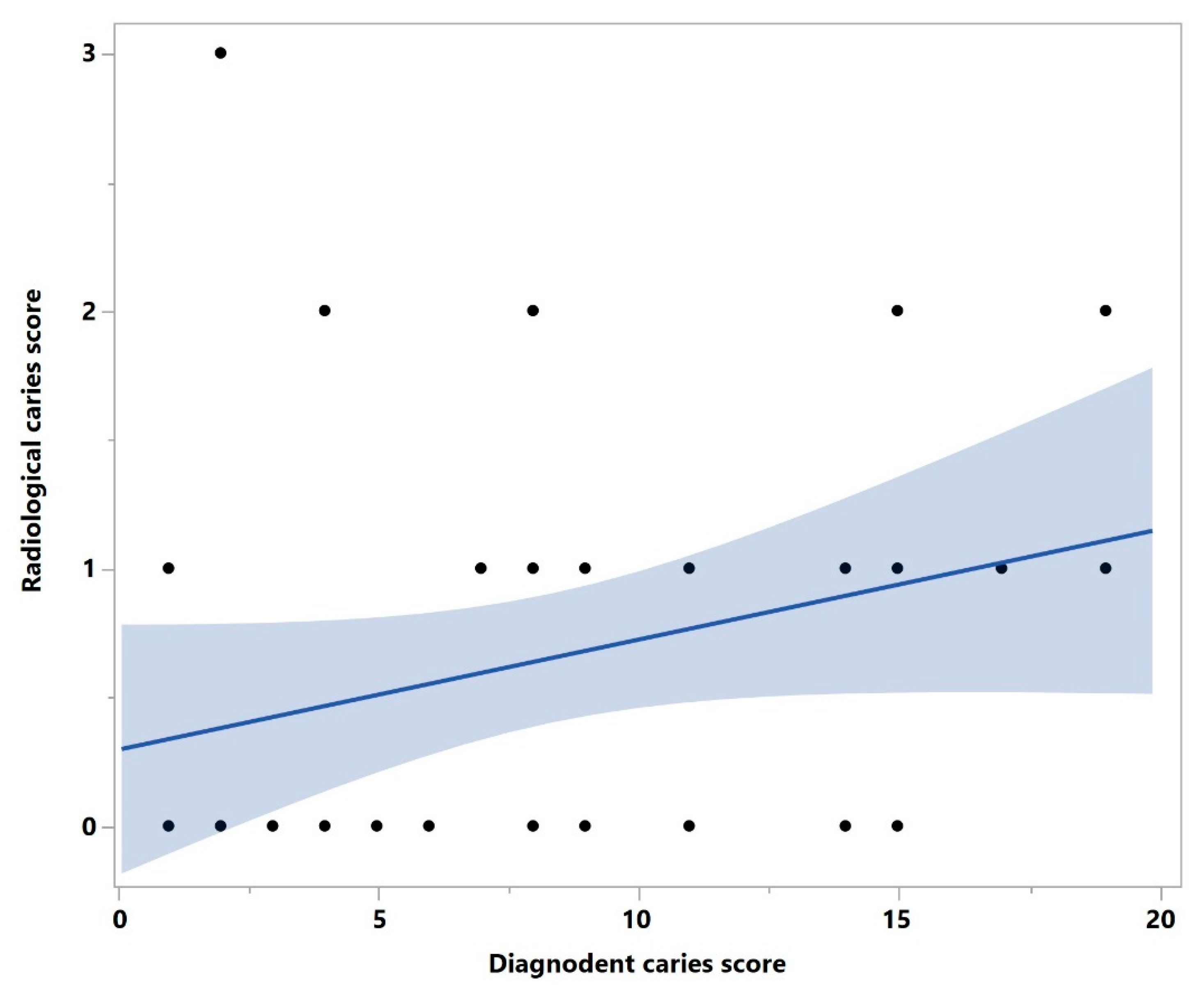
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erzurumlu, Z.U.; Sagirkaya, C.E.; Erzurumlu, K. Evaluation of radiopacities of CAD/CAM restorative materials and resin cements by digital radiography. Clin. Oral. Investig. 2021, 25, 5735–5741. [Google Scholar] [CrossRef]
- Sannino, G.; Germano, F.; Arcuri, L.; Bigelli, E.; Arcuri, C.; Barlattani, A. Cerec CAD/CAM chairside system. ORAL Implantol. 2014, 7, 57. [Google Scholar]
- Hosney, S.; Abouelseoud, H.K.; El-Mowafy, O. Radiopacity of Resin Cements Using Digital Radiography. J. Esthet. Restor. Dent. 2017, 29, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, R.B.; Carvalho, R.F.; Yamamoto, F.; Almeida, S.M.; Verner, F.S. Evaluation of Radiopacity of Luting Cements Submitted to Different Aging Procedures. J. Prosthodont. 2018, 27, 853–859. [Google Scholar] [CrossRef]
- Mayer, J.; Stawarczyk, B.; Vogt, K.; Hickel, R.; Edelhoff, D.; Reymus, M. Influence of cleaning methods after 3D printing on two-body wear and fracture load of resin-based temporary crown and bridge material. Clin. Oral. Investig. 2021, 25, 5987–5996. [Google Scholar] [CrossRef]
- Kessler, A.; Reymus, M.; Hickel, R.; Kunzelmann, K.H. Three-body wear of 3D printed temporary materials. Dent. Mater. 2019, 35, 1805–1812. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Daher, R.; Ardu, S.; di Bella, E.; Krejci, I.; Duc, O. Efficiency of 3D-printed composite resin restorations compared with subtractive materials: Evaluation of fatigue behavior, cost, and time of production. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Filli, T.; Imfeld, C.; Schmidlin, P.R. Composite vertical bite reconstructions in eroded dentitions after 5.5 years: A case series. J. Oral. Rehabil. 2012, 39, 73–79. [Google Scholar] [CrossRef]
- Edelhoff, D.; Beuer, F.; Schweiger, J.; Brix, O.; Stimmelmayr, M.; Guth, J.F. CAD/CAM-generated high-density polymer restorations for the pretreatment of complex cases: A case report. Quintessence Int. 2012, 43, 457–467. [Google Scholar]
- Soares, C.J.; Santana, F.R.; Fonseca, R.B.; Martins, L.R.; Neto, F.H. In vitro analysis of the radiodensity of indirect composites and ceramic inlay systems and its influence on the detection of cement overhangs. Clin. Oral. Investig. 2007, 11, 331–336. [Google Scholar] [CrossRef]
- Dukic, W.; Delija, B.; Derossi, D.; Dadic, I. Radiopacity of composite dental materials using a digital X-ray system. Dent. Mater. J. 2012, 31, 47–53. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Branco, C.A.; Soares, P.V.; Correr-Sobrinho, L.; Haiter-Neto, F.; Fernandes-Neto, A.J.; Soares, C.J. Radiodensity of base, liner and luting dental materials. Clin. Oral. Investig. 2006, 10, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Yaylacı, A.; Karaarslan, E.S.; Hatırlı, H. Evaluation of the radiopacity of restorative materials with different structures and thicknesses using a digital radiography system. Imaging Sci. Dent. 2021, 51, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Rasimick, B.J.; Gu, S.; Deutsch, A.S.; Musikant, B.L. Measuring the radiopacity of luting cements, dowels, and core build-up materials with a digital radiography system using a CCD sensor. J. Prosthodont. 2007, 16, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.F.; Brasileiro, I.V.; dos Anjos Pontual, M.L.; dos Anjos Pontual, A.; da Silveira, M.M. Influence of materials radiopacity in the radiographic diagnosis of secondary caries: Evaluation in film and two digital systems. Dentomaxillofac. Radiol. 2011, 40, 344–350. [Google Scholar] [CrossRef]
- Mejàre, I.; Källest, L.C.; Stenlund, H. Incidence and progression of approximal caries from 11 to 22 years of age in Sweden: A prospective radiographic study. Caries Res. 1999, 33, 93–100. [Google Scholar] [CrossRef]
- Pitts, N.B. Monitoring of caries progression in permanent and primary posterior approximal enamel by bitewing radiography. Community Dent. Oral. Epidemiol. 1983, 11, 228–235. [Google Scholar] [CrossRef]
- Shwartz, M.; Gröndahl, H.G.; Pliskin, J.S.; Boffa, J. A longitudinal analysis from bite-wing radiographs of the rate of progression of approximal carious lesions through human dental enamel. Arch. Oral. Biol. 1984, 29, 529–536. [Google Scholar] [CrossRef]
- Kruger, J.; du Plessis, A.; van Zijl, G. An investigation into the porosity of extrusion-based 3D printed concrete. Addit. Manuf. 2021, 37, 101740. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Fuh, J.Y.H.; Lee, H.P. Effect of Porosity on Mechanical Properties of 3D Printed Polymers: Experiments and Micromechanical Modeling Based on X-ray Computed Tomography Analysis. Polymers 2019, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Luis, E.; Pan, H.M.; Sing, S.L.; Bastola, A.K.; Goh, G.D.; Goh, G.L.; Tan, H.K.J.; Bajpai, R.; Song, J.; Yeong, W.Y. Silicone 3D Printing: Process Optimization, Product Biocompatibility, and Reliability of Silicone Meniscus Implants. 3D Print. Addit. Manuf. 2019, 6, 319–332. [Google Scholar] [CrossRef]
- Buskes, J.A.; Christoffersen, J.; Arends, J. Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res. 1985, 19, 490–496. [Google Scholar] [CrossRef]
- Paris, S.; Meyer-Lueckel, H.; Mueller, J.; Hummel, M.; Kielbassa, A.M. Progression of sealed initial bovine enamel lesions under demineralizing conditions in vitro. Caries Res. 2006, 40, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Angnes, G.; Angnes, V.; Grande, R.H.; Battistella, M.; Loguercio, A.D.; Reis, A. Occlusal caries diagnosis in permanent teeth: An in vitro study. Braz. Oral. Res. 2005, 19, 243–248. [Google Scholar] [CrossRef][Green Version]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Revilla-León, M.; Fry, E.; Supaphakorn, A.; Barmak, A.B.; Kois, J.C. Manufacturing accuracy of the intaglio surface of definitive resin-ceramic crowns fabricated at different print orientations by using a stereolithography printer. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Revilla-León, M.; Supaphakorn, A.; Barmak, A.B.; Rutkunas, V.; Kois, J.C. Influence of print orientation on the intaglio surface accuracy (trueness and precision) of tilting stereolithography definitive resin-ceramic crowns. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Haddadi, Y.; Ranjkesh, B.; Isidor, F.; Bahrami, G. Marginal and internal fit of crowns based on additive or subtractive manufacturing. Biomater. Investig. Dent. 2021, 8, 87–91. [Google Scholar] [CrossRef]
- Bae, E.J.; Jeong, I.D.; Kim, W.C.; Kim, J.H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.J.H.; Braian, M.; Larsson, C.; Wennerberg, A. Production tolerance of conventional and digital workflow in the manufacturing of glass ceramic crowns. Dent. Mater. 2019, 35, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Grzebieluch, W.; Kowalewski, P.; Grygier, D.; Rutkowska-Gorczyca, M.; Kozakiewicz, M.; Jurczyszyn, K. Printable and Machinable Dental Restorative Composites for CAD/CAM Application-Comparison of Mechanical Properties, Fractographic, Texture and Fractal Dimension Analysis. Materials 2021, 14, 4919. [Google Scholar] [CrossRef]
- Heffernan, M.J.; Aquilino, S.A.; Diaz-Arnold, A.M.; Haselton, D.R.; Stanford, C.M.; Vargas, M.A. Relative translucency of six all-ceramic systems. Part I: Core materials. J. Prosthet. Dent. 2002, 88, 4–9. [Google Scholar] [CrossRef]
- Liu, D.; Šavija, B.; Smith, G.E.; Flewitt, P.E.J.; Lowe, T.; Schlangen, E. Towards understanding the influence of porosity on mechanical and fracture behaviour of quasi-brittle materials: Experiments and modelling. Int. J. Fract. 2017, 205, 57–72. [Google Scholar] [CrossRef]
- Chandler, H.W.; Merchant, I.J.; Henderson, R.J.; Macphee, D.E. Enhanced crack-bridging by unbonded inclusions in a brittle matrix. J. Eur. Ceram. Soc. 2002, 22, 129–134. [Google Scholar] [CrossRef]
- du Plessis, A.; Boshoff, W.P. A review of X-ray computed tomography of concrete and asphalt construction materials. Constr. Build. Mater. 2019, 199, 637–651. [Google Scholar] [CrossRef]
- Thompson, A.; Maskery, I.; Leach, R.K. X-ray computed tomography for additive manufacturing: A review. Meas. Sci. Technol. 2016, 27, 072001. [Google Scholar] [CrossRef]
- Sichani, A.V.; Javadinejad, S.; Ghafari, R. Diagnostic value of DIAGNOdent in detecting caries under composite restorations of primary molars. Dent. Res. J. 2016, 13, 327–332. [Google Scholar] [CrossRef]
- Shi, X.Q.; Welander, U.; Angmar-Månsson, B. Occlusal caries detection with KaVo DIAGNOdent and radiography: An in vitro comparison. Caries Res. 2000, 34, 151–158. [Google Scholar] [CrossRef]
- Rahiotis, C.; Vougiouklakis, G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J. Dent. 2007, 35, 695–698. [Google Scholar] [CrossRef]
- Virajsilp, V.; Thearmontree, A.; Aryatawong, S.; Paiboonwarachat, D. Comparison of proximal caries detection in primary teeth between laser fluorescence and bitewing radiography. Pediatr. Dent. 2005, 27, 493–499. [Google Scholar]
- Attrill, D.C.; Ashley, P.F. Occlusal caries detection in primary teeth: A comparison of DIAGNOdent with conventional methods. Br. Dent. J. 2001, 190, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, V.; Seppä, L.; Hausen, H. Clinical study of the use of the laser fluorescence device DIAGNOdent for detection of occlusal caries in children. Caries Res. 2003, 37, 17–23. [Google Scholar] [CrossRef]
- Bamzahim, M.; Aljehani, A.; Shi, X.Q. Clinical performance of DIAGnodent in the detection of secondary carious lesions. Acta Odontol. Scand. 2005, 63, 26–30. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Neuhaus, K.W.; Hug, I.; Stich, H.; Seemann, R.; Lussi, A. In vitro detection of secondary caries associated with composite restorations on approximal surfaces using laser fluorescence. Oper. Dent. 2010, 35, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Pontual, A.A.; de Melo, D.P.; de Almeida, S.M.; Bóscolo, F.N.; Neto, F.H. Comparison of digital systems and conventional dental film for the detection of approximal enamel caries. Dentomaxillofac. Radiol. 2010, 39, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Senel, B.; Kamburoglu, K.; Uçok, O.; Yüksel, S.P.; Ozen, T.; Avsever, H. Diagnostic accuracy of different imaging modalities in detection of proximal caries. Dentomaxillofac. Radiol. 2010, 39, 501–511. [Google Scholar] [CrossRef]
- Lussi, A.; Imwinkelried, S.; Pitts, N.; Longbottom, C.; Reich, E. Performance and reproducibility of a laser fluorescence system for detection of occlusal caries in vitro. Caries Res. 1999, 33, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kavvadia, K.; Lagouvardos, P. Clinical performance of a diode laser fluorescence device for the detection of occlusal caries in primary teeth. Int. J. Paediatr. Dent. 2008, 18, 197–204. [Google Scholar] [CrossRef]
- Lepidi, L.; Galli, M.; Mastrangelo, F.; Venezia, P.; Joda, T.; Wang, H.L.; Li, J. Virtual Articulators and Virtual Mounting Procedures: Where Do We Stand? J. Prosthodont. 2021, 30, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kochanowski, M.; Barankiewicz, A.; Sadowska, P.; Dejak, B. Digital planning protocol for functional and esthetic prosthetic treatment. Int. J. Comput. Dent. 2022, 26, 61–73. [Google Scholar] [CrossRef]




| Material | Composition | Manufacturer | Code |
|---|---|---|---|
| VarseoSmile Crown plus | Ceramic-filled (30–50 wt% inorganic fillers; particle size 0.7 µm) silanized dental glass, methyl benzoylfor-mate, diphenyl (2, 4, 6-trimethylbenzoyl) phosphine oxide hybrid material | Bego, Bremen, Germany | VSCP |
| Vita Enamic | Polymer infiltrated (UDMA, TEGDMA 14 wt%) feldspar ceramic network (86 wt%) | Vita Zahnfabrik, Bad Sackingen, Germany | VE |
| Score | Description |
|---|---|
| 0 | No visible radiolucency |
| 1 | Radiolucency in the outer half (<half of the enamel) |
| 2 | Radiolucency in the inner half (<half of the enamel up to the enamel-dentin border) |
| 3 | Radiolucency in the dentin (broken enamel-dentin border but without obvious spread in the dentin) |
| 4 | Radiolucency with obvious spread in the outer half of the dentin (<halfway through the pulp) |
| 5 | Radiolucency with obvious spread in the inner half of the dentin (>halfway through the pulp) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prause, E.; Hey, J.; Schmidt, F.; Nicic, R.; Beuer, F.; Unkovskiy, A. The Assessability of Approximal Secondary Caries of Non-Invasive 3D-Printed Veneers Depending on the Restoration Thickness—An In Vitro Study. Bioengineering 2023, 10, 992. https://doi.org/10.3390/bioengineering10090992
Prause E, Hey J, Schmidt F, Nicic R, Beuer F, Unkovskiy A. The Assessability of Approximal Secondary Caries of Non-Invasive 3D-Printed Veneers Depending on the Restoration Thickness—An In Vitro Study. Bioengineering. 2023; 10(9):992. https://doi.org/10.3390/bioengineering10090992
Chicago/Turabian StylePrause, Elisabeth, Jeremias Hey, Franziska Schmidt, Robert Nicic, Florian Beuer, and Alexey Unkovskiy. 2023. "The Assessability of Approximal Secondary Caries of Non-Invasive 3D-Printed Veneers Depending on the Restoration Thickness—An In Vitro Study" Bioengineering 10, no. 9: 992. https://doi.org/10.3390/bioengineering10090992
APA StylePrause, E., Hey, J., Schmidt, F., Nicic, R., Beuer, F., & Unkovskiy, A. (2023). The Assessability of Approximal Secondary Caries of Non-Invasive 3D-Printed Veneers Depending on the Restoration Thickness—An In Vitro Study. Bioengineering, 10(9), 992. https://doi.org/10.3390/bioengineering10090992








