Abstract
Background: Bone defect therapy is a common clinical challenge for orthopedic and clinical physicians worldwide, and the therapeutic effect affects the physiological function and healthy life quality of millions of patients. Compared with traditional autogenous bone transplants, bone xenografts are attracting attention due to their advantages of unlimited availability and avoidance of secondary damage. However, there is currently a lack of bibliometric analysis on bone xenograft. This study aimed to use bibliometric methods to analyze the literature on bone xenograft from 2013 to 2023, to explore the current status, hotspots, and future trends of research in this field, and to promote its development and progress. Methods: Using the Web of Science Core Collection database, we retrieved and collected publication data related to xenogeneic bone grafting materials worldwide from January 2013 to March 2023. Origin (2021), CiteSpace (6.2.R2 standard), and an online bibliometric platform were used for bibliometric analysis and data visualization. Results: A total of 3395 documents were retrieved, and 686 eligible papers were selected. The country and institutions with the highest number of publications and centrality were the United States (125 papers, centrality = 0.44) and the University of Zurich (29 papers, centrality = 0.28), respectively. The most cited author was Araujo MG (163 times), and the author with the most significant centrality was Froum SJ (centrality = 0.09). The main keyword clusters were “tissue engineering”, “sinus floor elevation”, “dental implants”, “tooth extraction”, and “bone substitutes”. The most significant bursting keywords in the last three years were “platelet rich fibrin”. Conclusions: Research on bone xenograft is steadily growing and will continue to rise. Currently, research hotspots and directions are mainly focused on dental implants related to bone-augmentation techniques and bone tissue engineering. In the future, research hotspots and directions may focus on decellularization technology and investigations involving platelet-rich fibrin.
1. Introduction
Bone defects represent a common clinical challenge for orthopedic and clinical physicians worldwide [1]. Millions of patients suffer from bone defects each year due to trauma, infection, tumor resection, or congenital malformations, with the global burden of these diseases continuing to rise [2]. Successful treatment of bone defects is crucial for restoring the structural integrity, function, and overall quality of life of the affected patients [3,4]. Bone regeneration is an important method and research direction for treating bone defects [5,6,7]. Autologous bone graft has traditionally been the gold standard for bone regeneration, but they are not always feasible options due to limited donors, prolonged surgical time, and potential donor site morbidity [8]. Therefore, alternative bone graft materials such as bone xenografts have been explored as potential substitutes [9]. In recent years, bone xenografts have become a viable alternative to autografts for bone grafts due to their availability, cost-effectiveness, and reduced morbidity at donor sites [10]. However, despite the increasing use of bone xenografts, it is still necessary to understand the current research status of xenografts, including the hotspots and trends.
Bibliometrics is a quantitative method for analyzing scientific literature and has been proven to be an indispensable tool for tracking research trend evolution and identifying high-impact nodes in a given field [11]. Therefore, this bibliometric study aims to reveal the hotspots and trends in xenograft research to provide valuable insights for researchers, clinical physicians, and policymakers [12]. By studying the advances in this rapidly developing life science field, we can better understand the current state of the art and identify opportunities for future innovations.
In this bibliometric study, we will analyze scientific publications related to bone xenograft over the past decade. Our analysis will focus on exploring publication trends and highlighting contributing countries, institutions, and authors. We will also use various statistical tools to identify the most relevant research topics and keywords related to bone xenograft. In addition, we will recognize prominent research themes, collaborations, and potential directions for future research. Overall, the results of this bibliometric study can provide important insights into the current research status of bone xenograft, including the hotspots and trends (Figure 1). These insights can help improve and develop new bone xenografts for clinical use and ultimately improve patient outcomes.
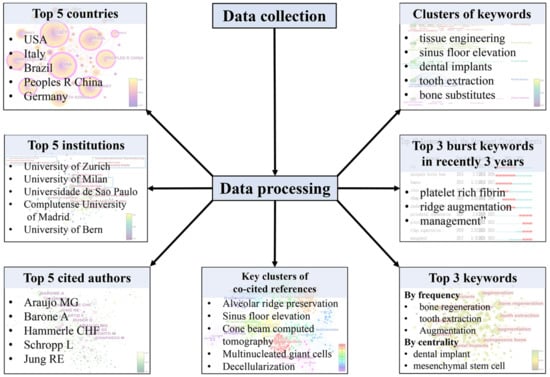
Figure 1.
Flow diagram and results of bibliometric analysis.
2. Materials and Methods
2.1. Data Collection
The first author conducted a literature search on the platform of Web of Science (WoS) in the “Web of Science Core Collection (WoSCC)” database using the search formula “((ALL = (xenogeneic bone)) OR ALL = (bone xenograft)) OR ALL = (bone xenotransplantation)” on 28 March 2023. The search period was from 2013 to 2023, and there were no restrictions on article types. A total of 3395 articles were retrieved. Two researchers conducted independent screening based on inclusion and exclusion criteria, and after review and verification, 686 articles were selected. The inclusion criteria were: (1) the article type was a research paper or review paper. (2) The research content was related to “research and application of bone xenografts of bone defects”. The exclusion criteria were: (1) Meeting abstract, proceeding paper, letter, news item, editorial materials, book chapter, and retracted publications. (2) Duplicates. (3) Articles unrelated to bone xenografts, such as organ transplantation, bone marrow transplantation, cancer bone metastasis, cardiovascular diseases, and cartilage tissue engineering. The screened literature was downloaded in the format of “fully documented and cited references”, including information such as title, abstract, keywords, publication year, authors, nationality, journal name, research direction, publishing institution, funding agencies, and references. That was saved as a plain text file in the raw analysis data sample (Figure 2).
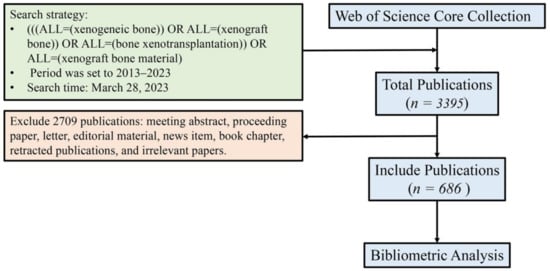
Figure 2.
Process of literature search and filtration.
2.2. Bibliometric and Visualization Analysis
Information on the number of annual publications, research types, and other relevant data was extracted from the sample data. The data analysis software Origin (2021) was used to conduct a descriptive analysis of the data and create bar graphs. Additionally, we conducted visualization analysis using the CiteSpace (6.2.R2 Standard) software and the bibliometric online platform https://bibliometric.com/, accessed on 31 March 2023. With CiteSpace, we explored the collaboration or co-citation network of countries, institutions, authors, references, and keywords while identifying references and keywords clusters and burst items. On the https://bibliometric.com/ online platform, we conducted country cooperation relationship analysis to obtain statistical data and visualize knowledge maps.
The knowledge map produced by CiteSpace provides research information conducted over a specific time range, with node or edge colors referencing the color bar indicating the year. Node size represents the frequency of occurrence, with larger nodes indicating higher frequency. The circular layers of the node represent the annual rings, with a purple circular layer marking the centrality (when the number of centralities is larger than 0.1) and its width indicating the magnitude of the centrality, reflecting the structural and influential aspects of countries, institutions, authors, keywords, and references.
By analyzing the knowledge map and relevant literature, we interpret the current research status of bone xenografts and analyze their hotspots and trends.
3. Results
3.1. Publication of Annual Trend
Annual publication trends were analyzed by counting the number of papers published each year using documents obtained from the WoSCC. To reflect changes in the volume of the literature published over the past decade, a column chart and cumulative publication line graph were plotted (Figure 3). The chart shows a steady upward trend in the number of publications per year. According to the analysis of the WoS platform, the H-index of bone xenograft has been 41 over the past decade.
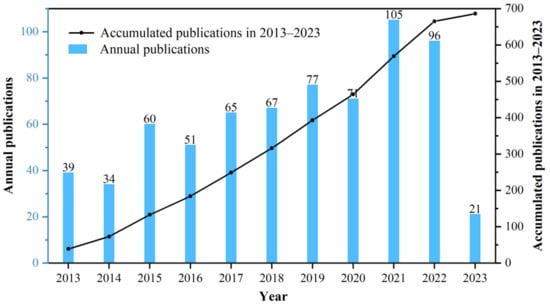
Figure 3.
The annual trends of publications during 2012–2023.
3.2. Analysis of National (Regional) Collaboration
To identify the countries and regions with a significant number of publications and influential contributions of bone xenografts, we conducted a country (regional) cooperation analysis of the relevant literature. The national cooperation network diagram indicates a total of 63 nodes, 245 connections, and a network density of 0.1254 (Figure 4). Figure 5 presents the publication volume and centrality of the top 10 countries in terms of publication output. The United States is the most productive and influential country of international cooperation on bone xenograft, with 125 papers and a centrality of 0.44. Following the United States, the top five countries include Italy (123 papers, 0.16 centrality), Brazil (75 papers, 0.11 centrality), China (74 papers, 0.10 centrality), Germany (74 papers, 0.33 centrality), and South Korea (64 papers, 0.01 centrality). Moreover, countries with significant centrality (>0.1) include the United States, Germany, Switzerland (63 papers, 0.18 centrality), Italy, Brazil, Spain (62 papers, 0.11 centrality), France (15 papers, 0.11 centrality), and China. Notably, two highly cited papers published by France are “High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results” [13] and “MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold” [14].
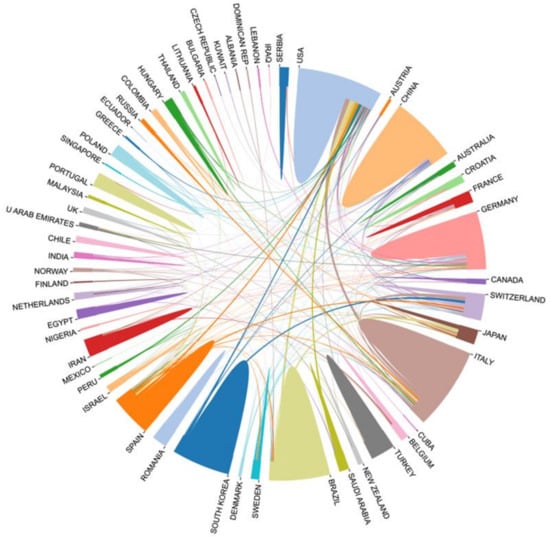
Figure 4.
National cooperation network diagram.
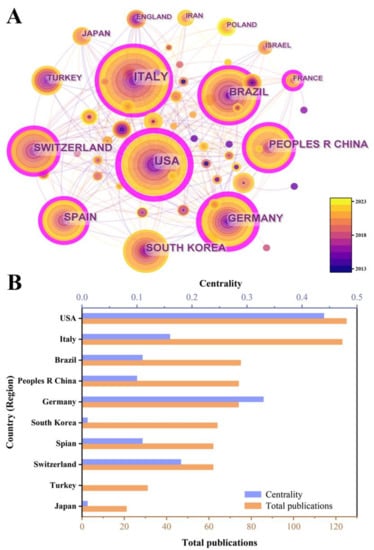
Figure 5.
The publication number of the top 10 countries. (A) Knowledge map visualized by CiteSpace. (B) Bar chart.
3.3. Analysis of Institution Collaboration
To identify institutions with significant influence, we conducted an analysis of institutional co-occurrence based on relevant literature (Figure 6). Figure 6A displays the main institutional collaboration network of bone defects using bone xenografts, resulting in 285 nodes, 436 connections, and a density value of 0.0109. Figure 6B presents the publication volume and centrality of the top 10 institutions in terms of publication output. The institution with the strongest contribution and influence is the University of Zurich, with 29 publications and a centrality of 0.3, which indicates significant centrality. Other institutions that have made significant contributions include the University of Milan, the University of Sao Paulo, etc. In 2023, there was a collaboration between Peking University and China-Japan Friendship Hospital that resulted in the publication of a paper titled “Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians” [15], while institutions in Spain collaborated to publish “Physico-chemical and biological characterization of a new bovine bone mineral matrix available for human usage” [16].
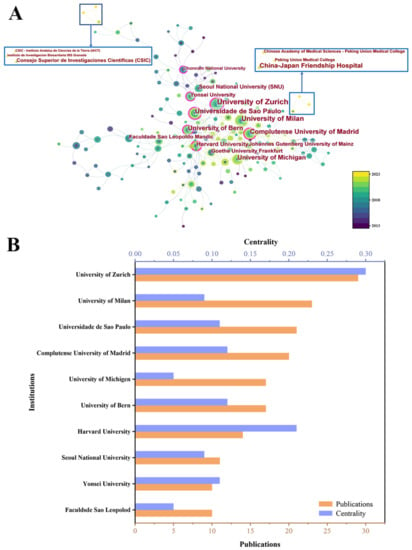
Figure 6.
(A) Institution cooperation network diagram. (B) The publication number of the top 10 institutions.
3.4. Analysis of Author Collaboration
To identify highly influential authors in bone xenograft, we conducted a co-authorship analysis (Figure 7). We generated a co-citation network map comprising 476 nodes and 3210 connections (Figure 7A), with a density value of 0.0284. Figure 7B shows the citation counts and centrality of the top 10 most cited authors. Among them, Araujo MG was the most cited author in the past decade, with 163 citations and a centrality of 0.08. His most cited publication was “Dimensional ridge alterations following tooth extraction: An experimental study in the dog”, which explored the histological changes in alveolar bone absorption and remodeling after tooth extraction [17]. Froum SJ had the highest centrality with 46 citations and a centrality of 0.09. His most cited publication was “Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants”, which found that the average height of the interproximal papilla between adjacent implants was 3.4 mm, ranging from 1 mm to 7 mm [18].
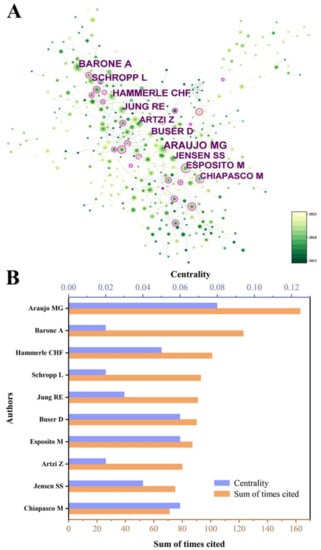
Figure 7.
(A) The author co-cited distribution map. (B) The cited times of the top 10 authors.
3.5. Analysis of Reference Burstiness and Clusters
We conducted burstiness and cluster analyses of our research data to identify highly central literature and provide a basis for analyzing research hotspots and trends (Figure 8 and Figure 9). Figure 8 shows the distribution of reference co-citations, with a total of 521 nodes and 2084 connections and a density value of 0.154. The top-ranked literature was a 2014 publication by Avila-Ortiz G et al. titled “Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis” [19], with a centrality of 0.20. This study evaluated the impact of filling tooth extraction sockets with bone graft materials to prevent post-extraction alveolar ridge volume loss and found that alveolar ridge preservation (ARP) can effectively limit physiological atrophy compared to simple extraction [19].
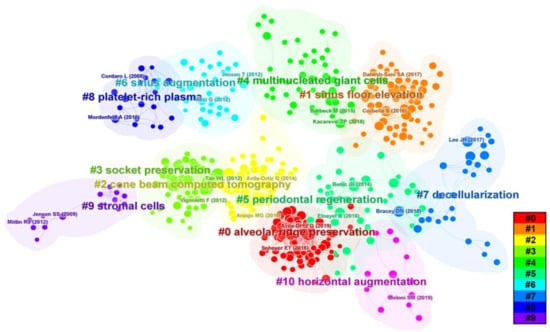
Figure 8.
References co-cited distribution map.
The second-ranked literature, with a centrality of 0.16, was a 2016 publication by Vittorio Favero et al. titled “Sinus floor elevation outcomes following perforation of the Schneiderian membrane. An experimental study in sheep” [20]. This study assessed the effect of covering collagen membranes on bone formation after sinus membrane perforation in sheep and found that using collagen membranes on relatively small sinus mucosal perforations may result in greater new bone formation [20]. The third-ranked literature, with a centrality of 0.14, was a 2017 publication by Tim Fienitz et al. titled “Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus elevation procedures. A prospective, randomized-controlled, clinical multicenter study” [21]. This study investigated the effectiveness of sintered and non-sintered bovine bone substitutes in maxillary sinus floor elevation (MSFE) and found that both groups had comparable new bone formation and volume stability [21]. We also conducted a clustering analysis of co-cited literature to identify the research foundation and focus areas. As shown in Figure 8, there are 10 main clusters, with earlier research hotspots on stromal cells, platelet-rich fibrin (PRF), socket preservation, and MSFE on the left. Recent hotspots appear on the right, including decellularization and multinucleated giant cells (MGCs), among others.
This study analyzed the top 10 most prominent publications in the past decade, with a focus on the evaluation of the effectiveness of ARP after tooth extraction [19,22,23,24,25] (Figure 9).
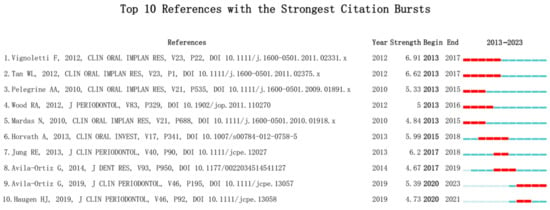
Figure 9.
Top 10 References with the Strongest Citation Bursts [19,22,23,24,25,26,27,28,29,30].
3.6. Analysis of Keyword Burstiness and Clusters
We conducted a co-occurrence analysis on the acquired data to identify hot topics and urgent issues (Figure 10). To further obtain recent hot keywords, we applied the burst detection algorithm based on co-occurrence. A total of 298 nodes and 2733 connections were obtained, with a density value of 0.0618. The top three keywords in terms of frequency are “bone regeneration” (125 times), “tooth extraction” (116 times), and “augmentation” (112 times). The top two keywords in terms of centrality are “dental implant” (0.07) and “mesenchymal stem cell” (0.06).
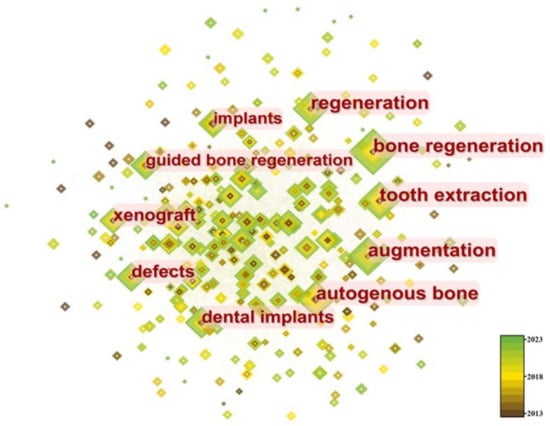
Figure 10.
The co-occurrence network of keywords.
We analyzed the top ten keywords with the highest burstiness level and found that three emerging keywords were “platelet rich fibrin”, “ridge augmentation”, and “management”. Among them, the most significant keyword was “platelet rich fibrin” (Figure 11).
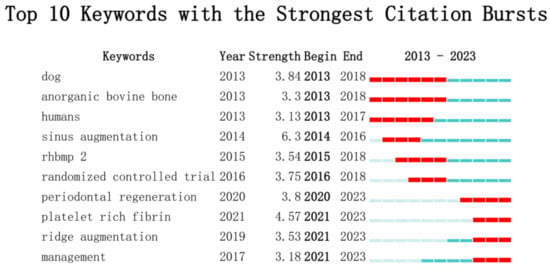
Figure 11.
Top 10 Keywords with the Strongest Citation Bursts.
Cluster analysis was performed on keywords, as shown in Figure 12, resulting in five main clusters: “tissue engineering”, “sinus floor elevation”, “dental implants”, “tooth extraction”, and “bone substitutes”. These clusters are primarily focused on dentistry.
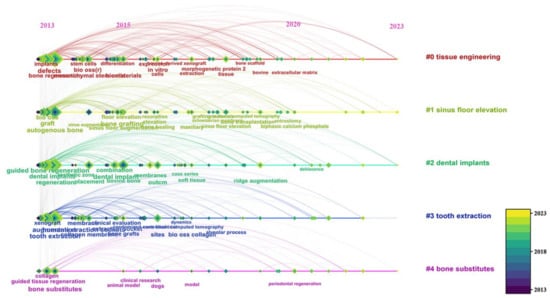
Figure 12.
Time distribution of keywords in different clusters.
4. Discussion
4.1. Global Research Status of Bone Xenograft
Bone grafts are defined as living tissues that promote bone healing and can be transplanted alone or in combination with other materials into bone defects [31]. The number of bone defect surgeries and related research has been increasing year by year [32,33]. Animal-derived bone grafts, widely used in clinical practice [34], have maintained stable development in the past decade (Figure 3), mainly focused on the field of dentistry (Figure 10). Among the 63 countries participating in the research, developed countries such as the United States, Germany, and Italy have a leading position in terms of publication volume and impact (Figure 4 and Figure 5), which is related to their early start of dentistry and a large proportion of medical investment. Notably, despite having fewer publications, France still has significant centrality (Figure 5), thanks to two high-quality papers that explored the effects of bone xenograft on periodontal ligament stem cells and MGCs, contributing to the study of the regenerative properties and biocompatibility of bone xenograft.
With economic development and improved medical level, developing countries like China and Brazil account for a relatively large proportion of research (Figure 4 and Figure 5). Professor Araujo MG from Brazil has made outstanding contributions (Figure 7), with his multiple studies on socket healing and ridge augmentation being widely cited. It is worth noting that the remaining five authors in the top 10 list had high centrality and citation counts, indicating their substantial contribution to the field of bone xenograft. Although China has not yet formed an institution or author with marking centrality (Figure 6), several Chinese research institutions recently cooperated in studying the safety and effectiveness of Bio-oss and Bio-guide for mid-facial augmentation in plastic surgery, promoting the research and application of xenogeneic bone graft materials in plastic surgery. While cooperation between countries is relatively close, it is mainly concentrated among countries with greater influence. However, the cooperation between countries with lower rankings remains relatively loose, and the development between countries is imbalanced (Figure 4). Therefore, for countries and regions with less influence, it is necessary to continue to increase financial investment, enhance institutional and international cooperation, and promote the research and development of bone xenografts.
4.2. Analysis of Hotspots and Trends in Bone Xenograft
The analysis of references reveals that bone xenograft has been a long-standing research hotspot in bone-augmentation technologies such as ARP, ridge augmentation, and MSFE. Come beam computed tomography (CBCT) has emerged as a popular non-invasive three-dimensional (3D) measurement method in bone xenograft research due to its convenience, non-invasiveness, and multi-dimensional advantages over clinical exploration and histological evaluations (Figure 8). Early studies indicate significant absorption of alveolar bone occurs within 3 to 6 months after tooth extraction [26]. To determine the effectiveness of bone-augmentation procedures and ensure implant stability, numerous researchers have studied the impact of various bone-augmentation techniques. CBCT enables researchers to quantitatively and intuitively measure and track changes in the contour of the alveolar ridge following tooth extraction and changes in bone elevation after MSFE [24,35], confirming the effectiveness of bone xenograft in various bone-augmentation techniques [36,37,38,39,40,41,42].
Ideal bone graft materials for bone-augmentation techniques should possess properties such as biocompatibility, good mechanical performance, osteoconductive, osteoinductive capabilities, and appropriate degradation rates [30]. Bone xenografts from various sources can effectively promote the healing of bone defects by embedding newly formed bone, exhibit low degradation rates, and provide excellent mechanical support to maintain bone repair space and implant stability [43,44,45,46,47]. However, the osteoinductivity of bone xenografts has not yet reached the ideal standards achieved by autologous or allogeneic bone materials [42,47,48,49,50,51]. Heat treatment or sintering is a commonly used physical purification method for bone xenografts. Research findings reveal that xenograft bone materials purified by high-temperature sintering could cause more numerous MGCs to aggregate under high temperatures, leading to pro- or anti-inflammatory effects that may affect tissue regeneration [13,52,53]. MGCs continue to be one of the major areas of research focus, and their roles and impacts on tissue regeneration and material degradation require further study (Figure 8). In this context, further investigation and improvement are necessary to enhance the tissue reactivity and osteoinductivity of bone xenografts in the future. In addition to analyzing references, we conducted reference co-citation analysis and found that decellularization technology is an emerging research hotspot in recent years (Figure 8). Decellularization is a process that removes cells and their related components from the extracellular matrix (ECM) through physical, chemical, or enzymatic treatments, especially DNA and RNA, to produce natural matrices with intact mechanical properties [54]. This technology is also one of the pathways used for the purification of bone xenografts [55]. Porcine bone tissue has similar bone microstructures to humans. However, the presence of α1, 3 Gal epitopes can cause hyperacute rejection in humans [56,57]. Therefore, researchers have employed decellularization technology to purify porcine bone xenografts [58]. Compared to deproteinized bovine bone grafts prepared via high-temperature sintering (300–1300 °C), the decellularized porcine bone ECM retains various proteins that support intracellular and extracellular signaling pathways, such as Chondroadherin, Lumican, and Biglycan. Additionally, it preserves the natural microstructure as much as possible, showing better new bone regeneration ability in preclinical studies [59]. Researchers have used 3D printing technology to prepare xenogeneic composite scaffolds by combining decellularized porcine bone with polycaprolactone, resulting in scaffolds with lower immunogenicity, better osteogenic performance, and higher degradation rates [56]. Considering these results, decellularized xenogeneic bone grafts hold immense potential for repairing and regenerating bone defects. However, various decellularization methods may still damage the ECM or not eliminate immune risks due to residual cell components [60,61]. Future clinical studies and applications of decellularization in bone defect repair must focus on providing long-term experimental data and conducting more clinical trials with the development of molecular biology and cell biology, tissue engineering has gradually delved into the study of bone xenografts, which is one of the long-term research directions (Figure 12). Mesenchymal stem cells (MSCs) have gained extensive attention due to their ability for self-renewal and multipotent differentiation [62]. Several preclinical or clinical studies have shown that MSCs from different sources, such as adipose tissue and bone marrow, significantly promote bone regeneration of xenografts [63,64,65]. However, some scholars believe that cell therapy still faces issues such as high cost, inconvenient regulation, and long treatment times. Further optimization is needed in aspects such as cell selection, delivery, vitality, and phenotypic stability before it can be widely applied in clinics [66,67]. Therefore, the concept of a cell-free strategy has been receiving increasing attention from scholars. The cell-free bone biomimetic scaffold aims to interact with surrounding cells and tissues through the scaffold and its loaded growth factors or other bioactive substances to promote vascularization and osteogenesis, thereby changing the traditional recovery process of diseases or injuries [68].
In the past 10 years, researchers have attempted various bioactive substances to enhance the osteoinductive and osteoconductive properties of xenografts, including bone morphogenetic protein 2, parathyroid hormone, 4-hexylresorcinol, and titanium particles, all of which have achieved certain results [64,69,70,71,72,73,74,75]. Through the analysis of keyword burstiness, we found that platelet-rich fibrin (PRF) has become one of the emerging research hotspots of bone xenograft in the past three years (Figure 11). PRF is a second-generation autologous platelet concentrate named PRF in 2005 and is obtained by the centrifugal processing of blood. It is rich in platelets, white blood cells, mononuclear cells, and various cytokines, with advantages such as simple preparation, no immunogenicity, and easy clinical application [76,77]. In vitro, studies have shown that PRF exhibits properties such as promoting angiogenesis and has achieved good results when used in conjunction with xenografts in studies on ARP, guided bone regeneration, periodontal regeneration, MSFE, and accelerated orthodontic tooth movement [15,46,78,79,80,81]. Studies have found that different centrifugal processes may alter the 3D network structure of PRF, which may potentially affect the efficacy of related bone-augmentation techniques [72,73,74,75,76,77,78,79,80,81,82,83,84]. Based on the above background, PRF has great research potential in enhancing the performance of bone xenografts and is one of the future research hotspots.
4.3. Advantages and Limitations
Bone xenografts are one of the major bone substitute materials used clinically. This study focuses on the analysis of the current research status of bone xenografts, and the research results can provide data support and new ideas for the improvement of new bone grafts. However, there are still some limitations in this study. Firstly, the data sources only include the literature published in the WoSCC database, which may miss some relevant studies from other databases, affecting the comprehensiveness of this study’s analysis. Secondly, the citation count of literature may increase over time, which may result in relatively lower citation counts for some recent important literature and not being prominently reflected in the analysis results.
5. Conclusions
In conclusion, between 2013 and 2023, the research scale of bone xenograft has experienced a steadily increased. The research hotspot and directions are mainly focused on the dental field, encompassing bone-augmentation techniques related to implants and bone tissue engineering. The application of CBCT has significantly supported the development of this field, eliciting anticipation for the application of new devices and technologies in future research.
The development of various decellularization techniques is one of the current research directions. However, promoting the clinical application of these technologies requires further studies due to the lack of relevant clinical research.
PRF has been the focus point field of bone xenograft in recent years. In addition to various clinical studies, significant research space is available for adjusting the 3D network structure and exploring its effects.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. J.L., Y.Z., S.C. and S.W.: involved in the data curation, validation, formal analysis, investigation, resources, visualization, and writing original manuscript of the article. W.Z.: reviewed the manuscript. Q.Z.: involved in the conception and design of the article and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Science and Technology Project of Guangzhou City (Grant No. 20202A011026), and the Science and Technology Planning Projects of Guangzhou City, China (Grant Nos. 202201020203, 202201020117).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| WoS | web of science |
| WoSCC | web of science core collection |
| ARP | alveolar ridge preservation |
| MSFE | maxillary sinus floor elevation |
| PRF | platelet rich fibrin |
| MGCs | multinucleated giant cells |
| CBCT | cone beam computer tomography |
| 3D | three-dimensional |
| ECM | extracellular matrix |
| MSCs | Mesenchymal stem cells |
References
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.H.; Keller, J. Current and Future Concepts for the Treatment of Impaired Fracture Healing. Int. J. Mol. Sci. 2019, 20, 5805. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tare, R.S.; Yang, L.Y.; Williams, D.F.; Ou, K.L.; Oreffo, R.O. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; di Blasio, M.; Isola, G.; Cicciu, M. Conservative treatment of temporomandibular joint condylar fractures: A systematic review conducted according to PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. J. Oral Rehabil. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, L.; Xiao, Y. Porous Nanomaterials Targeting Autophagy in Bone Regeneration. Pharmaceutics 2021, 13, 1572. [Google Scholar] [CrossRef]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef]
- Zhang, Q.; Xin, M.; Yang, S.; Wu, Q.; Xiang, X.; Wang, T.; Zhong, W.; Helder, M.N.; Jaspers, R.T.; Pathak, J.L.; et al. Silica nanocarrier-mediated intracellular delivery of rapamycin promotes autophagy-mediated M2 macrophage polarization to regulate bone regeneration. Mater. Today Bio 2023, 20, 100623. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Artas, G.; Gul, M.; Acikan, I.; Kirtay, M.; Bozoglan, A.; Simsek, S.; Yaman, F.; Dundar, S. A comparison of different bone graft materials in peri-implant guided bone regeneration. Braz. Oral Res. 2018, 32, e59. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravida, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ninkov, A.; Frank, J.R.; Maggio, L.A. Bibliometrics: Methods for studying academic publishing. Perspect. Med. Educ. 2022, 11, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.F.; Walker, C.K. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy 2015, 35, 551–559. [Google Scholar] [CrossRef]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3D Hydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, K.; Liu, R.X.; Xu, B.H. Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. J. Clin. Med. 2023, 12, 959. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Martin-Morales, N.; Olaechea, A.; Hernandez-Cortes, P.; Verdugo-Escamilla, C.; Martinez-Ruiz, F.; Carrillo-Galvez, A.B.; O’Valle, F.; Padial-Molina, M. Physico-chemical and biological characterization of a new bovine bone mineral matrix available for human usage. Clin. Implant. Dent. Relat. Res. 2023, 25, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Tarnow, D.; Elian, N.; Fletcher, P.; Froum, S.; Magner, A.; Cho, S.C.; Salama, M.; Salama, H.; Garber, D.A. Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants. J. Periodontol. 2003, 74, 1785–1788. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Lang, N.P.; Canullo, L.; Urbizo Velez, J.; Bengazi, F.; Botticelli, D. Sinus floor elevation outcomes following perforation of the Schneiderian membrane. An experimental study in sheep. Clin. Oral Implant. Res. 2016, 27, 233–240. [Google Scholar] [CrossRef]
- Fienitz, T.; Moses, O.; Klemm, C.; Happe, A.; Ferrari, D.; Kreppel, M.; Ormianer, Z.; Gal, M.; Rothamel, D. Histological and radiological evaluation of sintered and non-sintered deproteinized bovine bone substitute materials in sinus augmentation procedures. A prospective, randomized-controlled, clinical multicenter study. Clin. Oral Investig. 2017, 21, 787–794. [Google Scholar] [CrossRef]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Mardas, N.; Mezzomo, L.A.; Needleman, I.G.; Donos, N. Alveolar ridge preservation. A systematic review. Clin. Oral Investig. 2013, 17, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Philipp, A.; Annen, B.M.; Signorelli, L.; Thoma, D.S.; Hammerle, C.H.; Attin, T.; Schmidlin, P. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: A randomized controlled clinical trial. J. Clin. Periodontol. 2013, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 195–223. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral. Implant. Res. 2012, 23 (Suppl. S5), 1–21. [Google Scholar] [CrossRef]
- Pelegrine, A.A.; da Costa, C.E.; Correa, M.E.; Marques, J.F., Jr. Clinical and histomorphometric evaluation of extraction sockets treated with an autologous bone marrow graft. Clin. Oral Implants Res. 2010, 21, 535–542. [Google Scholar] [CrossRef]
- Wood, R.A.; Mealey, B.L. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J. Periodontol. 2012, 83, 329–336. [Google Scholar] [CrossRef]
- Mardas, N.; Chadha, V.; Donos, N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: A randomized, controlled clinical trial. Clin. Oral Implants Res. 2010, 21, 688–698. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Huang, M.; Li, Z.; Shen, Z.; Feng, J.; Chen, H.; Wu, J.; Gao, J.; Wen, Z.; et al. Research hotspots and trends of bone defects based on Web of Science: A bibliometric analysis. J. Orthop. Surg. Res. 2020, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Bracey, D.N.; Cignetti, N.E.; Jinnah, A.H.; Stone, A.V.; Gyr, B.M.; Whitlock, P.W.; Scott, A.T. Bone xenotransplantation: A review of the history, orthopedic clinical literature, and a single-center case series. Xenotransplantation 2020, 27, e12600. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chung, J.H.; Shin, S.Y.; Shin, S.I.; Kye, S.B.; Kim, N.K.; Kwon, T.G.; Paeng, J.Y.; Kim, J.W.; Oh, O.H.; et al. Efficacy of rhBMP-2/Hydroxyapatite on Sinus Floor Augmentation: A Multicenter, Randomized Controlled Clinical Trial. J. Dent. Res. 2015, 94 (Suppl. S9), 158S–165S. [Google Scholar] [CrossRef] [PubMed]
- Bow, A.; Anderson, D.E.; Dhar, M. Commercially available bone graft substitutes: The impact of origin and processing on graft functionality. Drug Metab. Rev. 2019, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Lai, V.J.; Michalek, J.E.; Liu, Q.Q.; Mealey, B.L. Ridge preservation following tooth extraction using bovine xenograft compared with porcine xenograft: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 361–368. [Google Scholar] [CrossRef]
- Dos Santos, F.R.; Minto, B.W.; da Silva, S.W.G.; Coelho, L.D.; Rossignoli, P.P.; Costa, J.S.; Taba, M.; Dias, L.G.G.G. Caprine demineralized bone matrix (DBMc) in the repair of non-critical bone defects in rabbit tibias. A new bone xenograft. Acta Cir. Bras. 2020, 35, e202000801. [Google Scholar] [CrossRef] [PubMed]
- Gashtasbi, F.; Hasannia, S.; Hasannia, S.; Mahdi Dehghan, M.; Sarkarat, F.; Shali, A. Comparative study of impact of animal source on physical, structural, and biological properties of bone xenograft. Xenotransplantation 2020, 27, e12628. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L.; Cardaropoli, G. Socket preservation using bovine bone mineral and collagen membrane: A randomized controlled clinical trial with histologic analysis. Int. J. Periodontics Restor. Dent. 2012, 32, 421–430. [Google Scholar]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.C.; Mealey, B.L. Histologic comparison of healing following tooth extraction with ridge preservation using two different xenograft protocols. J. Periodontol. 2013, 84, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Leventis, M.; Fairbairn, P.; Mangham, C.; Galanos, A.; Vasiliadis, O.; Papavasileiou, D.; Horowitz, R. Bone Healing in Rabbit Calvaria Defects Using a Synthetic Bone Substitute: A Histological and Micro-CT Comparative Study. Materials 2018, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, E.T.; Heard, R.; Janakievski, J.; Mandelaris, G.; Nevins, M.L.; Pickering, S.R.; Richardson, C.R.; Pope, B.; Toback, G.; Velasquez, D.; et al. A randomized, controlled, multicentre clinical trial of post-extraction alveolar ridge preservation. J. Clin. Periodontol. 2016, 43, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Jimbo, R.; Gangolli, R.; Perez, L.; Manne, L.; Yoo, D.; Lorenzoni, F.; Witek, L.; Coelho, P.G. Evaluation of bone response to various anorganic bovine bone xenografts: An experimental calvaria defect study. Int. J. Oral Maxillofac. Surg. 2014, 43, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, T.L.; de Molon, R.S.; Paim, P.R.; Marcantonio, E.; Marcantonio, E., Jr.; Faeda, R.S. Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: A prospective, randomized and controlled split-mouth clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lan, L.; Miron, R.J.; Wei, L.; Zhang, M.; Zhang, Y. Variability in Particle Degradation of Four Commonly Employed Dental Bone Grafts. Clin. Implant. Dent. Relat. Res. 2015, 17, 996–1003. [Google Scholar] [CrossRef]
- Djordjevic, F.; Mihailovic, B.; Mladenovic, R.; Dubovina, D.; Kostic, M.; Stanisic, J.; Vlahovic, Z. CBCT analysis of bone density in bicortical defects after augmentation with alloplastic and xenogeneic bone substitutes—A study on domestic pigs. Vojnosanit. Pregl. 2021, 78, 1200–1206. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Salama, M.; Chrepa, V.; Hinrichs, J.E.; Gaillard, P. A Randomized, Blinded, Controlled Clinical Study of Particulate Anorganic Bovine Bone Mineral and Calcium Phosphosilicate Putty Bone Substitutes for Socket Preservation. Int. J. Oral Max Impl. 2014, 29, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Azpur, G.; de la Fuente, A.; Chavez, E.; Valdivia, E.; Khouly, I. Horizontal ridge augmentation with guided bone regeneration using particulate xenogenic bone substitutes with or without autogenous block grafts: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Alayan, J.; Ivanovski, S. A prospective controlled trial comparing xenograft/autogenous bone and collagen-stabilized xenograft for maxillary sinus augmentation-Complications, patient-reported outcomes and volumetric analysis. Clin. Oral Implant. Res. 2018, 29, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Peric Kacarevic, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.X.; Gao, C.Y.; Wang, Y.Y.; Wang, Y.L.; Mao, C.; Wang, Q.; Economidou, S.N.; Douroumis, D.; Wen, F.; Tan, L.P.; et al. Investigation of bone reconstruction using an attenuated immunogenicity xenogenic composite scaffold fabricated by 3D printing. Bio-Des. Manuf. 2020, 3, 396–409. [Google Scholar] [CrossRef]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Smith, T.L.; Ornelles, D.A.; Deora, R.; Parks, G.D.; van Dyke, M.E.; Whitlock, P.W. A porcine xenograft-derived bone scaffold is a biocompatible bone graft substitute: An assessment of cytocompatibility and the alpha-Gal epitope. Xenotransplantation 2019, 26, e12534. [Google Scholar] [CrossRef] [PubMed]
- Bracey, D.N.; Seyler, T.M.; Jinnah, A.H.; Lively, M.O.; Willey, J.S.; Smith, T.L.; van Dyke, M.E.; Whitlock, P.W. A Decellularized Porcine Xenograft-Derived Bone Scaffold for Clinical Use as a Bone Graft Substitute: A Critical Evaluation of Processing and Structure. J. Funct. Biomater. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chen, M.Y.; Hsieh, D.J.; Periasamy, S.; Yen, K.C.; Chuang, C.T.; Wang, H.C.; Tseng, F.W.; Kuo, J.C.; Chien, H.H. Evaluating the bone-regenerative role of the decellularized porcine bone xenograft in a canine extraction socket model. Clin. Exp. Dent. Res. 2021, 7, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int J. Oral Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Pelegrine, A.A.; Peruzzo, D.; Martinez, E.F.; Oliveira, R.D.E.; Aloise, A.C.; Ferreira, L.M. Adipose Mesenchymal Stem Cells Associated with Xenograft in a Guided Bone Regeneration Model: A Histomorphometric Study in Rabbit Calvaria. Int. J. Oral Max Impl. 2015, 30, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Mazza, E.; Colombo, M.; Mazzola, S.; Mineo, G.V.; Giannoudis, P.V. Treatment of AVN using the induction chamber technique and a biological-based approach: Indications and clinical results. Injury 2014, 45, 369–373. [Google Scholar] [CrossRef]
- Kloss, F.R.; Kammerer, P.W.; Kloss-Brandstatter, A. Risk Factors for Complications Following Staged Alveolar Ridge Augmentation and Dental Implantation: A Retrospective Evaluation of 151 Cases with Allogeneic and 70 Cases with Autogenous Bone Blocks. J. Clin. Med. 2023, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.N.; Dusserre, N.; Maruszewski, M.; L’Heureux, N. Cell-based therapeutics from an economic perspective: Primed for a commercial success or a research sinkhole? Regen. Med. 2008, 3, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gorman, J.H., III; Gorman, R.C. Acellular biomaterials: An evolving alternative to cell-based therapies. Sci. Transl. Med. 2013, 5, 176ps174. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal. Res. 2012, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Yon, J.; Lee, J.S.; Lim, H.C.; Kim, M.S.; Hong, J.Y.; Choi, S.H.; Jung, U.W. Pre-clinical evaluation of the osteogenic potential of bone morphogenetic protein-2 loaded onto a particulate porcine bone biomaterial. J. Clin. Periodontol. 2015, 42, 81–88. [Google Scholar] [CrossRef]
- Kuroshima, S.; Al-Salihi, Z.; Yamashita, J. Parathyroid hormone related to bone regeneration in grafted and nongrafted tooth extraction sockets in rats. Implant Dent. 2013, 22, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bashara, H.; Wohlfahrt, J.C.; Polyzois, I.; Lyngstadaas, S.P.; Renvert, S.; Claffey, N. The effect of permanent grafting materials on the preservation of the buccal bone plate after tooth extraction: An experimental study in the dog. Clin. Oral Implant. Res. 2012, 23, 911–917. [Google Scholar] [CrossRef]
- Bienz, S.P.; Payer, M.; Hjerppe, J.; Husler, J.; Jakse, N.; Schmidlin, P.R.; Hammerle, C.H.F.; Jung, R.E.; Thoma, D.S. Primary bone augmentation leads to equally stable marginal tissue conditions comparing the use of xenograft blocks infused with BMP-2 and autogenous bone blocks: A 3D analysis after 3 years. Clin. Oral Implant. Res. 2021, 32, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Paeng, K.W.; Jung, U.W.; Benic, G.I. Effectiveness of xenogeneic and synthetic bone-block substitute materials with/without recombinant human bone morphogenetic protein-2: A preclinical study using a rabbit calvarium model. J. Clin. Periodontol. 2021, 48, 1126–1136. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, S.G.; Park, N.R.; Choi, J.Y. Porcine Bone Incorporated with 4-Hexylresorcinol Increases New Bone Formation by Suppression of the Nuclear Factor Kappa B Signaling Pathway. J. Craniofac. Surg. 2018, 29, 1983–1990. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.F.; Araujo Peres, J.A.; Queiroz, M.S. Advances in separation methods for the use of platelet-rich fibrin in tissue repair: An integrative review. Gen. Dent. 2023, 71, 65–69. [Google Scholar] [PubMed]
- Yashwant, V.A.; Balu, P.; Kumar, R.S.; Ammayappan, P.; Murugaboopathy, V. Effectiveness of platelet rich fibrin versus demineralized bone xenograft in periodontally accelerated osteogenic orthodontics. Angle Orthod. 2022, 92, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Carames, J.M.M.; Vieira, F.A.; Carames, G.B.; Pinto, A.C.; Francisco, H.C.O.; Marques, D. Guided Bone Regeneration in the Edentulous Atrophic Maxilla Using Deproteinized Bovine Bone Mineral (DBBM) Combined with Platelet-Rich Fibrin (PRF)-A Prospective Study. J. Clin. Med. 2022, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Chen, C.J.; Barootchi, S.; Kim, D.M. Efficacy of biologics for the treatment of periodontal infrabony defects: An American Academy of Periodontology best evidence systematic review and network meta-analysis. J. Periodontol. 2022, 93, 1803–1826. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; El Bagdadi, K.; Sader, R.; Choukroun, J.; James Kirkpatrick, C.; Ghanaati, S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.V.; Vaishnavi, V.; YSH, S.C. Regenerative Capacity of Leukocyte-rich and Platelet-rich Fibrin in Indirect Sinus Elevation Procedure May be Dependent on Model-Specific Modification of the Centrifugation Cycle. Contemp. Clin. Dent. 2019, 10, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Kono, M.; Katagiri, H.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Histological comparison of Platelet rich fibrin clots prepared by fixed-angle versus horizontal centrifugation. Platelets 2021, 32, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jimenez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).