Current Biomaterials for Wound Healing
Abstract
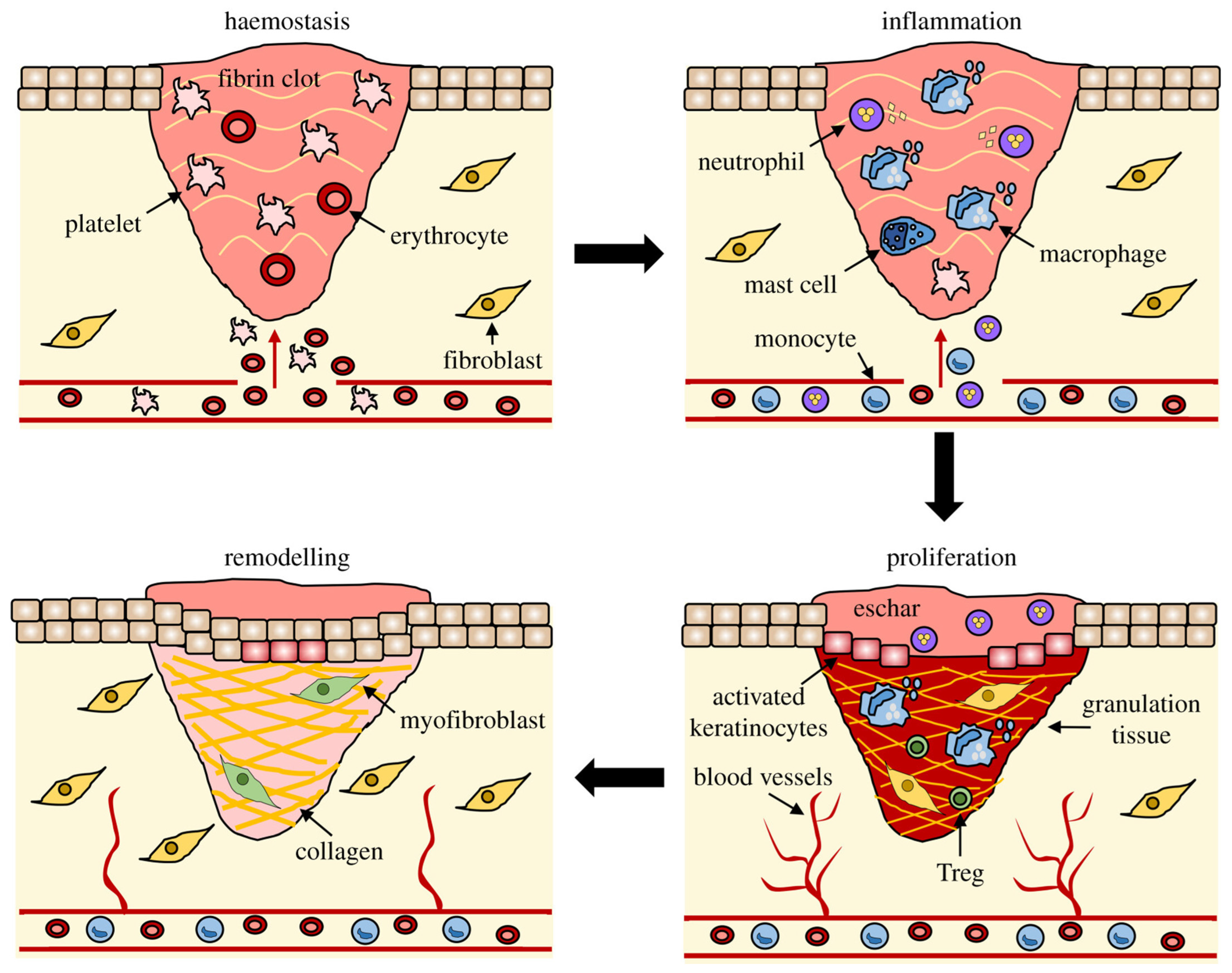
:1. Introduction
2. Properties of Biomaterials for Wound Healing
2.1. Synthetic Biomaterials in Wound Healing
2.2. Biomaterials as a Delivery System
2.3. Collagen in Wound Healing
2.4. Cellulose in Wound Healing
2.5. Silk in Wound Healing
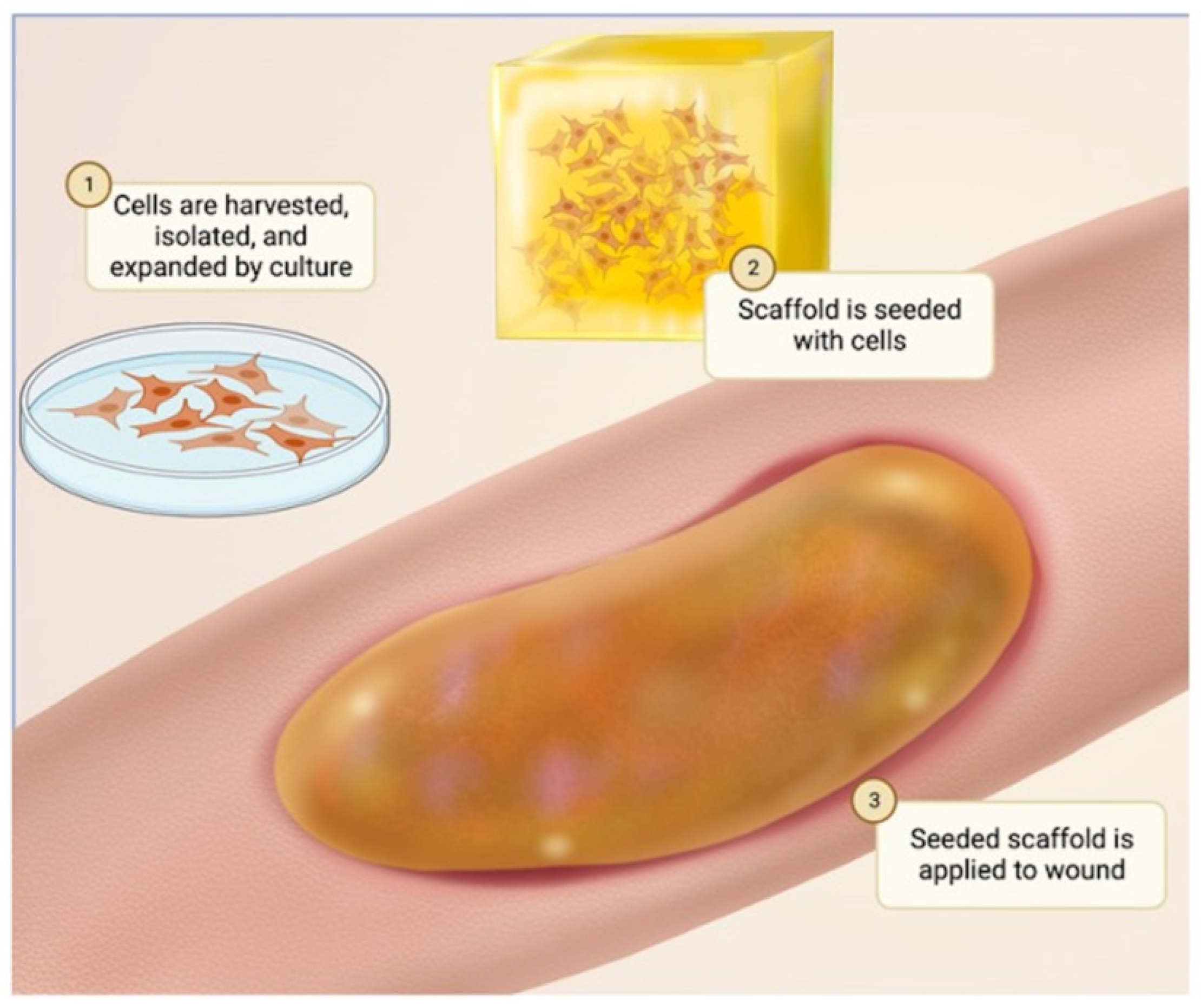
3. Cell Sources in Biomaterials for Skin Tissue Engineering
3.1. Keratinocytes
3.2. Fibroblasts
3.3. ADSCs
3.4. HFSCs
4. Skin Substitutes Currently Used in Clinical Practice
4.1. Acellular Skin Substitutes
4.2. Integra and AlloDerm
4.3. Decellularized ECM
4.4. Cultured Epidermal Autografts
4.5. Cultured Skin Substitutes
4.6. Allogenic Skin Substitute
5. Experimental 3D Bioprinting Printing in Skin Tissue Engineering
Experimental Tissue Enhancement in Wound Healing
6. Wound Dressings
6.1. Maintenance with Molded Silicone Dressings
6.2. Wound Maintenance with Hydrogel Dressings
7. Challenges in Skin Tissue Engineering
7.1. Limitation in Vascularization in Skin Tissue Engineering
7.2. Immune Rejection in Skin Tissue Enginneering
7.3. Limitations in Skin Appendage Regeneration
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System; Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 2012. [Google Scholar] [CrossRef]
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015, 103, 72–88. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hilmi, A.B.; Halim, A.S. Vital roles of stem cells and biomaterials in skin tissue engineering. World J. Stem Cells 2015, 7, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Callà, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled release of 18-β-glycyrrhetic acid by nanodelivery systems increases cytotoxicity on oral carcinoma cell line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chung, J.W.Y.; Yan, V.C.M.; Wong, T.K.S. Polylactic Acid-Based Biomaterials in Wound Healing: A Systematic Review. Adv. Skin. Wound Care 2023, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kinoshita, M.; Miyazaki, H.; Saito, A.; Fujie, T.; Iwaya, K.; Takeoka, S.; Saitoh, D. Application of poly-L-lactic acid nanosheet as a material for wound dressing. Plast. Reconstr. Surg. 2013, 131, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kibe, T.; Koga, T.; Nishihara, K.; Fuchigami, T.; Yoshimura, T.; Taguchi, T.; Nakamura, N. Examination of the early wound healing process under different wound dressing conditions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 123, 310–319. [Google Scholar] [CrossRef]
- Xie, Z.; Paras, C.B.; Weng, H.; Punnakitikashem, P.; Su, L.C.; Vu, K.; Tang, L.; Yang, J.; Nguyen, K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013, 9, 9351–9359. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.W.; Xu, T.; Li, L.; Liu, P.; Li, Y. Effect of bovine bone collagen oligopeptides on wound healing in mice. Aging 2021, 13, 9028–9042. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Tarrahi, R.; Khataee, A.; Karimi, A.; Yoon, Y. The latest achievements in plant cellulose-based biomaterials for tissue engineering focusing on skin repair. Chemosphere 2022, 288, 132529. [Google Scholar] [CrossRef]
- Cao, Y.M.; Liu, M.Y.; Xue, Z.W.; Qiu, Y.; Li, J.; Wang, Y.; Wu, Q.K. Surface-structured bacterial cellulose loaded with hUSCs accelerate skin wound healing by promoting angiogenesis in rats. Biochem. Biophys. Res. Commun. 2019, 516, 1167–1174. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Tamariz-Domínguez, E.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Growth factors and extracellular matrix proteins during wound healing promoted with frozen cultured sheets of human epidermal keratinocytes. Cell Tissue Res. 2002, 307, 79–89. [Google Scholar] [CrossRef]
- Wong, T.; McGrath, J.A.; Navsaria, H. The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 2007, 156, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Mineo, A.; Suzuki, R.; Kuroyanagi, Y. Development of an artificial dermis composed of hyaluronic acid and collagen. J. Biomater. Sci. Polym. Ed. 2013, 24, 726–740. [Google Scholar] [CrossRef]
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef]
- Hassan, W.U.; Greiser, U.; Wang, W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014, 22, 313–325. [Google Scholar] [CrossRef]
- Vu, N.B.; Nguyen, H.T.; Palumbo, R.; Pellicano, R.; Fagoonee, S.; Pham, P.V. Stem cell-derived exosomes for wound healing: Current status and promising directions. Minerva Med. 2021, 112, 384–400. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Sung, J.H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert. Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ozpur, M.A.; Guneren, E.; Canter, H.I.; Karaaltin, M.V.; Ovali, E.; Yogun, F.N.; Baygol, E.G.; Kaplan, S. Generation of Skin Tissue Using Adipose Tissue-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Li, K.N.; Tumbar, T. Hair follicle stem cells as a skin-organizing signaling center during adult homeostasis. EMBO J. 2021, 40, e107135. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Wang, B.; Qinglai, T.; Yang, Q.; Li, M.; Zeng, S.; Yang, X.; Xiao, Z.; Tong, X.; Lei, L.; Li, S. Functional acellular matrix for tissue repair. Mater. Today Bio 2023, 18, 100530. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The Basics of Integra Dermal Regeneration Template and its Expanding Clinical Applications. Semin. Plast. Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef]
- Shakespeare, P.G. The role of skin substitutes in the treatment of burn injuries. Clin. Dermatol. 2005, 23, 413–418. [Google Scholar] [CrossRef]
- Rennekampff, H.O.; Kiessig, V.; Griffey, S.; Greenleaf, G.; Hansbrough, J.F. Acellular human dermis promotes cultured keratinocyte engraftment. J. Burn. Care Rehabil. 1997, 18, 535–544. [Google Scholar] [CrossRef]
- Singh, H.; Purohit, S.D.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Mishra, N.C. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 210–219. [Google Scholar] [CrossRef]
- Zhang, Q.; Johnson, J.A.; Dunne, L.W.; Chen, Y.; Iyyanki, T.; Wu, Y.; Chang, E.I.; Branch-Brooks, C.D.; Robb, G.L.; Butler, C.E. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016, 35, 166–184. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Mcheik, J.N.; Barrault, C.; Levard, G.; Morel, F.; Bernard, F.X.; Lecron, J.C. Epidermal healing in burns: Autologous keratinocyte transplantation as a standard procedure: Update and perspective. Plast. Reconstr. Surg. Glob. Open 2014, 2, e218. [Google Scholar] [CrossRef] [PubMed]
- Fujito, H.; Yamanaka, H.; Tsuge, I.; Katsube, M.; Sakamoto, M.; Fujimoto, M.; Morimoto, N. A Case of a Giant Congenital Melanocytic Nevus Treated by Curettage with the Application of Cultured Epidermal Autografts before 6 Months of Age. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3600. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Chang, P.; Li, S.; Sun, Q.; Guo, K.; Wang, H.; Zhang, L.; Xie, Y.; Zheng, X.; Liu, Y. Large full-thickness wounded skin regeneration using 3D-printed elastic scaffold with minimal functional unit of skin. J. Tissue Eng. 2022, 13, 20417314211063022. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Alam, F.; Butt, H. Smart 3D Printed Auxetic Hydrogel Skin Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 5545–5553. [Google Scholar] [CrossRef]
- Poinas, A.; Perrot, P.; Lorant, J.; Nerrière, O.; Nguyen, J.M.; Saiagh, S.; Frenard, C.; Leduc, A.; Malard, O.; Espitalier, F.; et al. CICAFAST: Comparison of a biological dressing composed of fetal fibroblasts and keratinocytes on a split-thickness skin graft donor site versus a traditional dressing: A randomized controlled trial. Trials 2019, 20, 612. [Google Scholar] [CrossRef]
- Reksodiputro, M.; Widodo, D.; Bashiruddin, J.; Siregar, N.; Malik, S. PRFM enhance wound healing process in skin graft. Facial Plast. Surg. 2014, 30, 670–675. [Google Scholar] [CrossRef]
- Hahn, H.M.; Jeong, Y.S.; Lee, I.J.; Kim, M.J.; Lim, H. Efficacy of split-thickness skin graft combined with novel sheet-type reprocessed micronized acellular dermal matrix. BMC Surg. 2022, 22, 358. [Google Scholar] [CrossRef]
- Götting, M.; Zibell, R.; Jungehülsing, M. Individually moulded silicone dressing in full thickness skin grafts. J. Otolaryngol. Head. Neck Surg. 2022, 51, 33. [Google Scholar] [CrossRef]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Amirsadeghi, A.; Jafari, A.; Eggermont, L.J.; Hashemi, S.S.; Bencherif, S.A.; Khorram, M. Vascularization strategies for skin tissue engineering. Biomater. Sci. 2020, 8, 4073–4094. [Google Scholar] [CrossRef]
- Schumann, P.; Lindhorst, D.; Kampmann, A.; Gellrich, N.C.; Krone-Wolf, S.; Meyer-Lindenberg, A.; von See, C.; Gander, T.; Lanzer, M.; Rücker, M.; et al. Decelerated vascularization in tissue-engineered constructs in association with diabetes mellitus in vivo. J. Diabetes Complicat. 2015, 29, 855–864. [Google Scholar] [CrossRef]
- Suematsu, Y.; Nagano, H.; Kiyosawa, T.; Takeoka, S.; Fujie, T. Angiogenic efficacy of ASC spheroids filtrated on porous nanosheets for the treatment of a diabetic skin ulcer. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1245–1254. [Google Scholar] [CrossRef]
- Robering, J.W.; Al-Abboodi, M.; Titzmann, A.; Horn, I.; Beier, J.P.; Horch, R.E.; Kengelbach-Weigand, A.; Boos, A.M. Tissue Engineering of Lymphatic Vasculature in the Arteriovenous Loop Model of the Rat. Tissue Eng. Part. A 2021, 27, 129–141. [Google Scholar] [CrossRef]
- Wagner, M.E.H.; Kampmann, A.; Schumann-Moor, K.; Gellrich, N.C.; Tavassol, F.; Schmeltekop, F.; Rücker, M.; Lanzer, M.; Gander, T.; Essig, H.; et al. Cell seeding accelerates the vascularization of tissue engineering constructs in hypertensive mice. Hypertens. Res. 2021, 44, 23–35. [Google Scholar] [CrossRef]
- Chogan, F.; Chen, Y.; Wood, F.; Jeschke, M.G. Skin Tissue Engineering Advances in Burns: A Brief Introduction to the Past, the Present, and the Future Potential. J. Burn. Care Res. 2023, 44, S1–S4. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Mahajan, N.; Atala, A.; Murphy, S.V. Advances in Skin Tissue Engineering and Regenerative Medicine. J. Burn. Care Res. 2023, 44, S33–S41. [Google Scholar] [CrossRef]
- Yang, G.; Mahadik, B.; Choi, J.Y.; Fisher, J.P. Vascularization in tissue engineering: Fundamentals and state-of-art. Prog. Biomed. Eng. 2020, 2, 012002. [Google Scholar] [CrossRef]
- Łabuś, W.; Kitala, D.; Szapski, M.; Klama-Baryła, A.; Kraut, M.; Smętek, W. Tissue Engineering in Skin Substitute. Adv. Exp. Med. Biol. 2021, 1345, 193–208. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Hosseini, M.; Koehler, K.R.; Shafiee, A. Biofabrication of Human Skin with Its Appendages. Adv. Healthc. Mater. 2022, 11, e2201626. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques 2011, 51, 239–240, 242, 244 passim. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Qian, E.; Kang, Y. Effects of Macro-/Micro-Channels on Vascularization and Immune Response of Tissue Engineering Scaffolds. Cells 2021, 10, 1514. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, J.; Liu, C.; Ma, C.; Bai, F.; Leng, X.; Chen, Z.; Xie, Z.; Mi, J.; Wu, X. Early-stage bilayer tissue-engineered skin substitute formed by adult skin progenitor cells produces an improved skin structure in vivo. Stem Cell Res. Ther. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- BILLINGHAM, R.E.; RUSSELL, P.S. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits’ skin. Nature 1956, 177, 791–792. [Google Scholar] [CrossRef]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue engineering: Current strategies and future directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, X.; Xia, X.; Han, M.; Li, F.; Li, C.; Li, Y.; Gao, D. Organoid technology for tissue engineering. J. Mol. Cell Biol. 2020, 12, 569–579. [Google Scholar] [CrossRef]
- Kolagar, T.A.; Farzaneh, M.; Nikkar, N.; Khoshnam, S.E. Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances and Limitations. Curr. Stem Cell Res. Ther. 2020, 15, 102–110. [Google Scholar] [CrossRef]
- Tong, A.; Voronov, R. A Minireview of Microfluidic Scaffold Materials in Tissue Engineering. Front. Mol. Biosci. 2021, 8, 783268. [Google Scholar] [CrossRef]
- Wilkins, R.G.; Unverdorben, M. Wound cleaning and wound healing: A concise review. Adv. Skin. Wound Care 2013, 26, 160–163. [Google Scholar] [CrossRef]


| Property | Effect on Wound Healing |
|---|---|
|
|
|
|
|
|
|
|
|
|
| Cell Type | Key Properties |
|---|---|
|
|
| |
|
|
|
|
| Skin Substitute | Key Properties | Examples |
|---|---|---|
| Allograft |
| |
| Cultured Epithelial Autograft (CEA) |
|
|
| Synthetic Skin Substitutes |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Downer, M.; Berry, C.E.; Parker, J.B.; Kameni, L.; Griffin, M. Current Biomaterials for Wound Healing. Bioengineering 2023, 10, 1378. https://doi.org/10.3390/bioengineering10121378
Downer M, Berry CE, Parker JB, Kameni L, Griffin M. Current Biomaterials for Wound Healing. Bioengineering. 2023; 10(12):1378. https://doi.org/10.3390/bioengineering10121378
Chicago/Turabian StyleDowner, Mauricio, Charlotte E. Berry, Jennifer B. Parker, Lionel Kameni, and Michelle Griffin. 2023. "Current Biomaterials for Wound Healing" Bioengineering 10, no. 12: 1378. https://doi.org/10.3390/bioengineering10121378
APA StyleDowner, M., Berry, C. E., Parker, J. B., Kameni, L., & Griffin, M. (2023). Current Biomaterials for Wound Healing. Bioengineering, 10(12), 1378. https://doi.org/10.3390/bioengineering10121378





