Comparison of Cost and Potency of Human Mesenchymal Stromal Cell Conditioned Medium Derived from 2- and 3-Dimensional Cultures
Abstract
1. Introduction
2. Materials and Methods
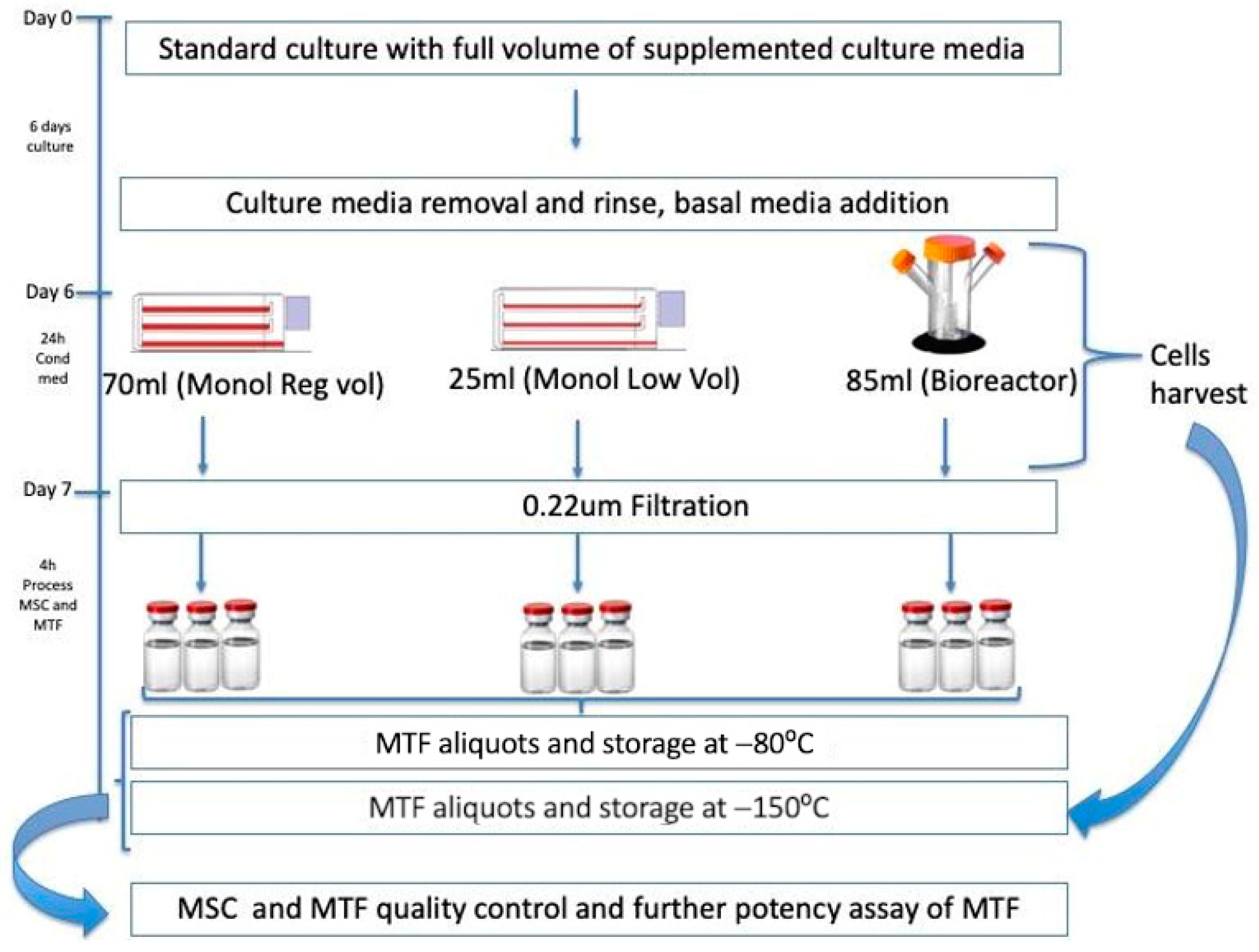
2.1. Cell Culture and MTFs Production
2.2. In Vitro Potency Assays for MTFs Using PHA on PBMNCs and Macrophages with LPS
2.3. Statistical Analysis
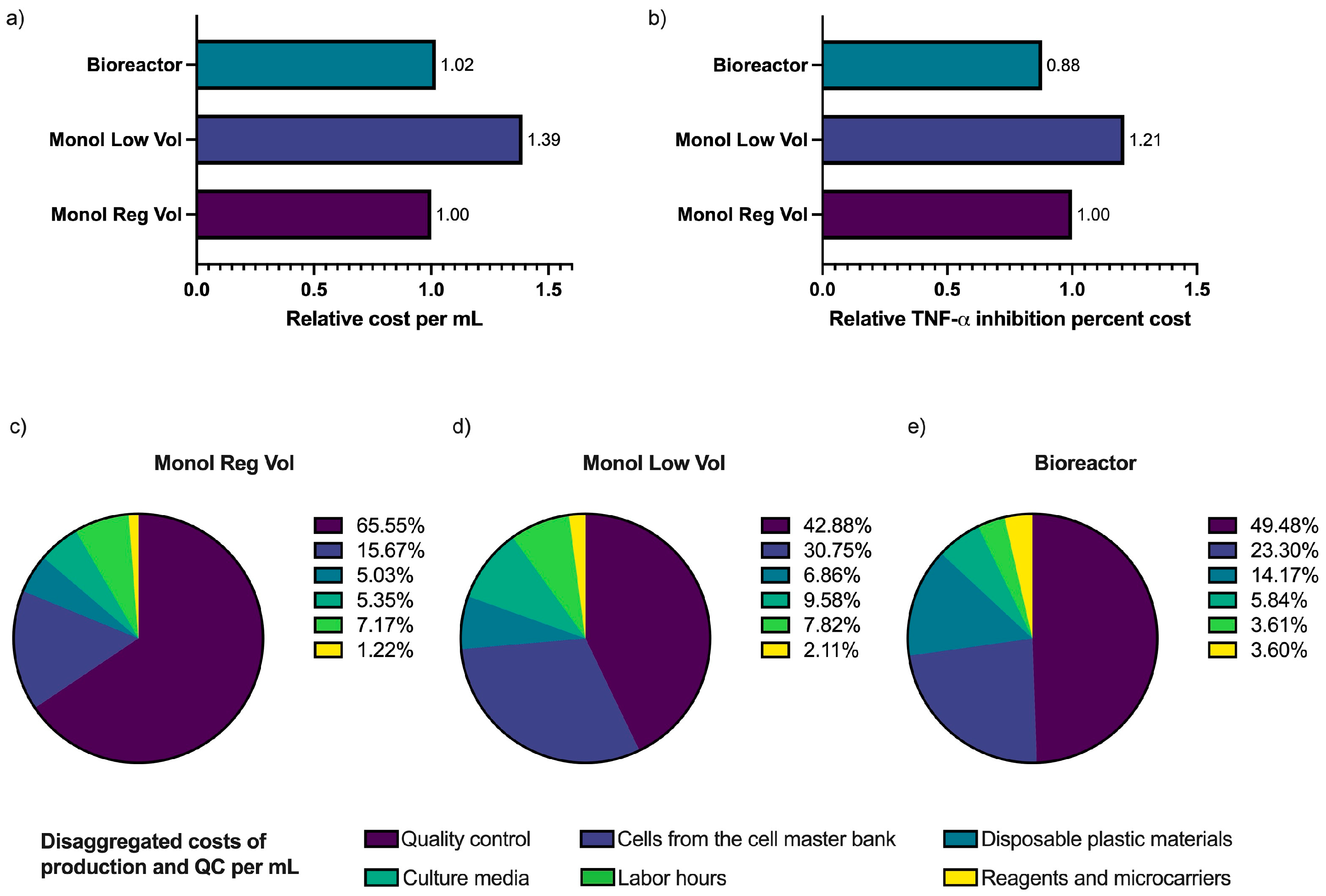
2.4. Comparison of the Cost of Each Anti-Inflammatory Unit Production
3. Results
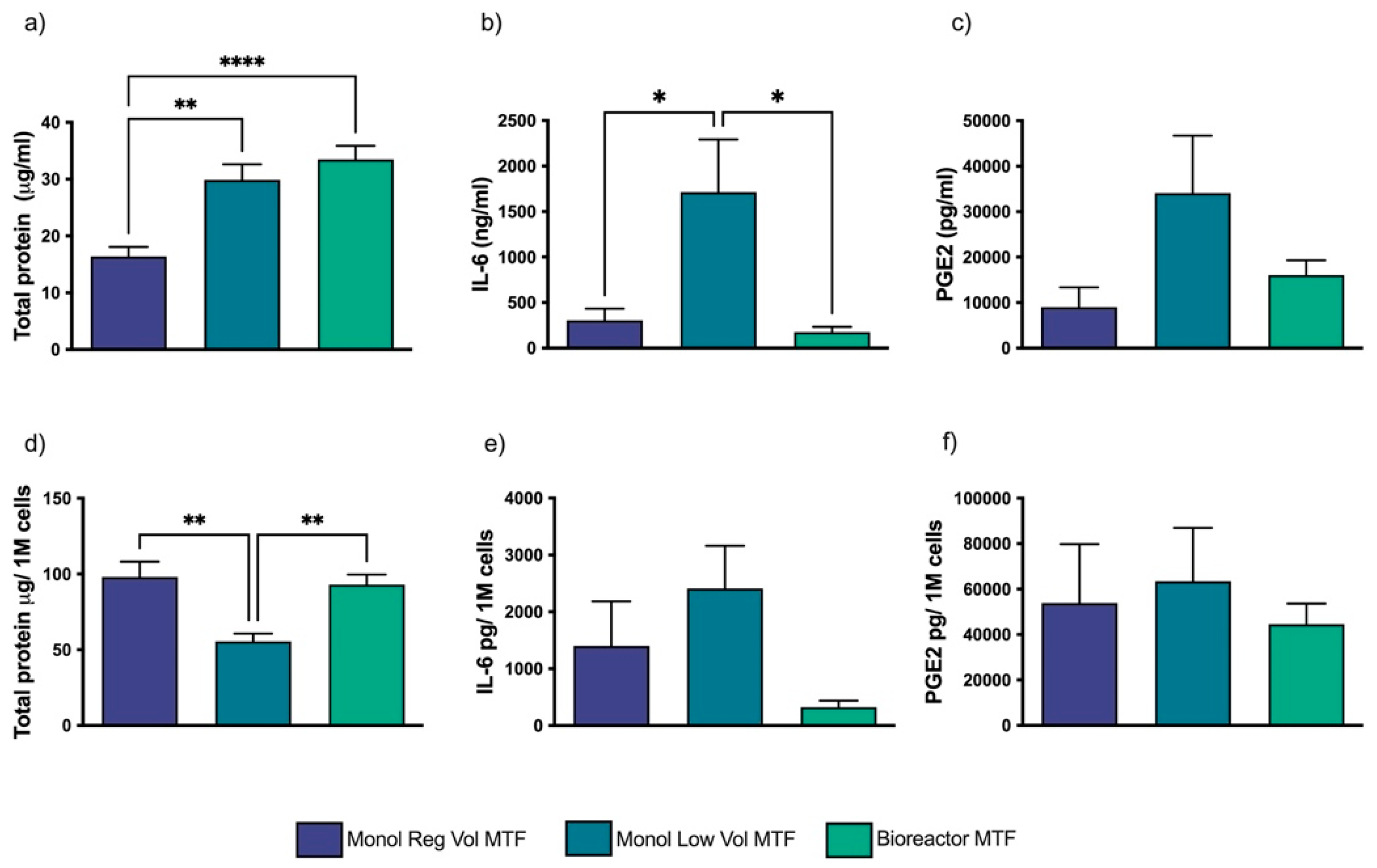
3.1. MTFs Produced in Different Culture Setups Are Compositionally Different
3.2. The 3D Dynamic Culture-Produced MTFs Partially Inhibited PHA’s Effect on PBMNCs
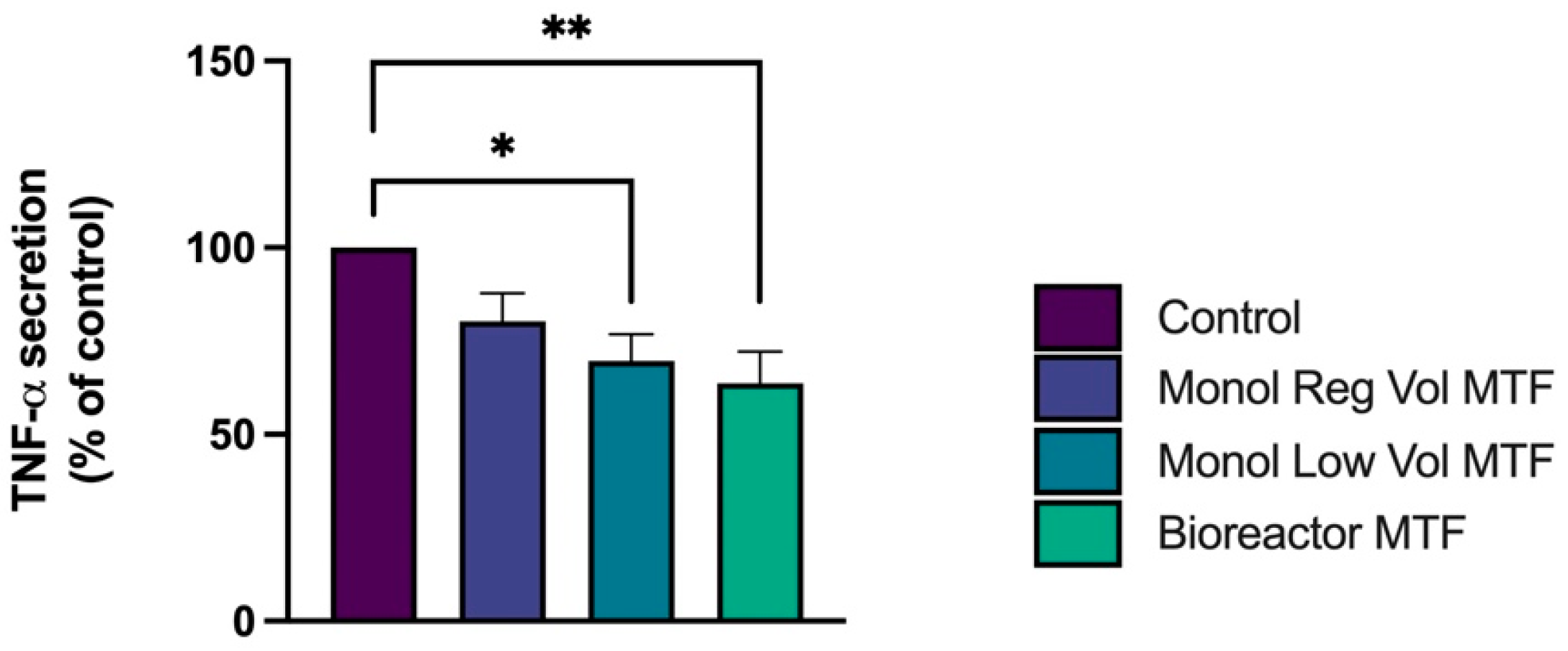
3.3. TNF-α Release Is Decreased by MTFs in LPS-Activated THP-1 Macrophages
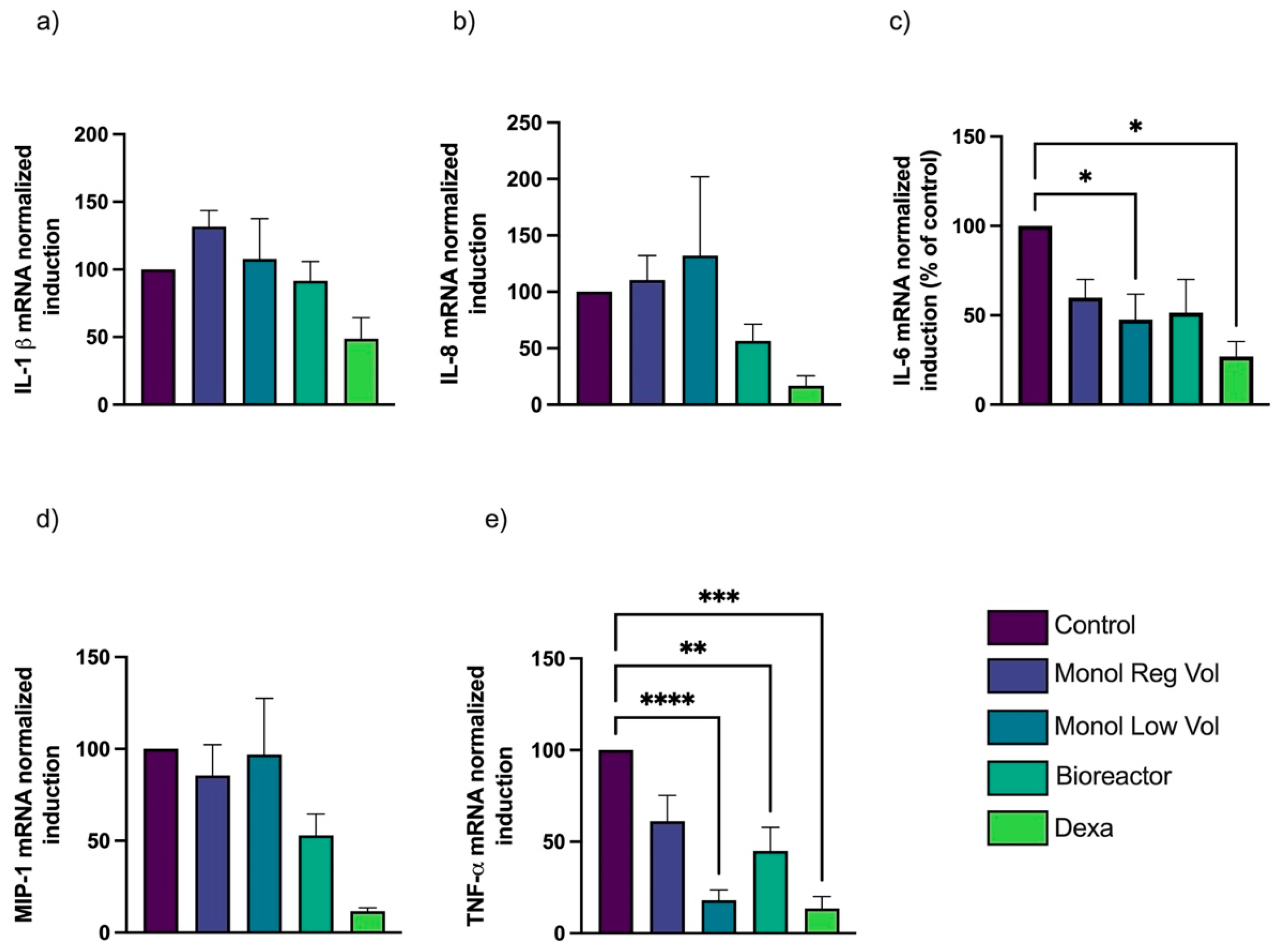
3.4. Inflammatory Cytokines Are Inhibited by MTFs, and the Effect Is Greater with 3D Dynamic Culture
3.5. The 3D Dynamic Culture Produces TNF-α Inhibitory MTFs More Efficiently Than Monolayer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Potency Assays of MTFs from Monolayer and Bioreactor Systems
Appendix A.1. PHA Inflammation Assay
Appendix A.2. THP-1 Macrophages Differentiation and LPS Assay
Appendix A.3. Real-Time PCR Analysis
- β-actin forward: 5′-ATTGCCGACAGGATGCAGAA-3′
- β-actin reverse: 5′-GCTGATCCA CATCTGCTGGAA-3′
- IL-6 forward: 5′-TTCAATGAGGAGACTTGCCTG-3′
- IL-6 reverse: 5′-ACAACAACAATCTGAGGTGCC-3′
- IL-8 forward: 5′-AAGGAACCATCTCACTGTGTGTAAAC-3′
- IL-8 reverse: 5′-ATCAGGAAGGCT GCCAAGAG-3′
- MIP-1α forward: 5′-TTGTGATTGTTTGCTCTGAGAGTTC-3′
- MIP-1α reverse: 5′-CGG TCGTCACCAGACACACT-3′
- IL-1β forward: 5′-TACAAGGAGAAGAAAGTAATGACAA-3′
- IL-1β reverse: 5′-AGCTTGTTATTGATTTCTATCTTGT-3′
- TNF- α forward: 5′-CTCCTCACCCACACCATCAGCCGCA-3′
- TNF- α reverse: 5′-ATAGATGGGCTCATACCA GGGCTTG-3′
References
- El-Gabalawy, H.; Guenther, L.C.; Bernstein, C.N. Epidemiology of immune-mediated inflammatory diseases: Incidence, prevalence, natural history, and comorbidities. J. Rheumatol. Suppl. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.; Pike, C.; Kaufman, R.; Maynchenko, M.; Kidolezi, Y.; Cifaldi, M. Societal cost of rheumatoid arthritis patients in the US. Curr. Med. Res. Opin. 2009, 26, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Jeemon, P.; Mini, G.K.; Thankappan, K.R.; Sylaja, P.G. GBD 2017 Causes of Death Collaborators, Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current Therapeutic Options in the Treatment of Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, N.; Proudfoot, C.; Fournier, M.; Chen, C.-I.; Kuznik, A.; Kiss, Z.; Gal, P.; Michaud, K. Economic Evaluation of Sarilumab in the Treatment of Adult Patients with Moderately-to-Severely Active Rheumatoid Arthritis Who Have an Inadequate Response to Conventional Synthetic Disease-Modifying Antirheumatic Drugs. Adv. Ther. 2019, 36, 1337–1357. [Google Scholar] [CrossRef]
- Wang, L.-T.; Ting, C.-H.; Yen, M.-L.; Liu, K.-J.; Sytwu, H.-K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H.-Y.; Liao, C.; Lin, W.; Lin, S. Clinical Efficacy and Safety of Mesenchymal Stem Cells for Systemic Lupus Erythematosus. Stem Cells Int. 2020, 2020, 6518508. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 11592. [Google Scholar] [CrossRef]
- Riordan, N.H.; Morales, I.; Fernández, G.; Allen, N.; Fearnot, N.E.; Leckrone, M.E.; Markovich, D.J.; Mansfield, D.; Avila, D.; Patel, A.N.; et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med. 2018, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.Z.-H.; Johnson, S.; Dave, M. Efficacy and Safety of Mesenchymal Stem/Stromal Cell Therapy for Inflammatory Bowel Diseases: An Up-to-Date Systematic Review. Biomolecules 2021, 11, 82. [Google Scholar] [CrossRef]
- Hua, C.; Chen, S.; Cheng, H. Therapeutic potential of mesenchymal stem cells for refractory inflammatory and immune skin diseases. Hum. Vaccines Immunother. 2022, 18, 2144667. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Prolif. 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Mallis, P.; Michalopoulos, E.; Chatzistamatiou, T.; Giokas, C.S. Interplay between mesenchymal stromal cells and immune system: Clinical applications in immune-related diseases. Explor. Immunol. 2021, 1, 112–139. [Google Scholar] [CrossRef]
- Facchin, F.; Bianconi, E.; Romano, M.; Impellizzeri, A.; Alviano, F.; Maioli, M.; Canaider, S.; Ventura, C. Comparison of Oxidative Stress Effects on Senescence Patterning of Human Adult and Perinatal Tissue-Derived Stem Cells in Short and Long-term Cultures. Int. J. Med. Sci. 2018, 15, 1486–1501. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2020, 52, 93–101. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Griffin, M.D.; Ritter, T.; Ryan, A.E. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front. Immunol. 2017, 8, 1626. [Google Scholar] [CrossRef]
- Coppin, L.; Sokal, E.; Stéphenne, X. Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives. Cells 2019, 8, 1160. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Chatzistamatiou, T.; Dimou, Z.; Sarri, E.-F.; Georgiou, E.; Salagianni, M.; Triantafyllia, V.; Andreakos, E.; Stavropoulos-Giokas, C.; Michalopoulos, E. Mesenchymal stromal cell delivery as a potential therapeutic strategy against COVID-19: Promising evidence from in vitro results. World J. Biol. Chem. 2022, 13, 47–65. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.-K.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Shabbir, A.; Zisa, D.; Suzuki, G.; Lee, T.; Lin, H.; Angeli, M.; Chung, K.J.; Ejimadu, C.; Rosa, A.R.; Golpanian, S.; et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am. J. Physiol. Circ. Physiol. 2009, 296, H1888–H1897. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M.; et al. Adult Human Mesenchymal Stem Cells Added to Corticosteroid Therapy for the Treatment of Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Park, W.S.; Ahn, S.Y.; Sung, S.I.; Ahn, J.-Y.; Chang, Y.S. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 2017, 83, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rybkowska, P.; Radoszkiewicz, K.; Kawalec, M.; Dymkowska, D.; Zabłocka, B.; Zabłocki, K.; Sarnowska, A. The Metabolic Changes between Monolayer (2D) and Three-Dimensional (3D) Culture Conditions in Human Mesenchymal Stem/Stromal Cells Derived from Adipose Tissue. Cells 2023, 12, 178. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Bazhanov, N.; Kuhlman, J.; Prockop, D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells 2013, 31, 2443–2456. [Google Scholar] [CrossRef]
- Campbell, A.; Brieva, T.; Raviv, L.; Rowley, J.; Niss, K.; Brandwein, H.; Oh, S.; Karnieli, O. Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl. Med. 2015, 4, 1155–1163. [Google Scholar] [CrossRef]
- Das, R.; Roosloot, R.; Van Pel, M.; Schepers, K.; Driessen, M.; Fibbe, W.E.; De Bruijn, J.D.; Roelofs, H. Preparing for cell culture scale-out: Establishing parity of bioreactor- and flask-expanded mesenchymal stromal cell cultures. J. Transl. Med. 2019, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Álvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [PubMed]
- Pacienza, N.; Lee, R.H.; Bae, E.-H.; Kim, D.-K.; Liu, Q.; Prockop, D.J.; Yannarelli, G. In Vitro Macrophage Assay Predicts the In Vivo Anti-inflammatory Potential of Exosomes from Human Mesenchymal Stromal Cells. Mol. Ther. Methods Clin. Dev. 2019, 13, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, J.; Li, Y.; Duan, W.-M.; Bi, J.; Qu, T. Human umbilical cord-derived mesenchymal stem cells suppress proliferation of PHA-activated lymphocytes in vitro by inducing CD4+CD25highCD45RA+ regulatory T cell production and modulating cytokine secretion. Cell. Immunol. 2016, 302, 26–31. [Google Scholar] [CrossRef]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H. Macrophage inflammatory assay. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- De Wolf, C.; Van De Bovenkamp, M.; Hoefnagel, M. Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy 2017, 19, 784–797. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q.; et al. Process development for expansion of human mesenchymal stromal cells in a 50L single-use stirred tank bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Lembong, J.; Kirian, R.; Takacs, J.D.; Olsen, T.R.; Lock, L.T.; Rowley, J.A.; Ahsan, T. Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Sulaiman, N.; Fauzi, M.B.; Law, J.X.; Ng, M.H.; Idrus, R.B.H.; Yazid, M.D. Three dimensional microcarrier system in mesenchymal stem cell culture: A systematic review. Cell Biosci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Tsai, A.-C.; Jeske, R.; Chen, X.; Yuan, X.; Li, Y. Influence of Microenvironment on Mesenchymal Stem Cell Therapeutic Potency: From Planar Culture to Microcarriers. Front. Bioeng. Biotechnol. 2020, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-M.; Schilling, T.; Benisch, P.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Limbert, C.; Seufert, J.; Kassem, M.; Schütze, N.; et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem. Biophys. Res. Commun. 2007, 363, 209–215. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, H.; Chen, Z.; Wang, Y.; Lin, J.; Xu, S.; Zhang, C.; Wang, B.; Yuan, Z.; Feng, X.; et al. High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol. Med. Rep. 2017, 16, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Showalter, M.R.; Wancewicz, B.; Fiehn, O.; Archard, J.A.; Clayton, S.; Wagner, J.; Deng, P.; Halmai, J.; Fink, K.D.; Bauer, G.; et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem. Biophys. Res. Commun. 2019, 512, 729–735. [Google Scholar] [CrossRef]
- Egger, D.; Lavrentieva, A.; Kugelmeier, P.; Kasper, C. Physiologic isolation and expansion of human mesenchymal stem/stromal cells for manufacturing of cell-based therapy products. Eng. Life Sci. 2022, 22, 361–372. [Google Scholar] [CrossRef]
- Mi, F.; Gong, L. Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci. Rep. 2017, 37, BSR20170181. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, S.; Li, C.; Ye, Z.; Lin, B.; Liang, Y.; Wang, B.; Ma, Y.; Chai, X.; Zhang, X.; et al. Mesenchymal stem/stromal cells-derived IL-6 promotes nasopharyngeal carcinoma growth and resistance to cisplatin via upregulating CD73 expression. J. Cancer 2020, 11, 2068–2079. [Google Scholar] [CrossRef]
- Estúa-Acosta, G.A.; Buentello-Volante, B.; Magaña-Guerrero, F.S.; Flores, J.E.-A.; Vivanco-Rojas, O.; Castro-Salas, I.; Zarco-Ávila, K.; García-Mejía, M.A.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cell-Synthesized PGE2 Exerts an Immunomodulatory Effect on Neutrophil Extracellular Trap in a PAD-4-Dependent Pathway through EP2 and EP4. Cells 2022, 11, 2831. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Ding, H.; Shi, X.; Ren, H. Mesenchymal stem cell-secreted prostaglandin E2 ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res. Ther. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.D.; Centanni, J.M.; Bhatia, N.; Emler, C.A.; Drier, D.; Leverson, G.E.; McKenna, D.H.; Gee, A.P.; Lindblad, R.; Hei, D.J.; et al. A reproducible immunopotency assay to measure mesenchymal stromal cell–mediated T-cell suppression. Cytotherapy 2014, 17, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.V.G.; Riewaldt, J.; Wehner, R.; Schmitz, M.; Odendahl, M.; Bornhäuser, M.; Tonn, T. Gamma irradiation preserves immunosuppressive potential and inhibits clonogenic capacity of human bone marrow-derived mesenchymal stromal cells. J. Cell. Mol. Med. 2014, 18, 1184–1193. [Google Scholar] [CrossRef]
- von Bahr, L.; Sundberg, B.; Lönnies, L.; Sander, B.; Karbach, H.; Hägglund, H.; Ljungman, P.; Gustafsson, B.; Karlsson, H.; Le Blanc, K.; et al. Long-Term Complications, Immunologic Effects, and Role of Passage for Outcome in Mesenchymal Stromal Cell Therapy. Biol. Blood Marrow Transplant. 2012, 18, 557–564. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Warrington, T.P.; Bostwick, J.M. Psychiatric adverse effects of corticosteroids. Mayo Clin. Proc. 2006, 81, 1361–1367. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Galipeau, J. Macrophages at the Nexus of Mesenchymal Stromal Cell Potency: The Emerging Role of Chemokine Cooperativity. Stem Cells 2021, 39, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Rauwel, B.; Baron, M.; Boyer, J.F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Yip, R.M.L.; Yim, C.W. Role of Interleukin 6 Inhibitors in the Management of Rheumatoid Arthritis. JCR J. Clin. Rheumatol. 2021, 27, e516–e524. [Google Scholar] [CrossRef]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflamm. 2011, 2011, 908468. [Google Scholar] [CrossRef] [PubMed]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, X.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis. Markers 2019, 2019, 8023460. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-Y.; Yu, H.-Y.; Lee, W.-C.; Hsiao, C.-E.; Wu, C.-L.; Cheng, H.-T.; Lin, L.-J.; Li, F.; Chou, Y.-T.; Cheng, J.-W. A novel CXCL8 analog is effective in inhibiting the growth via cell cycle arrest and attenuating invasion of Lewis lung carcinoma. OncoTargets Ther. 2019, 12, 7611–7621. [Google Scholar] [CrossRef]
- Burke, H.; Freeman, A.; Cellura, D.C.; Stuart, B.L.; Brendish, N.J.; Poole, S.; Borca, F.; Phan, H.T.T.; Sheard, N.; Williams, S.; et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir. Res. 2020, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Sutton, M.T.; Fletcher, D.R.; Reese-Koc, J.; Roesch, E.A.; Lazarus, H.M.; Chmiel, J.F.; Caplan, A.I. Human Mesenchymal Stem Cell (hMSC) Donor Potency Selection for the “First in Cystic Fibrosis” Phase I Clinical Trial (CEASE-CF). Pharmaceuticals 2023, 16, 220. [Google Scholar] [CrossRef]
- Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Kudriaeva, A.; Miftakhova, R.; Rybalov, A.; Bulatov, E. Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies. Bioengineering 2022, 9, 808. [Google Scholar] [CrossRef]
- Eaker, S.; Abraham, E.; Allickson, J.; Brieva, T.A.; Baksh, D.; Heathman, T.R.; Mistry, B.; Zhang, N. Bioreactors for cell therapies: Current status and future advances. Cytotherapy 2017, 19, 9–18. [Google Scholar] [CrossRef]
- Romano, B.; Elangovan, S.; Erreni, M.; Sala, E.; Petti, L.; Kunderfranco, P.; Massimino, L.; Restelli, S.; Sinha, S.; Lucchetti, D.; et al. TNF-Stimulated Gene-6 Is a Key Regulator in Switching Stemness and Biological Properties of Mesenchymal Stem Cells. Stem Cells 2019, 37, 973–987. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The prevalence and long-term health effects of Long COVID among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. Eclinicalmedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Mallis, P.; Michalopoulos, E.; Chatzistamatiou, T.; Stavropoulos-Giokas, C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J. Stem Cells 2020, 12, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Doens, D.; Santamaría, R.; Ramos, M.; Restrepo, C.M.; de Arruda, L.B.; Lleonart, R.; Gutiérrez, M.; Fernández, P.L. A Pseudopterane Diterpene Isolated from the Octocoral Pseudopterogorgia acerosa Inhibits the Inflammatory Response Mediated by TLR-Ligands and TNF-Alpha in Macrophages. PLoS ONE 2013, 8, e84107. [Google Scholar] [CrossRef]


| Average Viability (%) | Viability CV (%) | Average Doubling Time (h) | Doubling Time CV (%) | |
|---|---|---|---|---|
| Lot 1 | 91.10 | 3.71 | 31.49 | 11.84 |
| Lot 2 | 85.67 | 4.82 | 30.58 | 8.92 |
| Lot 3 | 86.20 | 0.91 | 31.21 | 2.80 |
| Inhibition Percentage * | |||
|---|---|---|---|
| Monol Reg Vol | Monol Low Vol | 3D Dynamic Culture | |
| TNF-α from ELISA | 49.96 | 71.36 | 63.86 |
| TNF-α from mRNA RT-PCR | 70.30 | 66.90 | 75.40 |
| Sum | 120.26 | 138.26 | 139.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrigal, M.; Fernández, P.L.; Lleonart, R.; Carreño, L.; Villalobos Gorday, K.A.; Rodríguez, E.; de Cupeiro, K.; Restrepo, C.M.; Rao, K.S.J.; Riordan, N.H. Comparison of Cost and Potency of Human Mesenchymal Stromal Cell Conditioned Medium Derived from 2- and 3-Dimensional Cultures. Bioengineering 2023, 10, 930. https://doi.org/10.3390/bioengineering10080930
Madrigal M, Fernández PL, Lleonart R, Carreño L, Villalobos Gorday KA, Rodríguez E, de Cupeiro K, Restrepo CM, Rao KSJ, Riordan NH. Comparison of Cost and Potency of Human Mesenchymal Stromal Cell Conditioned Medium Derived from 2- and 3-Dimensional Cultures. Bioengineering. 2023; 10(8):930. https://doi.org/10.3390/bioengineering10080930
Chicago/Turabian StyleMadrigal, Marialaura, Patricia L. Fernández, Ricardo Lleonart, Lizmar Carreño, Kaiser Alejandro Villalobos Gorday, Ellís Rodríguez, Kathya de Cupeiro, Carlos M. Restrepo, K. S. Jagannatha Rao, and Neil H. Riordan. 2023. "Comparison of Cost and Potency of Human Mesenchymal Stromal Cell Conditioned Medium Derived from 2- and 3-Dimensional Cultures" Bioengineering 10, no. 8: 930. https://doi.org/10.3390/bioengineering10080930
APA StyleMadrigal, M., Fernández, P. L., Lleonart, R., Carreño, L., Villalobos Gorday, K. A., Rodríguez, E., de Cupeiro, K., Restrepo, C. M., Rao, K. S. J., & Riordan, N. H. (2023). Comparison of Cost and Potency of Human Mesenchymal Stromal Cell Conditioned Medium Derived from 2- and 3-Dimensional Cultures. Bioengineering, 10(8), 930. https://doi.org/10.3390/bioengineering10080930





