Abstract
Objectives: Composites are commonly used for tooth restorations, but recurrent caries often lead to restoration failures due to polymerization shrinkage-stress-induced marginal leakage. The aims of this research were to: (1) develop novel low-shrinkage-stress (L.S.S.) nanocomposites containing dimethylaminododecyl methacrylate (DMADDM) with nanoparticles of calcium fluoride (nCaF2) or amorphous calcium phosphate (NACP) for remineralization; (2) investigate antibacterial and cytocompatibility properties. Methods: Nanocomposites were made by mixing triethylene glycol divinylbenzyl ether with urethane dimethacrylate containing 3% DMADDM, 20% nCaF2, and 20% NACP. Flexural strength, elastic modulus, antibacterial properties against Streptococcus mutans biofilms, and cytotoxicity against human gingival fibroblasts and dental pulp stem cells were tested. Results: Nanocomposites with DMADDM and nCaF2 or NACP had flexural strengths matching commercial composite control without bioactivity. The new nanocomposite provided potent antibacterial properties, reducing biofilm CFU by 6 logs, and reducing lactic acid synthesis and metabolic function of biofilms by 90%, compared to controls (p < 0.05). The new nanocomposites produced excellent cell viability matching commercial control (p > 0.05). Conclusions: Bioactive L.S.S. antibacterial nanocomposites with nCaF2 and NACP had excellent bioactivity without compromising mechanical and cytocompatible properties. The new nanocomposites are promising for a wide range of dental restorations by improving marginal integrity by reducing shrinkage stress, defending tooth structures, and minimizing cariogenic biofilms.
1. Introduction
Methacrylate-derived composites are the predominant option to restore tooth structures [,]. Dental professionals prefer this material as it provides a range of advantages, including aesthetic appearance, resistance to wear, and capacity to adhere to both enamel and dentin [,]. However, composite restorations have a survival rate of 5 to 10 years, which is relatively low []. A significant factor in the low survival rate can be attributed to the occurrence of polymerization shrinkage stress, which may result in tooth fracture and recurrent caries [,]. Polymerization shrinkage stress occurs when monomer chains are polymerized during the polymerization process [,]. This leads to the composite material undergoing shrinkage. Consequently, this contributes to the development of residual shrinkage stresses, primarily arising from the bonding constraints at the interface between the restoration and the tooth [,,]. The stress resulting from the polymerization shrinkage could potentially cause detachment at the tooth–restoration interface, resulting in the formation of marginal gaps and micro-cracks [,]. The formation of marginal gaps can result in recurrent caries, which are more likely to happen if no bioactive material is used [,].
Several attempts have been employed to minimize shrinkage stress, and one of them involves utilizing resin systems with distinct polymerization characteristics, such as silorane resin-based [,], epoxy resin-based [], and step-growth thiolene resin-based []. In addition, the utilization of epoxy oligomers or polymeric nanogel fillers has demonstrated efficacy in mitigating shrinkage stress [,]. A novel low-shrinkage-stress (L.S.S.) resin has been recently developed, which is based on ether-based triethylene glycol divinylbenzyl ether (TEG-DVBE) and urethane dimethacrylate (UDMA) []. As a result of this formulation, the polymerization rate slowed down, giving the composite more time to achieve its stiffness stage []. By utilizing this method, stress relaxation was achieved, and the accumulation of excessive contraction stress was prevented []. In addition, UDMA enhanced the resistance of the composite to hydrolysis caused by saliva, whereas TEG-DVBE provided resistance to esterase degradation and other forms of hydrolytic challenges []. Another study involved the integration of this novel L.S.S. resin into a dental adhesive material. This particular adhesive exhibited reduced water sorption and solubility, as well as the formation of thicker hybrid layers and more resin tags in comparison to control adhesives [].
One way to improve the durability of resin-derived materials is by utilizing bioactive agents in their formulations [,]. Quaternary ammonium compounds have been widely used to reduce bacterial growth on treated surfaces such as medical devices and textiles [,,,]. Quaternary ammonium methacrylates (QAMs) are antibacterial monomers that have shown encouraging outcomes for potential clinical use, particularly in inhibiting dental biofilm [,,]. Monomers derived from QAMs, such as dimethylaminohexadecyl methacrylate (DMAHDM) and dimethylaminododecyl methacrylate (DMADDM), have demonstrated strong antibacterial effects against dental biofilm []. However, the high viscosity of DMAHDM may pose a challenge when attempting to mix it with other resin monomers. On the other hand, DMADDM is more easily mixed and also possesses contact-killing antimicrobial properties that can lead to bacterial death. Attempts have been made to incorporate DMADDM into dental materials, such as denture base materials, coating agents for dental implants, and resin-based dental composites to develop a bioactive dental material [,,]. A recent study has shown that adding 3% DMADDM to an L.S.S. composite resulted in a potent antibacterial activity against Streptococcus mutans (S. mutans) biofilms while retaining outstanding mechanical qualities, low polymerization stresses, and an outstanding degree of conversion [].
Remineralizing fillers, such as nanoparticles of calcium fluoride nanoparticles (nCaF2) or amorphous calcium phosphate (NACP), are another strategy for imparting bioactivity in resin-based materials. Composites containing these fillers may change the dynamic process of remineralization/demineralization toward remineralization by releasing high levels of remineralizing ions [,,]. nCaF2 promotes high fluoride (F) ion release, promoting remineralization and reducing bacterial acid production, while NACP has been demonstrated to protect enamel hardness and marginal dentin against demineralization [,,]. Such an approach has the potential to reduce demineralization and improve the durability of resin-based materials. A literature search, however, showed no reports on integrating nCaF2 or NACP with DMADDM into the L.S.S. nanocomposite.
Currently, there is a rising inclination towards the creation of dental restorations that have better durability. One way to achieve this is by developing bioactive nanocomposites with low-polymerization stress. This approach holds the potential to improve the marginal seal and inhibit the onset of secondary caries [,,]. Earlier research found that utilizing a composite with low polymerization shrinkage stress may improve sealing performance, perhaps preventing secondary caries formation [,]. Furthermore, the addition of DMADDM with resin-based materials has demonstrated remarkable antibiofilm activity while not adversely affecting the mechanical properties []. Another approach to inhibiting the progression of secondary caries is incorporating nCaF2 and NACP into resin-based materials, which have demonstrated promising effects in remineralizing tooth structure [,]. A previous investigation explored the incorporation of NACP with L.S.S. composite, which provides a high release of phosphate and calcium ions over 35 days []. However, to date, there has been no report on the antibacterial activity, mechanical properties, and cytocompatibility of L.S.S. nanocomposite with the incorporation of DMADDM with nCaF2 or NACP.
Consequently, the objective of this study was to explore how the incorporation of DMADDM with nCaF2 or NACP impacts the cytocompatibility, antibacterial properties, and mechanical properties of L.S.S. nanocomposites. The following hypotheses were tested. (1) The combination of DMADDM with nCaF2 or NACP would not impair the mechanical characteristics of the L.S.S. nanocomposite. (2) The novel nanocomposite with DMADDM and nCaF2 or NACP would greatly reduce the biofilm viability, lactic acid synthesis, and metabolic function. (3) The viability of human gingival fibroblast and dental pulp stem cells would not be negatively impacted by the new L.S.S. nanocomposites.
2. Materials and Methods
2.1. Synthesis of a Nanocomposites
The resin matrix for the L.S.S. nanocomposite, referred to as “UV” resin, was created using 55.8% UDMA and 44.2% of TEG-DVBE (all mass %), which was based on previous studies []. Photoinitiators consisting of 0.8% of 4-N, N-dimethylaminobenzoate (4EDMAB; Millipore Sigma) and 0.2% camphorquinone (CQ, Milli-pore Sigma, Burlington, MA, USA) were incorporated.
The Menschutkin reaction was utilized to synthesize DMADDM, which involved combining tertiary amines with organo-halides []. To produce DMADDM, a mixture of 10 mmol of 1-bromododecane (BDD) (TCI America, Portland, OR, USA), 10 mmol of 2-(dimethylamino) ethyl methacrylate (DMAEMA, Aldrich, St. Louis, MO, USA), and 3 g of ethanol as the solvent were stirred in a glass vial at 70 °C for 24 h. After the ethanol was evaporated, the resultant DMADDM was a crystal-clear viscous liquid. []. DMADDM was added to the UV resin at a final DMADDM mass fraction of 3%.
The nCaF2 and NACP were produced using a spray-drying process, as previously reported [,]. The nCaF2 mean particle size was approximately 32 nm, and the NACP mean particle size was about 116 nm [,]. The filler level of nCaF2 and NACP was 20% mass fraction in the final composite to preserve strong mechanical characteristics and release a high amount of F, Ca, and P ions for remineralization. Furthermore, at a mass fraction of 45%, silanized barium boroaluminosilicate glass particles (Dentsply Sirona, Milford, DE, USA) were utilized to provide mechanical reinforcement. The total filler level of 65% was chosen based on preliminary experiments to ensure good handling properties. In addition, Heliomolar (Ivoclar, Mississauga, ON, Canada) was selected as a commercial comparison control composite since it comprises 66.7 wt% nano-silica and ytterbium trifluoride and could release fluoride []. Six composites were evaluated:
- Heliomolar composite (designated as “Commercial Control composite”);
- Experimental composite: 35% UV + 65% glass (designated as “Experimental Control Composite”);
- 35% UV + 20% NACP + 45% glass (designated as “NACP Nanocomposite”);
- 32% UV + 3% DMADDM + 20% NACP + 45% glass (designated as “NACP+DMADDM Nanocomposite”);
- 35% UV + 20% nCaF2 + 45% glass (designated as “nCaF2 Nanocomposite”);
- 32% UV + 3% DMADDM + 20% nCaF2 + 45% glass (designated as “nCaF2+DMADDM Nanocomposite”).
2.2. Mechanical Properties
To test the mechanical properties of the composite, a stainless-steel mold with specific dimensions of 2 × 2 × 25 mm3 was utilized to create the specimens, which were covered with mylar strips on both sides [,]. The samples were cured for 1 min (1200 mW/cm2, Labolight, DUO, GC, Tokyo, Japan) []. A computer-controlled Universal Testing Machine (Insight 1, MTS, Cary, NC, USA) was used to conduct a three-point flexural test to measure the flexural strength and elastic modulus (n = 6), following previous studies [,].
2.3. Samples Preparation for S. mutans Biofilm Testing
Disk-shaped composites were created with a diameter of 9 mm and a thickness of 2 mm. The surfaces of each sample were cured for 60 s (Labolight DUO, GC America, Alsip, IL, USA) and incubated in a 37 °C incubator for 24 h []. Next, the samples were placed in distilled water and agitated at 100 rpm for 1 h to eliminate any remaining uncured monomer []. The composite disks (n = 6) for each group were sterilized using ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC, USA). To ensure the complete release of entrapped ethylene oxide, the samples were degassed for seven days, following the manufacturer’s guidelines.
2.4. Inoculation of S. mutans and Biofilm Formation
Approval was obtained from the University of Maryland Baltimore Institutional Review Board to use bacterial species in the study. S. mutans (UA159) was selected as it is one of the organisms commonly associated with dental caries []. S. mutans was cultured overnight (16–18 h) in brain heart infusion (BHI) broth (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C with 5% CO2 for all biofilm assays [,]. To adjust the inoculum to 107 colony-forming unit counts CFU/mL, based on the standard curve of OD600nm versus the CFU/mL graph, a spectrophotometer (Genesys 10S, Thermo Scientific, Waltham, MA, USA) was used []. The composite samples were placed in the well of 24-well plates, immersed with a 1.5 mL BHI culture medium with 2% sucrose (wt/vol), and incubated for 24 h. The composite samples were then transferred to 24-well plates, immersed with 1.5 mL of fresh medium with sucrose, and incubated for another 24 h. According to a previous study, incubation for 48 h was sufficient to form relatively mature biofilms on composite disks [].
2.5. Examining S. mutans Biofilms Using Scanning Electron Microscopy (SEM)
At 48 h, biofilms on composites were washed with phosphate-buffered saline (PBS) and treated with 1% glutaraldehyde at 4 °C overnight. Subsequently, the composite samples were rinsed with PBS and dehydrated using a series of ethanol solutions. Next, the samples were rinsed with hexamethyldisilazane and allowed to dry overnight. The surface of the samples was sputter-coated with platinum. SEM (Quanta 200, FEI Company, Hillsboro, OR, USA) was utilized to visualize biofilms on composites [].
2.6. S. mutans Biofilm Colony-Forming Units (CFU)
The composite samples containing 48-h biofilms were moved to a fresh plate containing PBS, and the biofilms were harvested by scraping and sonicating/vortexing (FS-30, Fisher, Pittsburg, PA, USA) []. The bacterial suspensions were diluted serially (101–106-fold) and spread onto BHI agar plates, which were then incubated for 48 h at 37 °C with 5% CO2. The colony number was counted, and the biofilm (CFU) was determined []. The CFU experiment was performed in triplicate.
2.7. Metabolic Activity of S. mutans Biofilms
Biofilm metabolic activity was assessed using a colorimetric assay involving 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) []. The composite samples with biofilms were placed in a fresh 24-well plate and treated with 1 mL of MTT dye (0.5 mg/mL MTT in PBS). The plate was then incubated at 37 °C in a 5% CO2 environment. After 1 h, the samples were transferred to another plate containing 1 mL of dimethyl sulfoxide (DMSO) and kept in the dark at room temperature for 20 min to dissolve the formazan crystals []. To determine the absorbance, 200 μL of the DMSO solution was collected from each specimen and transferred to a 96-well plate. The absorbance was measured at 540 nm using a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA). A higher absorbance indicates a higher concentration of formazan, indicating increased metabolic activity of the biofilm on the disk []. The metabolic activity experiment was performed in triplicate.
2.8. Lactic Acid Production by S. mutans Biofilms
The composites with biofilms at 48 h were moved to 24-well plates containing buffered peptone water (BPW, Aldrich, St. Louis, MO, USA) with 0.2% sucrose and incubated for 3 h at 37 °C in 5% CO2 []. To determine lactate concentrations in BPW, the optical density was measured at 340 nm using a microplate reader (Spectra-Max M5) via lactate dehydrogenase enzymatic assay, following previously described methods []. The lactic acid production experiment was performed in triplicate.
2.9. Cytotoxicity of Human Gingival Fibroblasts and Dental Pulp Stem Cells
Human gingival fibroblast (HGF, P10866, Innoprot, Bizkaia, Spain) and dental pulp stem cells (DPSC, PT-5025, Lonza, Basel, Switzerland) were utilized to evaluate cytotoxicity. The fibroblast medium (Sciencell Research Laboratories, Carlsbad, CA, USA), consisting of 1 wt.% fibroblast growth supplement, 2 wt.% fetal bovine serum, 100 IU/mL streptomycin, and 100 IU/mL penicillin, was used to culture HGF (passage 7) []. DPSCs (passage 6) were cultured in DPSC basal medium (DPSC, PT-5025, Lonza, Basel, Switzerland) supplemented with 2 mM L-glutamine, 2 wt.% fetal bovine serum, 100 mM ascorbic acid solution, GA-1000, 100 IU/mL streptomycin, and 100 IU/mL penicillin []. Cell seeding was performed when the viability of the cells had exceeded 90%.
In a 96-well plate with the corresponding medium, cells were seeded at a density of 5000 cells per well and incubated for 24 h at 37 °C with 5% CO2 [,,]. Disk-shaped composite samples (n = 3, diameter of 4 mm, and thickness of 1 mm) were sterilized in the same manner as previously described for antibacterial testing. After that, the samples were put in 4 mL of medium and left at 37 °C for 24 h, yielding a surface area to solution ratio of 0.63 cm2/mL, which falls within the required range of 0.5–6 cm2/mL as per ISO 10993-12:2021 []. Next, 100 mL of each sample’s original extracts were added to cultured cells and incubated for 24 h at 37 °C with 5% CO2. After incubation, each well received 10 mL of cell counting kit-8 (CCK-8, Dojindo, Rockville, MD, USA) and was incubated for 2 h under the same conditions []. The absorbance at 450 nm was used to evaluate the degree of cellular dehydrogenase activity in the culture medium, while the control group consisted of cells cultured in media alone []. The cell viability experiment was performed in triplicate.
2.10. Statistics
The statistical analyses, including normality verification and power analysis, were performed using Sigma Plot (SYSTAT, Chicago, IL, USA). One-way analyses of variance (ANOVA) and Tukey’s comparison tests were performed to determine the significant differences between groups in load-bearing properties, antibacterial effects, and cell viability. The data were deemed statistically significant at a p-value of below 0.05.
3. Results
3.1. Mechanical Properties
Figure 1 depicts the flexural strengths and modulus of elasticity of the composites (mean ± sd; n = 6): (A) flexural strength and (B) elastic modulus. The incorporation of nCaF2 and NACP with and without DMADDM in the L.S.S. nanocomposite resulted in flexural strength values comparable to the commercial control composite (p > 0.05). These outcomes demonstrate that our novel formulations did not affect flexural strength.
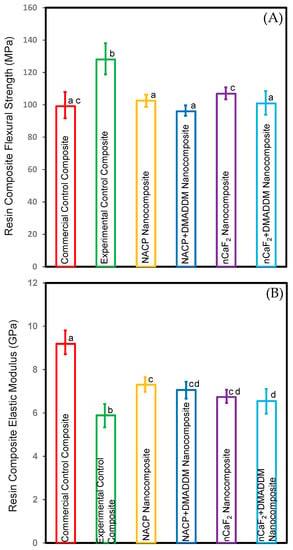
Figure 1.
Composite mechanical properties: (A) flexural strength, and (B) elastic modulus (mean ± sd; n = 6). Incorporating DMADDM with nCaF2 or NACP into an L.S.S. nanocomposite did not compromise the flexural strength compared to commercial control composite (p > 0.05). Incorporating nCaF2 or NACP with DMADDM did not compromise the elastic modulus compared to the experimental control composite (p < 0.05). Dissimilar letters represent values that are significantly different from each other (p < 0.05).
The commercial control composite had much higher elastic modulus values than all other testing groups (p < 0.05). However, these results demonstrated that incorporating nCaF2 and NACP with and without DMADDM into the L.S.S. nanocomposite increased the elastic modulus compared to the experimental control composite (p < 0.05).
3.2. Examination of S. mutans Biofilms Using SEM
Figure 2 displays the SEM findings after 48 h of S. mutans biofilms (n = 6). The nCaF2 nanocomposite, NACP nanocomposite, and control composite groups demonstrated significant biofilm formation after 48 h. Conversely, our result showed that incorporating DMADDM with either nCaF2 or NACP into the L.S.S. resin matrix resulted in minimal bacterial biofilm formation.
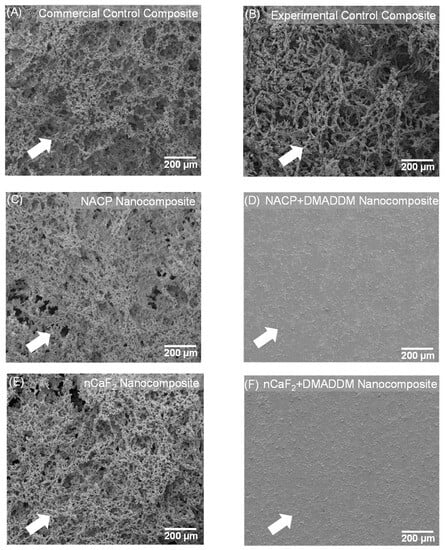
Figure 2.
SEM examination of biofilms on composites at 2 days (n = 6). (A) Commercial control composite. (B) Experimental control composite. (C) NACP nanocomposite. (D) NACP+DMADDM nanocomposite. (E) nCaF2 nanocomposite. (F) nCaF2+DMADDM nanocomposite. DMADDM greatly reduced S. mutans biofilm growth on composites. In comparison, NACP nanocomposite, nCaF2 nanocomposite, and control composite groups were covered with biofilms.
3.3. S. mutans Biofilm CFU
Figure 3 depicts the CFU findings of the S. mutans biofilm (mean ± sd; n = 6). Incorporating nCaF2 and NACP into an L.S.S. nanocomposite showed no antibacterial effect without incorporating DMADDM. However, our results demonstrated that incorporating DMADDM with either nCaF2 or NACP into an L.S.S. nanocomposite significantly decreased the CFU count for S. mutans biofilm by 6 logs when compared to other groups (p < 0.05).
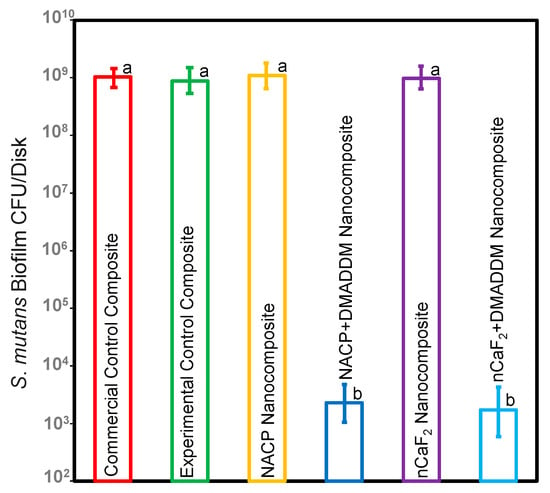
Figure 3.
CFU of S. mutans biofilms on composites (mean ± sd; n = 6). The nCaF2 nanocomposite, NACP nanocomposite, and control composite had the highest biofilm growth. DMADDM inhibited S. mutans biofilm growth by 6 logs compared to commercial control composite (p < 0.05). Dissimilar letters represent values that are significantly different from each other (p < 0.05).
3.4. Metabolic Function of S. mutans Biofilms
Figure 4 depicts the bacterial metabolic function of biofilms on composite samples after two days (mean ± sd; n = 6). Integrating DMADDM with either nCaF2 or NACP into an L.S.S. nanocomposite considerably lowered metabolic activities by roughly 90% when compared to other groups (p < 0.05). However, there was no statistically significant variation in metabolic activity between these groups (p > 0.05).
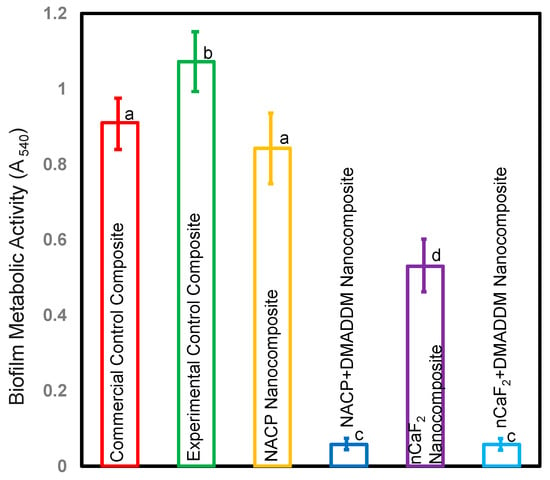
Figure 4.
Metabolic activity of biofilms on composites after two days (mean ± sd; n = 6). Incorporating DMADDM with either nCaF2 or NACP into an L.S.S. nanocomposite diminished the metabolic function of S. mutans by over 90% compared with other groups (p < 0.05). Different letters represent data that are significantly different from each other (p < 0.05).
3.5. Production of Lactic Acid by S. mutans Biofilms
Figure 5 depicts the lactic acid production of S. mutans biofilms adhered to composites (mean ± sd; n = 6). The experimental control composite, commercial control composite, and NACP nanocomposite groups had the highest acid production (p < 0.5). Adding nCaF2 reduced acid generation compared to the commercial control composite (p < 0.5). Nevertheless, adding DMADDM into an L.S.S. nanocomposite containing either nCaF2 or NACP dramatically decreased lactic acid generation by more than 92% when compared to other groups (p < 0.5). Our results revealed that the incorporation of DMADDM demonstrated the highest biofilm reduction.
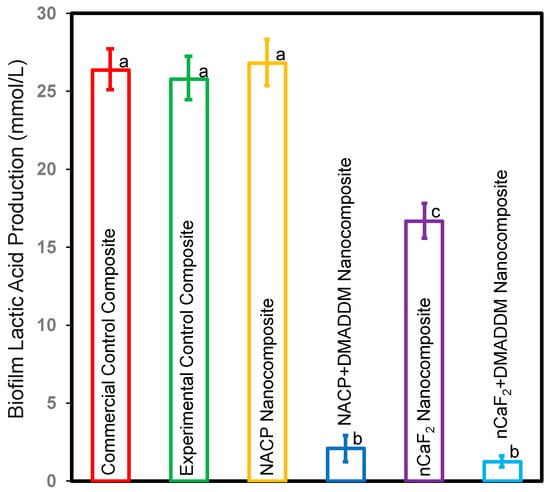
Figure 5.
Lactic acid production of S. mutans biofilms on composites (mean ± sd; n = 6). The NACP nanocomposite and control composite groups had the highest lactic acid production (p < 0.05). The nCaF2 nanocomposite reduced lactic acid production compared to control groups (p < 0.05). However, incorporating DMADDM diminished lactic acid production by over 92% compared to other groups (p < 0.05). Different letters represent data that are significantly different from each other (p < 0.05).
3.6. Cytotoxicity Test
The viability toward the newly developed L.S.S. nanocomposite: (A) HGF, (B) DPSCs (mean ± sd; 3 experimental repeats, each with n = 3 technical replicates) plots in Figure 6. The NACP nanocomposite group demonstrated significantly increased HGF and DPSCs cell viability compared to the commercial control composite (p < 0.5). However, all other novel nanocomposite groups showed comparable cell viability to the commercial control composite (p > 0.5).
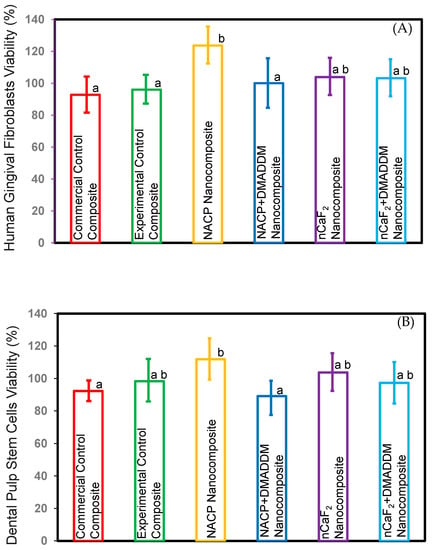
Figure 6.
Cell viability of composites. (A) HGF, (B) DPSCs (mean ± sd; n = 3 × 3). These results showed that the NACP nanocomposite had a significant increase in viability compared to the commercial control composite (p < 0.05). All the other novel nanocomposite groups showed comparable viability to commercial control composite (p > 0.05). Different letters represent data that are significantly different from each other (p < 0.05).
4. Discussion
For the first time, the present work explored the effect of incorporating nCaF2 and NACP with and without DMADDM into an L.S.S. nanocomposite. Incorporating nCaF2, NACP, and DMADDM into an L.S.S. nanocomposite may potentially decrease polymerization shrinkage stress, marginal leakage, and secondary caries [,]. As a result, the clinical durability of composite restorations could be improved []. In the current study, we studied the antibacterial property and cytocompatibility of a novel L.S.S. nanocomposite, incorporating 20% nCaF2 or 20% NACP, with and without 3% DMADDM. The capability of L.S.S. nanocomposite to suppress S. mutans biofilm without negatively influencing mechanical characteristics and cytocompatibility was achieved, and the study hypotheses were proved. Incorporating DMADDM with nCaF2 or NACP into the L.S.S. nanocomposite resulted in a significant reduction of S. mutans biofilm growth compared to the control groups. This reduction in biofilm growth was observed while maintaining mechanical properties that aligned with clinical standards. Furthermore, the addition of DMADDM with nCaF2 or NACP was associated with a decrease in metabolic and lactic acid production activity of S. mutans biofilm, suggesting potential for preventing secondary caries.
Several attempts have been made to develop a low-shrinkage composite, such as the use of epoxy oligomers fillers [], polymeric nanogel fillers [], the epoxy resin-base [], silorane resin-base [], and step-growth thiolene resin-base []. However, these approaches negatively affect the mechanical properties without improving the margin integrity []. Recently, UDMA and TEGDVBE monomers were combined to create a composite with minimal shrinkage stress []. This composite demonstrated low polymerization stress while maintaining a high degree of conversion and excellent mechanical properties []. In addition, several attempts have been made to create a bioactive L.S.S. composite by adding DMAHDM to the L.S.S. resin. However, the lengthy chain of DMAHDM can elevate the viscosity of the resin, which can decrease the degree of conversion and filler load that can be incorporated into the composite []. Moreover, the antibacterial outcomes of adding DMAHDM to the resin composite may not be consistent due to the molecule’s long chain length. This extended chain may decrease the charge density of quaternary ammonium at the surface, which could impact the composite’s antibacterial properties []. Recently, a bioactive L.S.S. incorporating DMADDM demonstrated a substantial reduction of 46% in polymerization shrinkage stresses in comparison to a conventional resin-based control composite []. In addition, the incorporation of 3% DMADDM exhibits potent antibacterial properties without negatively impacting the degree of polymerization conversion and mechanical properties []. However, no report has investigated the effect of incorporating nCaF2 or NACP with DMADDM into an L.S.S. nanocomposite.
One of the strategies to impart bioactivity in resin-based materials is the incorporation of remineralizing fillers in the composite system [,,]. Nanocomposites containing nCaF2 or NACP can alter the dynamic process of remineralization/demineralization in favor of remineralization [,]. This is achieved by releasing a significant amount of remineralizing ions [,]. The current research involved the incorporation of nCaF2 and NACP particles into an antibacterial nanocomposite that has low polymerization stress. The purpose of this was to produce a smart material that effectively interacts with the oral environment. Composites containing nCaF2 and NACP could potentially help to raise the pH and modify the microenvironment around the dental plaque []. Furthermore, incorporating nCaF2 and NACP into the composite may provide another potential benefit: the ability to recharge with Ca, P, and F ions, resulting in an extended release of these ions over time [,]. In addition, the incorporation of NACP into an L.S.S. nanocomposite demonstrated good mechanical properties even after 20,000 thermal cycles []. Another study found that incorporating 20% nCaF2 into the composite demonstrated excellent mechanical properties even after 105 thermal cycles []. The current study was designed to explore the antibacterial property and cytocompatibility of an L.S.S. nanocomposite containing nCaF2 or NACP with or without DMADDM. Incorporating nCaF2 and NACP with and without DMADDM achieved comparable flexural strength to commercial control composite (p > 0.05). Conversely, the newly developed L.S.S. composite exhibited a lower modulus of elasticity when compared to the commercial control composite (p < 0.05). We hypothesize that one possible reason for this difference is the variation in the size and type of the filler particles used in these composites []. Additionally, it is possible that the different resin matrices used in the commercial control composite could also contribute to the enhancement of its elastic modulus [,]. In previous studies, the addition of 15% nCaF2 to a conventional composite did not compromise the mechanical characteristics [,]. However, in the present study, there was a slight decrease in flexural strength at 20% nCaF2 in the L.S.S. nanocomposite when compared to the experimental control. This difference was likely due to the relatively lower concentration of silane-treated barium boroaluminosilicate glass particles for reinforcement in the current study, which could decrease the mechanical properties.
Dental plaque may have a negative impact on the durability of dental restorations through the development of recurrent caries and the production of enzymes that could cause material degradation [,,]. Therefore, developing a novel bioactive material that possesses remineralization and antibacterial properties, as well as the ability to reduce polymerization stress, could increase the clinical longevity of the composite restoration. In an earlier investigation, chlorhexidine was introduced to a composite with a low-shrinkage-stress resin system to develop an antibacterial effect []. However, they found it has a short-time effect []. Another effort was made to use zinc oxide and silver nanoparticles as antibacterial additives in resin-based materials [,,]. However, these nanoparticles were observed to detach from the surface of the material relatively quickly, which resulted in increased porosity and a decrease in mechanical strength [,]. To address this significant issue, a novel approach was developed, which involved the use of QAMs [,,]. These compounds could be covalently bonded with dental resins through copolymerization, resulting in long-lasting antibacterial effects []. The addition of QAMs in resin-based materials exhibited a strong antibacterial impact by employing a contact-killing mechanism []. QAMs have a positively charged quaternary amine N+ that can attach to the negatively charged membrane of bacterial cells []. This alters the balance of essential ions such as Na+, Ca2+, Mg2+, and K+ and results in leakage from the cytoplasm, which ultimately causes the bacterial membrane to break down []. Earlier research studies have indicated that the antibacterial efficacy of QAMs can be enhanced by increasing their chain length, as this increases their hydrophobicity, thereby enabling them to penetrate the hydrophobic bacterial cell membrane more effectively [,]. Furthermore, a previous study showed that a resin containing DMADDM maintained strong antibacterial effects even after six months of water aging while still possessing good mechanical properties []. The current study thoroughly investigated the effects of incorporating nCaF2 or NACP with or without DMADDM into an L.S.S. nanocomposite. Incorporating DMADDM with nCaF2 or NACP into an L.S.S. nanocomposite demonstrated significant antibacterial activity against S. mutans biofilm. This formulation significantly diminished the CFU count by 6 logs compared to the commercial control composite (p < 0.05). Furthermore, our findings demonstrated that the addition of DMADDM in combination with nCaF2 or NACP resulted in a reduction of over 85% in metabolic activities and lactic acid production compared to the commercial control composite. Moreover, SEM images validate the existence of S. mutans biofilms on the surfaces of nCaF2 nanocomposite, NACP nanocomposite, and control composite groups. Conversely, the incorporation of DMADDM along with nCaF2 or NACP resulted in a decrease in the attachment of bacteria to the surface. Nevertheless, our findings indicated that there was no significant difference in antibacterial impact when integrating DMADDM to either nCaF2 or NACP against S. mutans biofilms (p > 0.05). This formulation could be beneficial in various applications due to its L.S.S. and excellent bioactivity. For example, it could serve as a base for bulk dental restorations, as a composite restoration in Class V cases, and as a flowable composite for pits and fissures, etc. Additionally, its antibacterial properties could aid in reducing plaque accumulation, especially in patients with poor oral hygiene, potentially leading to a decrease in recurrent caries and gingival inflammation [,].
Despite the potential of the novel L.S.S. nanocomposite demonstrated in this study, it is crucial for the material to be non-toxic to human cells. This research represents the first investigation interested in the cytotoxic properties of this particular L.S.S. nanocomposite with antibacterial and remineralizing capabilities. This was achieved by utilizing the extract obtained directly from the cured disks through immersion in fibroblast medium and DPSC basal medium at 37 °C for 24 h. All the evaluated groups were classified as non-toxic in line with the recommendations of the International Organization for Standardization (ISO), as their cell viability measurements exceeded 75% []. As per the present study, it was found that the nCaF2 and NACP groups exhibited an improvement in cell viability when compared to the commercial control composite. The results from the present study are consistent with previous research that has shown that using calcium phosphate cement (CPC) for direct pulp capping may facilitate the differentiation of human dental pulp cells (HDPCs) into odontoblasts []. In addition, a previous study found that the release of calcium ions is considered crucial in the process of mineralization as it helps promote cellular migration and differentiation []. Furthermore, another study found that the use of nanocomposites and adhesives containing NACP and DMADDM resulted in lower levels of inflammatory response and increased tertiary dentin formation in a rat tooth cavity model []. These results suggest that using nCaF2, NACP, and DMADDM in restorative materials may have promising clinical applications in promoting pulp healing and tissue regeneration. The L.S.S. nanocomposite demonstrated strong antibacterial properties while maintaining excellent biocompatibility against HGF and DPSCs. However, further studies are needed to investigate the biocompatibility of this novel nanocomposite in vivo. These studies could provide valuable insights into the clinical performance of the composite and its potential use in restorative dentistry.
Integrating DMADDM with nCaF2 or NACP into the L.S.S. nanocomposite resulted in potent antibacterial characteristics without negatively impacting the load-bearing properties. The addition of DMADDM decreased biofilm CFU counts by 6 orders of magnitude compared to commercial composite. Furthermore, the new formulations showed excellent cytocompatibility. Therefore, developing an L.S.S. nanocomposite with antibacterial and remineralization properties can potentially address some of the major challenges associated with traditional composite restorations, such as secondary caries and restoration failure. Incorporating antibacterial agents together with remineralizing agents into an L.S.S. nanocomposite could reduce microleakage, inhibit bacterial growth, and promote remineralization of the tooth structure. This can lead to improved clinical outcomes and increased longevity of the restoration.
Additional research is required to address several limitations of the current study, including investigating the antibacterial effectiveness of the novel L.S.S. nanocomposite against multispecies biofilms that more closely resemble clinical conditions. Moreover, long-term investigations are still needed to assess the impact of incorporating a combination of nCaF2, NACP, and DMADDM into a single formulation on the antibacterial and mechanical properties of the L.S.S. nanocomposite. Furthermore, research is also needed to study the marginal seal of this new L.S.S. nanocomposite as compared to commercial composites []. Further studies are also needed to investigate the effects of integrating DMADDM with nCaF2 or NACP into the L.S.S. nanocomposite on the composite surface properties [] and color stability [].
5. Conclusions
This study developed a novel L.S.S. nanocomposite with antibacterial and remineralization capabilities. Incorporating DMADDM provided a potent antibacterial impact while maintaining mechanical properties. The new formulation achieved a reduction of 6-log in biofilm CFU, along with a significant decrease in biofilm lactic acid production and metabolic activity. In addition, the L.S.S. nanocomposite demonstrated excellent biocompatibility against HGF and DPSCs. The new bioactive L.S.S. nanocomposite has promise in various dental restorations to improve marginal integrity by reducing shrinkage stress, protecting tooth structures, and reducing cariogenic biofilms.
Author Contributions
A.A. contributed to data curation, investigation, writing—original draft; R.A. contributed to data curation, writing—original draft; A.A.B. contributed to data curation; L.M. contributed to data curation; A.S. contributed to visualization, resources; M.-A.J.-R. contributed to visualization, resources; R.M. contributed to visualization, resources; G.D.H. contributed to data curation, visualization; T.W.O. contributed to visualization, resources; J.S. contributed to supervision, visualization, resources; M.D.W. contributed to supervision, resources, data curation, formal analysis, project administration, writing—review, and editing; H.H.K.X. contributed to supervision, methodology, project administration, funding acquisition, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Maryland School of Dentistry bridge fund (HX) and the University of Maryland seed grant (HX).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Dentsply Sirona (Milford, DE) for the donation of the glass fillers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferracane, J.L. Resin Composite—State of the Art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.S.; Mai, Y.; Kim, H.; Tong, K.C.T.; Ng, D.; Hsiao, J.C.M. Review: Resin Composite Filling. Materials 2010, 3, 1228–1243. [Google Scholar] [CrossRef]
- Demarco, F.F.; Corrêa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J.M. Longevity of Posterior Composite Restorations: Not Only a Matter of Materials. Dent. Mater. 2012, 28, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors Involved in the Development of Polymerization Shrinkage Stress in Resin-Composites: A Systematic Review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial Activity and Bonding Characteristics of an Adhesive Resin Containing Antibacterial Monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.d.P.; Vilela, A.B.F.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization Shrinkage Stress of Composite Resins and Resin Cements—What Do We Need to Know? Braz. Oral. Res. 2017, 31, e62. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization Shrinkage and Polymerization Shrinkage Stress in Polymer-Based Restoratives. J. Dent. 1997, 25, 435–440. [Google Scholar] [CrossRef]
- Pereira, R.D.; Valdívia, A.D.C.M.; Bicalho, A.A.; Franco, S.D.; Tantbirojn, D.; Versluis, A.; Soares, C.J. Effect of Photoactivation Timing on the Mechanical Properties of Resin Cements and Bond Strength of Fiberglass Post to Root Dentin. Oper. Dent. 2015, 40, E206–E221. [Google Scholar] [CrossRef]
- Bicalho, A.; Pereira, R.; Zanatta, R.; Franco, S.; Tantbirojn, D.; Versluis, A.; Soares, C. Incremental Filling Technique and Composite Material—Part I: Cuspal Deformation, Bond Strength, and Physical Properties. Oper. Dent. 2014, 39, e71–e82. [Google Scholar] [CrossRef]
- Bicalho, A.; Valdívia, A.; Barreto, B.; Tantbirojn, D.; Versluis, A.; Soares, C. Incremental Filling Technique and Composite Material—Part II: Shrinkage and Shrinkage Stresses. Oper. Dent. 2014, 39, e83–e92. [Google Scholar] [CrossRef]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization Shrinkage Stress of Resin-Based Dental Materials: A Systematic Review and Meta-Analyses of Composition Strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Abdalla, M.A.; Heck, K.; Draenert, M.; Kaisarly, D. Current Strategies to Control Recurrent and Residual Caries with Resin Composite Restorations: Operator- and Material-Related Factors. J. Clin. Med. 2022, 11, 6591. [Google Scholar] [CrossRef]
- Chisini, L.A.; Collares, K.; Cademartori, M.G.; de Oliveira, L.J.C.; Conde, M.C.M.; Demarco, F.F.; Corrêa, M.B. Restorations in Primary Teeth: A Systematic Review on Survival and Reasons for Failures. Int. J. Paediatr. Dent. 2018, 28, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Arrais, C.A.G.; de Oliveira, M.T.; Mettenburg, D.; Rueggeberg, F.A.; Giannini, M. Silorane- and High Filled-Based “Low-Shrinkage” Resin Composites: Shrinkage, Flexural Strength and Modulus. Braz. Oral. Res. 2013, 27, 97–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maghaireh, G.A.; Taha, N.A.; Alzraikat, H. The Silorane-Based Resin Composites: A Review. Oper. Dent. 2017, 42, E24–E34. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Chen, R.-S.; Chang, Y.-L.; Chen, M.-H.; Cheng, K.-C.; Su, W.-F. Biphenyl Liquid Crystalline Epoxy Resin as a Low-Shrinkage Resin-Based Dental Restorative Nanocomposite. Acta Biomater. 2012, 8, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Investigations of Step-Growth Thiol-Ene Polymerizations for Novel Dental Restoratives. Dent. Mater. 2005, 21, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.R.; Garcia, J.W.; Barros, M.D.; Lewis, S.H.; Pfeifer, C.S.; Liu, J.; Stansbury, J.W. Control of Polymerization Shrinkage and Stress in Nanogel-Modified Monomer and Composite Materials. Dent. Mater. 2011, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Stansbury, J.W. Photopolymerization Shrinkage-Stress Reduction in Polymer-Based Dental Restoratives by Surface Modification of Fillers. Dent. Mater. 2021, 37, 578–587. [Google Scholar] [CrossRef]
- Yamauchi, S.; Wang, X.; Egusa, H.; Sun, J. High-Performance Dental Adhesives Containing an Ether-Based Monomer. J. Dent. Res. 2020, 99, 189–195. [Google Scholar] [CrossRef]
- Han, Q.; Li, B.; Zhou, X.; Ge, Y.; Wang, S.; Li, M.; Ren, B.; Wang, H.; Zhang, K.; Xu, H.H.K.; et al. Anti-Caries Effects of Dental Adhesives Containing Quaternary Ammonium Methacrylates with Different Chain Lengths. Materials 2017, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Burrow, M.F.; Matinlinna, J.P.; Wang, Y.; Tsoi, J.K.H. A Narrative Review of Bioactive Glass-Loaded Dental Resin Composites. J. Funct. Biomater. 2022, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, Ester-Free Monomers: Polymerization Kinetics, Mechanical Properties, Biocompatibility and Anti-Biofilm Activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles—A Review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial Activity of Dental Composites Containing Quaternary Ammonium Polyethylenimine Nanoparticles against Streptococcus Mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of Resin Composites and Adhesives by Oral Bacteria and Saliva: A Rationale for New Material Designs That Consider the Clinical Environment and Treatment Challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef]
- Song, B.K.; Cho, M.S.; Yoon, K.J.; Lee, D.C. Dispersion Polymerization of Acrylamide with Quaternary Ammonium Cationic Comonomer in Aqueous Solution. J. Appl. Polym. Sci. 2003, 87, 1101–1108. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacteria and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef]
- Li, F.; Wang, P.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Evaluation of Antibacterial and Remineralizing Nanocomposite and Adhesive in Rat Tooth Cavity Model. Acta Biomater. 2014, 10, 2804–2813. [Google Scholar] [CrossRef]
- Alhussein, A.; Alsahafi, R.; Wang, X.; Mitwalli, H.; Filemban, H.; Hack, G.D.; Oates, T.W.; Sun, J.; Weir, M.D.; Xu, H.H.K. Novel Dental Low-Shrinkage-Stress Composite with Antibacterial Dimethylaminododecyl Methacrylate Monomer. J. Funct. Biomater. 2023, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.D.; Moreau, J.L.; Levine, E.D.; Strassler, H.E.; Chow, L.C.; Xu, H.H.K. Nanocomposite Containing CaF(2) Nanoparticles: Thermal Cycling, Wear and Long-Term Water-Aging. Dent. Mater. 2012, 28, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Bhadila, G.; Wang, X.; Zhou, W.; Menon, D.; Melo, M.A.S.; Montaner, S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel Low-Shrinkage-Stress Nanocomposite with Remineralization and Antibacterial Abilities to Protect Marginal Enamel under Biofilm. J. Dent. 2020, 99, 103406. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, H.; Luo, T.; Hua, F.; He, H. Application of Amorphous Calcium Phosphate Agents in the Prevention and Treatment of Enamel Demineralization. Front. Bioeng. Biotechnol. 2022, 10, 853436. [Google Scholar] [CrossRef]
- Aoba, T. The Effect of Fluoride on Apatite Structure and Growth. Crit. Rev. Oral. Biol. Med. 1997, 8, 136–153. [Google Scholar] [CrossRef]
- Ferracane, J.L. Developing a More Complete Understanding of Stresses Produced in Dental Composites during Polymerization. Dent. Mater. 2005, 21, 36–42. [Google Scholar] [CrossRef]
- Malhotra, N.; Kundabala, M.; Shashirashmi, A. Strategies to Overcome Polymerization Shrinkage—Materials and Techniques. A Review. Dent. Update 2010, 37, 115–118, 120–122, 124–125. [Google Scholar] [CrossRef]
- Nanjundasetty, J.K.; Nanda, S.; Panuganti, V.; Marigowda, J.C. Marginal Sealing Ability of Silorane and Methacrylate Resin Composites in Class II Cavities: A Scanning Electron Microscopic Study. J. Conserv. Dent. 2013, 16, 503–508. [Google Scholar] [CrossRef]
- Clarin, A.; Ho, D.; Soong, J.; Looi, C.; Ipe, D.S.; Tadakamadla, S.K. The Antibacterial and Remineralizing Effects of Biomaterials Combined with DMAHDM Nanocomposite: A Systematic Review. Materials 2021, 14, 1688. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and Characterization of Dimethacrylates Containing Quaternary Ammonium Functionalities for Dental Applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.; Roh, B.-D.; Park, S.-H.; Park, J.-W. Inhibition of Streptococcus Mutans Biofilm Formation on Composite Resins Containing Ursolic Acid. Restor. Dent. Endod. 2013, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.; Horati, P.; Naik, B. Evaluation and Comparison of Flexural Strength of Cention N with Resin-Modified Glass-Ionomer Cement and Composite—An in Vitro Study. J. Conserv. Dent. 2022, 25, 288–291. [Google Scholar]
- Liu, C.; Niu, Y.; Zhou, X.; Zhang, K.; Cheng, L.; Li, M.; Li, Y.; Wang, R.; Yang, Y.; Xu, X. Hyperosmotic Response of Streptococcus Mutans: From Microscopic Physiology to Transcriptomic Profile. BMC Microbiol. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.-H.; Xing, X.-D.; Li, F.; Ma, S.; Qi, L.-L.; Chen, J.-H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral. Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef]
- Kadar, K.; Kiraly, M.; Porcsalmy, B.; Molnar, B.; Racz, G.Z.; Blazsek, J.; Kallo, K.; Szabo, E.L.; Gera, I.; Gerber, G.; et al. Differentiation Potential of Stem Cells from Human Dental Origin-Promise for Tissue Engineering. J. Physiol. Pharmacol. 2009, 60 (Suppl. 7), 167–175. [Google Scholar] [PubMed]
- Beltrami, R.; Colombo, M.; Rizzo, K.; Di Cristofaro, A.; Poggio, C.; Pietrocola, G. Cytotoxicity of Different Composite Resins on Human Gingival Fibroblast Cell Lines. Biomimetics 2021, 6, 26. [Google Scholar] [CrossRef]
- ISO Standard 10993-12; Biological Evaluation of Medical Devices: Sample Preparation and Reference Materials. International Organization for Standardization: Geneva, Switzerland, 2007.
- Schubert, A.; Ziegler, C.; Bernhard, A.; Bürgers, R.; Miosge, N. Cytotoxic Effects to Mouse and Human Gingival Fibroblasts of a Nanohybrid Ormocer versus Dimethacrylate-Based Composites. Clin. Oral. Investig. 2019, 23, 133–139. [Google Scholar] [CrossRef]
- Braga, R.R.; Ferracane, J.L. Alternatives in Polymerization Contraction Stress Management. Crit. Rev. Oral. Biol. Med. 2004, 15, 176–184. [Google Scholar] [CrossRef]
- Duarte de Oliveira, F.J.; Ferreira da Silva Filho, P.S.; Fernandes Costa, M.J.; Rabelo Caldas, M.R.G.; Dutra Borges, B.C.; Gadelha de Araújo, D.F. A Comprehensive Review of the Antibacterial Activity of Dimethylaminohexadecyl Methacrylate (DMAHDM) and Its Influence on Mechanical Properties of Resin-Based Dental Materials. Jpn. Dent. Sci. Rev. 2021, 57, 60–70. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional Fillers for Dental Resin Composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of Filler Characteristics on the Performance of Dental Composites: A Comprehensive Review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive Glass for Dentin Remineralization: A Systematic Review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Namba, N.; Yoshida, Y.; Nagaoka, N.; Takashima, S.; Matsuura-Yoshimoto, K.; Maeda, H.; Van Meerbeek, B.; Suzuki, K.; Takashiba, S. Antibacterial Effect of Bactericide Immobilized in Resin Matrix. Dent. Mater. 2009, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- AlSahafi, R.; Mitwalli, H.; Alhussein, A.; Balhaddad, A.A.; Alquria, T.A.; Melo, M.A.S.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Xu, H.H.K.; et al. Novel Rechargeable Nano-Calcium Phosphate and Nano-Calcium Fluoride Resin Cements. J. Dent. 2022, 126, 104312. [Google Scholar] [CrossRef] [PubMed]
- Filemban, H.; Bhadila, G.; Wang, X.; Melo, M.A.S.; Oates, T.W.; Hack, G.D.; Lynch, C.D.; Weir, M.D.; Sun, J.; Xu, H.H.K. Effects of Thermal Cycling on Mechanical and Antibacterial Durability of Bioactive Low-Shrinkage-Stress Nanocomposite. J. Dent. 2022, 124, 104218. [Google Scholar] [CrossRef]
- Pitel, M.L. Low-Shrink Composite Resins: A Review of Their History, Strategies for Managing Shrinkage, and Clinical Significance. Compend. Contin. Educ. Dent. 2013, 34, 578–590. [Google Scholar]
- Mitwalli, H.; AlSahafi, R.; Albeshir, E.G.; Dai, Q.; Sun, J.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel Nano Calcium Fluoride Remineralizing and Antibacterial Dental Composites. J. Dent. 2021, 113, 103789. [Google Scholar] [CrossRef]
- Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Effect of Composite Resin Biodegradation Products on Oral Streptococcal Growth. Biomaterials 2004, 25, 5467–5472. [Google Scholar] [CrossRef]
- Barbosa, R.P.d.S.; Pereira-Cenci, T.; da Silva, W.M.; Coelho-de-Souza, F.H.; Demarco, F.F.; Cenci, M.S. Effect of Cariogenic Biofilm Challenge on the Surface Hardness of Direct Restorative Materials in Situ. J. Dent. 2012, 40, 359–363. [Google Scholar] [CrossRef]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of Dental Composite Formulations to Their Degradation and the Release of Hydrolyzed Polymeric-Resin-Derived Products. Crit. Rev. Oral. Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Aljabo, A.; Xia, W.; Liaqat, S.; Khan, M.A.; Knowles, J.C.; Ashley, P.; Young, A.M. Conversion, Shrinkage, Water Sorption, Flexural Strength and Modulus of Re-Mineralizing Dental Composites. Dent. Mater. 2015, 31, 1279–1289. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I.; Yang, J. Antibacterial Dental Restorative Materials: A Review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar] [PubMed]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Mohammad Albar, N.H.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed. Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Effect of Water-Ageing on Dentine Bond Strength and Anti-Biofilm Activity of Bonding Agent Containing New Monomer Dimethylaminododecyl Methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Militi, A.; Nicita, F.; Bruno, G.; Tamà, C.; Lo Giudice, F.; Puleio, F.; Calapai, F.; Mannucci, C. Correlation between Oral Hygiene and IL-6 in Children. Dent. J. 2020, 8, 91. [Google Scholar] [CrossRef]
- Fischer, J.; Proefrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Improved Cytotoxicity Testing of Magnesium Materials. Mater. Sci. Engineering. B Solid-State Mater. Adv. Technol. 2011, 176. [Google Scholar] [CrossRef]
- Huang, H.; Luo, L.; Li, L.; Guan, Y.; Yan, Y.; Jiang, Z.; Jiang, B. Calcium Phosphate Cement Promotes Odontoblastic Differentiation of Dental Pulp Cells In Vitro and In Vivo. Coatings 2022, 12, 543. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Martins, C.S.; de Oliveira Cardoso Demarchi, A.C.; de Godoy, L.F.; Kuga, M.C.; Yamashita, J.C. Calcium and Hydroxide Release from Different Pulp-Capping Materials. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 104, e66–e69. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.; Hunecke, A. Influence of Curing Methods and Matrix Type on the Marginal Seal of Class II Resin-Based Composite Restorations in Vitro. Oper. Dent. 2006, 31, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Paolone, G.; Moratti, E.; Goracci, C.; Gherlone, E.; Vichi, A. Effect of Finishing Systems on Surface Roughness and Gloss of Full-Body Bulk-Fill Resin Composites. Materials 2020, 13, 5657. [Google Scholar] [CrossRef]
- Barutcigil, Ç.; Barutcigil, K.; Özarslan, M.M.; Dündar, A.; Yilmaz, B. Color of Bulk-Fill Composite Resin Restorative Materials. J. Esthet. Restor. Dent. 2018, 30, E3–E8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).