Abstract
Chronic wounds are associated with considerable patient morbidity and present a significant economic burden to the healthcare system. Often, chronic wounds are in a state of persistent inflammation and unable to progress to the next phase of wound healing. Placental-derived biomaterials are recognized for their biocompatibility, biodegradability, angiogenic, anti-inflammatory, antimicrobial, antifibrotic, immunomodulatory, and immune privileged properties. As such, placental-derived biomaterials have been used in wound management for more than a century. Placental-derived scaffolds are composed of extracellular matrix (ECM) that can mimic the native tissue, creating a reparative environment to promote ECM remodeling, cell migration, proliferation, and differentiation. Reliable evidence exists throughout the literature to support the safety and effectiveness of placental-derived biomaterials in wound healing. However, differences in source (i.e., anatomical regions of the placenta), preservation techniques, decellularization status, design, and clinical application have not been fully evaluated. This review provides an overview of wound healing and placental-derived biomaterials, summarizes the clinical results of placental-derived scaffolds in wound healing, and suggests directions for future work.
1. Introduction
Complex, hard-to-heal wounds present a significant clinical challenge and are associated with considerable patient morbidity. Often, chronic wounds are unable to progress past the inflammatory phase of wound healing. Consequently, the wound is burdened by elevated concentrations of pro-inflammatory cytokines and imbalanced proteolytic enzymes and protease inhibitors, resulting in high concentrations of matrix metalloproteinases (MMPs), which destroy the extracellular matrix (ECM) [1,2].
A promising strategy for the treatment of nonhealing wounds is the application of placental-derived biomaterials. Placental-derived biomaterials are known for their biocompatibility, biodegradability, and low immunogenicity [3], making them ideal for use in medical applications. Research has shown that placental-derived biomaterials can be used to promote wound healing by providing an ECM scaffold for tissue repair [4], exerting anti-inflammatory effects [4,5,6], facilitating cell migration [5,6], and promoting regeneration [7].
This review provides an overview of wound healing and placental-derived biomaterials, summarizes the clinical results of placental-derived scaffolds in wound healing, and presents directions for future work. The focus of this review is the clinical application of placental-derived scaffolds in wound healing. Although placental-derived cell-based therapy shows promising clinical application, it is beyond the scope of this review.
2. Overview of Wound Healing
Wound healing is the process by which the body repairs and regenerates damaged tissue after an injury. It is a complex and dynamic process that involves a variety of cellular and molecular events, including homeostasis, inflammation, cell migration, proliferation, and tissue remodeling. Wound healing is divided into three distinct phases: inflammatory, proliferative, and remodeling (Figure 1; Table 1) [8].
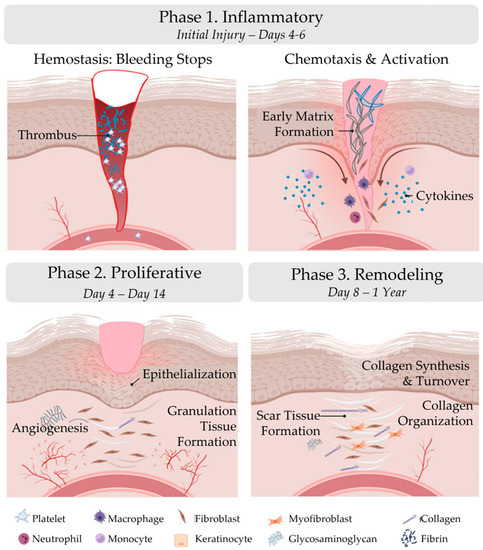
Figure 1.
Phases of wound healing. Wound healing is divided into three distinct phases: inflammatory, proliferative, and remodeling. The figure was originally published by Frontiers under the Creative Commons Attribution License (CC-BY), permitting unrestricted use [9]. The figure has been adapted.

Table 1.
Phases of wound healing. Wound healing is divided into three distinct phases: inflammatory, proliferative, and remodeling.
2.1. Inflammatory Phase
The inflammatory phase of wound healing includes hemostasis and inflammation. Immediately following injury to the skin the inflammatory phase is initiated. Wound formation activates a clotting cascade, involving the temporary release of vasoconstrictors to reduce bleeding, and a fibrin clot is formed. The fibrin clot consists of collagen, platelets, thrombin, and fibronectin, which stimulate the release of cytokines and growth factors, such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), transforming growth factor beta, and platelet factor-4 [8]. The fibrin clot serves as a scaffold for infiltrating cells, such as neutrophils, monocytes, fibroblasts, and endothelial cells, and concentrates the growth factors and cytokines [8,10]. After vasoconstriction, vasodilation occurs, which causes hyperemia and edema [8]. The released chemotactic factors and growth factors complete hemostasis and start inflammation [10].
Neutrophils are first recruited to the wound area and initiate phagocytosis. These cells release reactive oxygen species, proteases, and other enzymes that help debride the wound and remove any debris or bacteria. In addition, these cells release chemokines which serve as chemoattractants for other cell types and release pro-inflammatory cytokines. Next, leukocytes, including monocytes, are active in the wound area.
Macrophages are a type of white blood cell that play a key role in the body’s healing response [11]. Approximately 48–96 h after injury, monocytes differentiate into macrophages [8]. During wound healing, macrophages respond to temporal and spatial cues in their environment by altering their phenotypic polarization [12,13,14]. The M1 phenotype initiates the inflammatory response [10], and in the later phases of wound healing, the M1 phenotype transitions to the M2 phenotype, which facilitates tissue remodeling, repair, and the resolution of the healing process [15,16,17]. In chronic wounds, macrophages are believed to persist in an uncontrolled, pro-inflammatory (M1) activation state [1].
2.2. Proliferative Phase
The proliferative phase of wound healing is the building phase, characterized by epithelialization, angiogenesis, and granulation tissue formation. Fibroblasts and endothelial cells are the primary proliferating cells during this phase. Epithelialization begins as soon as the wound occurs and is stimulated by inflammatory cytokines and growth factors [8,18]. Keratinocytes are stimulated to migrate into the wound area, proliferate, and differentiate into the epidermis. Angiogenesis occurs simultaneously and is stimulated by local hypoxia, vascular endothelial growth factor, platelet-derived growth factor (PDGF), fibroblast growth factor-basic, and the serine protease thrombin [10,18,19]. Angiogenesis is marked by capillary formation and endothelial cell migration [8]. Lastly, granulation tissue formation occurs. Fibroblasts migrate to the wound site from adjacent tissues and proliferate [8,10,18]. In response to PDGF, fibroblasts synthesize a provisional matrix, consisting of type III collagen, glycosaminoglycans, and fibronectin [8].
2.3. Remodeling Phase
During the remodeling phase, granulation tissue formation ends, and wound maturation begins. This phase is characterized by the reorganization of collagen fibers and the contraction of the wound edges. The new tissue is remodeled and organized in an orderly manner to strengthen the repair [18]. Type III collagen is replaced with type I collagen [18], increasing the tensile strength of the wound. Matrix remodeling enzymes, particularly MMPs, play an important role in the remodeling of the local wound environment, as they break down ECM matrix components, such as collagen and elastin, allowing for tissue remodeling and the formation of new blood vessels. Even after a year, however, the wound tissue does not achieve the same strength as that of collagen from uninjured skin due to the formation of scar tissue.
2.4. Wound Formation
When the phases of wound healing are disrupted, abnormal healing occurs, and a chronic wound can develop. A chronic wound is a wound that fails to proceed through a normal, orderly, and timely sequence of repair within an expected timeframe, usually 3 months or longer [20,21]. Often, these wounds remain in a prolonged state of persistent inflammation and are unable to progress to the next phase of wound healing (Figure 2; List 1) [19]. This state of persistent inflammation is characterized by elevated pro-inflammatory cytokines, dysfunctional macrophages, high protease concentrations, and an abnormal ECM. In normal wound healing, the ECM regulates macrophage behavior. However, in a chronic wound, a vicious cycle exists between an abnormal ECM and uncontrolled M1 macrophages.
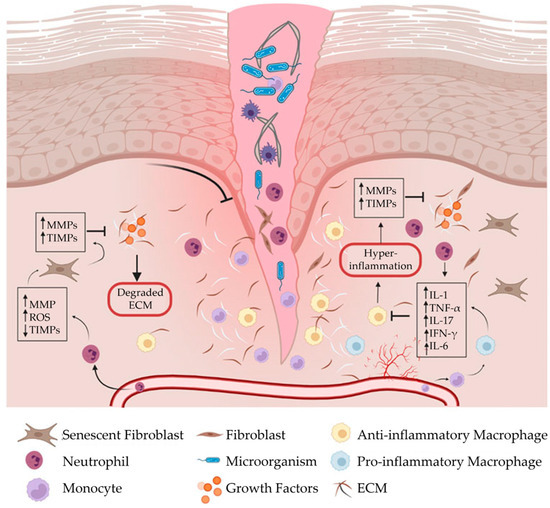
Figure 2.
Chronic wound formation. When the phases of wound healing are disrupted, abnormal healing occurs, and the wound fails to proceed through a normal, orderly, and timely sequence of repair. Often, chronic wounds remain in a prolonged state of persistent inflammation and are characterized by elevated concentrations of pro-inflammatory cytokines, pro-inflammatory (M1) macrophages, and proteases, which destroy the extracellular matrix. In normal wound healing, the extracellular matrix regulates macrophage behavior. In a chronic wound, however, a vicious cycle exists between a dysfunctional extracellular matrix and uncontrolled M1 macrophages. ECM, extracellular matrix; IFN-γ, interferon gamma; IL-1, interleukin-1; IL-6, interleukin-6; IL-17, interleukin-17; MMPs, matrix metalloproteinases; ROS, reactive oxygen species; TIMPs, tissue inhibitors of metalloproteinases; TNF-α, tumor necrosis factor-alpha. The figure was originally published by Frontiers under the Creative Commons Attribution License (CC-BY), permitting unrestricted use [9].
List 1. Characteristics of persistent inflammation [1,2]:
- Elevated pro-inflammatory cytokines;
- Dysfunctional macrophages;
- Imbalanced proteolytic enzymes and protease inhibitors;
- High concentrations of MMPs;
- Abnormal ECM.
2.5. Chronic Inflammation Leading to Fibrosis
Chronic inflammation plays a crucial role in the activation of fibrosis or scarring. When wound healing becomes dysregulated, collagen deposition can devolve into an irreversible accumulation of fibrotic connective tissue by ECM-producing myofibroblasts [22]. As highlighted by Wynn and Ramalingam [22], there are many underlying mechanisms and mediators that contribute to the pathogenesis of fibrosis. For example, during the inflammatory phase of wound healing, neutrophils are recruited to the wound area to initiate phagocytosis. While these cells release reactive oxygen species, proteases, and other enzymes that help debride the wound and remove any debris or bacteria, any prolonged disturbance could lead to excessive tissue damage, leading to fibrosis [22].
2.6. Factors That Impair Wound Healing
Impaired healing and wound formation occur when the body’s natural healing process is disrupted or compromised [23]. The main risk factors that can lead to chronic wound formation can be grouped into local factors and systemic factors (Table 2) [24]. These factors are often interrelated with systemic factors acting locally to influence wound healing [24]. Local factors include oxygenation, infection, and venous insufficiency [19,24,25]. Systemic factors include age, ambulatory status, comorbidities, medications, oncology interventions, and lifestyle habits [19,24,25]. When the healing process is impaired, wounds may take longer to heal or may not heal at all, which increases the risk of complications, such as infection, scarring, and even amputation. Effective treatment may involve addressing the underlying cause of the problem, as well as appropriate wound care to support healing and prevent further complications.

Table 2.
Factors that impair wound healing.
Many patients who experience chronic wounds have underlying medical conditions, such as diabetes, venous insufficiency, or obesity. The most common types of chronic wounds include arterial ulcers, diabetic ulcers, pressure ulcers, and venous ulcers [20,21].
2.7. Economic Impact of Chronic Wounds
It has been estimated that 1–2% of the general population in developed countries will experience a chronic wound [26,27]. In the United States, chronic nonhealing wounds impact 8.2 million Medicare beneficiaries with associated costs ranging from USD 28.1 to USD 96.8 billion [23]. The alarming number of patients affected by chronic nonhealing wounds is expected to rise because of the combined effects of an aging population and the rising rates of diabetes and obesity [28,29,30]. As such, chronic wounds represent a significant economic burden to the healthcare system [31].
2.8. Treatment of Chronic Wounds
The basic tenets of wound care follow the TIME principle: tissue debridement, infection control, moisture balance, and edges of the wound [20,32]. Once standard measures have been taken, an ulcer must be diagnosed, and treatment tailored to the specific type of ulcer. Those with arterial ulcers should be directed to a vascular surgeon for immediate attention [20]. Venous ulcers require compression, elevation of the lower limbs, and exercise if possible [20,33]. Diabetic foot ulcers require offloading and, if necessary, treatment of any underlying peripheral arterial disease [20,34,35]. Pressure ulcers should be managed with a repositioning schedule to reduce pressure on the affected area [20,36,37].
While the TIME principle remains a mainstay of treatment, several additional therapies have been suggested to improve wound healing (List 2).
List 2. Additional wound healing strategies:
- Negative pressure wound therapy [38,39];
- Hyperbaric oxygen therapy [40,41,42,43];
- Autologous platelet-rich plasma [44,45];
- Growth factors [46,47];
- Cell therapy [48,49];
- Scaffolds (e.g., autologous, biologic, and synthetic) [50,51,52,53].
3. Placental-Derived Biomaterials
3.1. Source of Placental-Derived Biomaterials
The placenta is a vital temporary embryonic and later fetal organ that connects the developing fetus to the maternal uterine wall in humans. Placental-derived biomaterials, also referred to as perinatal derivatives, include different placental tissues, sourced from the amniotic sac, amniotic fluid, placental disc, umbilical cord, or a combination of these sources [54,55] (Figure 3). This includes not only the tissues themselves but also the entities within these structures, including fluids, gels, and cells [55,56].
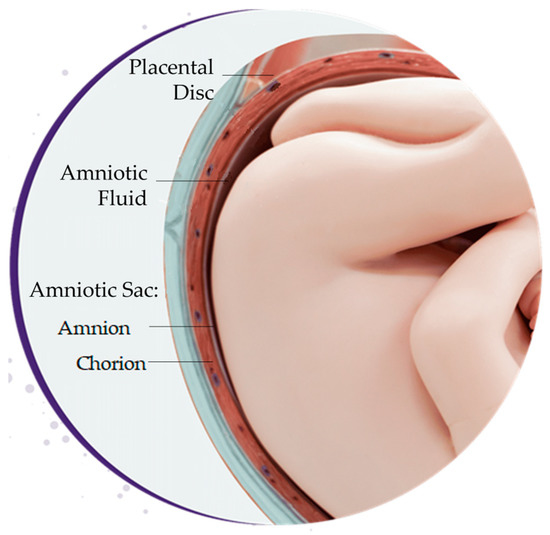
Figure 3.
Sources of placental-derived biomaterials. Placental-derived biomaterials can be sourced from the amniotic sac, amniotic fluid, placental disc, umbilical cord, or a combination of these sources. The amniotic sac is composed of the amnion and chorion layers.
3.1.1. Amniotic Fluid
The amniotic fluid surrounds the embryo and fetus during development and has a myriad of functions [57]. The amniotic fluid contains a variety of nutrients and growth factors that facilitate fetal growth, provides physical protection by cushioning the fetus and umbilical cord, and has antimicrobial properties, which protect the fetus from infection. The amniotic fluid is primarily composed of water and electrolytes, with signaling molecules, peptides, carbohydrates, lipids, proteins, and hormones making up a small percent [58,59]. Hyaluronic acid is also suspended within the amniotic fluid, increasing the viscosity [60].
3.1.2. Amniotic Sac
The amniotic sac is a thin semi-transparent membrane that holds the amniotic fluid for the developing fetus [61]. It is composed of an avascular layer, the amnion, and a highly vascularized layer, the chorion (Figure 4) [61,62].
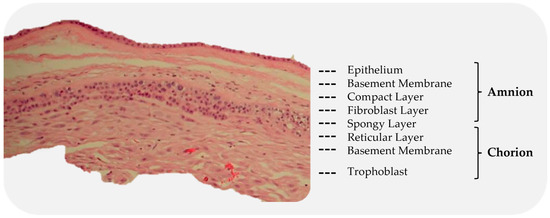
Figure 4.
Subdivision of the amnion and chorion layers. The red structures, visible in the chorion, are blood vessels.
The amniotic membrane (AM) is the innermost of the two membranes, delimiting the amniotic cavity and bathed in amniotic fluid [63]. The AM measures 0.02 mm–0.05 mm in thickness and has a multilayered architecture: an epithelium, a basement membrane, and a collagen-rich stromal layer. The epithelium is a monolayer of metabolically active cuboidal cells with microvilli present on the apical surface. The basement membrane is one of the thickest membranes in the human body [3]. It is made up of a rich collagen framework in addition to bioactive molecules, such as fibronectin and laminin [3,61]. The stromal layer can be further subdivided into three layers: a compact layer, a fibroblast layer, and a spongy layer. The compact layer provides the fibrous structure of the amnion [3]. Interstitial collagens (types I and III) form parallel bundles that provide mechanical integrity, while collagens type V and VI create filamentous connections between the interstitial collagens and the basement membrane [3,61]. The intermediate spongy layer is composed of a nonfibrillar meshwork of mostly type III collagen, as well as an abundance of proteoglycans and glycoproteins [3,64]. It loosely connects the amnion and chorion membranes, which allows the two membranes to be easily separated by blunt dissection [3] (Figure 5).
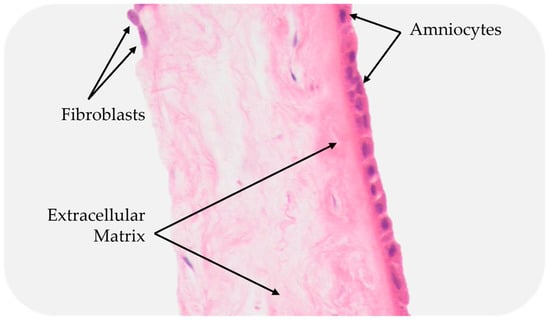
Figure 5.
Cross-section of a placental membrane with the chorion layer removed.
The chorion is the outermost membrane of the amniotic sac and is in contact with the amnion on the inner aspect and the maternal decidua on the outer [62]. Like the AM, the chorion membrane also has several layers: a reticular layer, a basement membrane, and a trophoblast layer. The reticular layer is the thickest layer of the chorion and is composed of collagen types I, III, IV, V, and VI and proteoglycans. The basement membrane is a dense layer of connective tissue (type IV collagen, fibronectin, and laminin) that adheres the trophoblasts and the reticular layer [62,64]. The trophoblast layer interfaces with the maternal decidua on the surface of the placental disc and consists of 2–10 layers of trophoblasts [61,62].
3.1.3. Placental Disc
The placental disc provides a link between the developing fetus and the mother, regulating nutrition, waste removal, hormonal balance, and the immune system while also acting as an immunologically privileged barrier to prevent direct contact between their respective blood supplies [65]. The placental disc is composed of a highly vascularized ECM, containing collagen types I, III, IV, and VI, as well as a vast distribution of noncollagenous glycoproteins and proteoglycans, such as fibronectin, fibrillin I, laminin, thrombospondin I, tenascin C, decorin, heparan sulfate proteoglycans, and elastin [66]. Within the placental disc, a variety of cell types can be found, such as trophoblasts (i.e., syncytiotrophoblasts/cytotrophoblasts), mesenchymal cells, and mesenchymal-derived macrophages, fibroblasts, and fetal vascular cells (i.e., vascular smooth muscle cells, perivascular cells, and endothelial cells) [67]. In addition to the various cell types, the placental disc is also rich in nutrients and cytokines [61].
3.1.4. Umbilical Cord
The umbilical cord contains three vessels, the umbilical vein and two umbilical arteries, which are embedded in Wharton’s Jelly and surrounded by a single epithelial layer, derived from the amnion [68,69]. The umbilical vein transports oxygenated blood from the placenta to the to the fetal heart, and the arteries return deoxygenated blood and waste away from the fetus and to the placenta [69]. Wharton’s Jelly is a mucoid connective tissue composed of a network of glycoprotein microfibrils and collagen fibrils [70]. Collagen types I, III, V, and VI have been identified in Wharton’s Jelly [71,72]. Hyaluronic acid, the most abundant glycosaminoglycan in Wharton’s Jelly [71], creates a hydrated gel around the fibroblasts and collagen fibrils, which maintains the architecture of the umbilical cord and provides protection from pressure [71,73,74]. The cell population of Wharton’s Jelly includes fibroblast-like cells, myofibroblast-like cells, and mesenchymal stem cells [73,75,76].
3.2. Properties of Placental-Derived Biomaterials
The human placenta is a temporary vital organ that is usually discarded as medical waste, making it an easily accessible, cost-effective, and ethical source of raw material. In addition to its availability, the placenta possesses several desirable biological properties that are innate to healing, including angiogenic, anti-inflammatory, antimicrobial, antifibrotic, and immunomodulatory with low immunogenicity (List 3). Complementary to the desirable biological properties, placental tissues have unique ECMs with notable structural and mechanical properties, including elasticity, stiffness, and tensile strength [77]. However, the ECM composition varies with the source, as described in the previous section.
List 3. Biologic properties of the placenta:
- Angiogenic [78];
- Anti-inflammatory [5,6,79,80,81];
- Antimicrobial [3,63,82,83,84];
- Antifibrotic [85,86];
- Immunomodulatory [4,87,88,89,90];
- Low immunogenicity [91].
In addition to the aforementioned properties, placental-derived biomaterials, containing viable cells, can also act through paracrine mechanisms [92]. The cells contained within placental-derived biomaterials stimulate tissue repair by mediating the release of trophic factors [93] and immunomodulation [94,95]. Moreover, the growth factors and cytokines released by placental-derived biomaterials facilitate anti-inflammatory and antimicrobial actions [96,97,98].
3.3. Differences among Placental-Derived Biomaterials
Despite a growing body of evidence demonstrating that placental-derived biomaterials have the capacity to enhance healing, the methods of processing and preparing the tissue are continually evolving. Variations in tissue source, preservation, decellularization, design, and application have the potential to affect the biological and mechanical characteristics of the tissue.
As previously noted, placental-derived biomaterials can be derived from a variety of sources, including the amniotic sac (e.g., amnion and chorion), amniotic fluid, and umbilical cord (e.g., umbilical cord blood, umbilical cord tissue, and Wharton’s Jelly) or a combination of these sources [54]. While this provides a plethora of biomaterials, it also introduces significant variability. Differences in composition exist among sources. For example, the AM has a collagen-rich ECM and contains several bioactive ECM molecules, such as fibronectin, laminin, elastin, and glycosaminoglycans [99], while Wharton’s Jelly is a mucoid connective tissue composed of a network of glycoprotein microfibrils and collagen fibrils [70]. In addition, research has shown that the immunomodulatory properties are source dependent [100]. Even when comparing biomaterials from the same source, interdonor and intradonor variability exists [101].
3.4. Preservation Method
Following delivery, the placenta is usually discarded as medical waste. Alternatively, placental-derived biomaterials can be obtained following normal, healthy, and full-term pregnancies. Comprehensive screening of the donors is required before the tissue is procured and processed. With appropriate consent, the placenta is collected after delivery and donated. After the tissue is collected, the placenta is transported for processing. Although the specific testing requirements may vary depending on local and regional guidelines, proper screening is required to test for infectious diseases, such as human immunodeficiency virus, hepatitis B virus, hepatitis C virus, West Nile, and syphilis. The tissue is sterilized to minimize the risk of disease transmission to recipients and is processed to allow prolonged storage. Tissue preservation is usually accomplished by one of several techniques, most commonly cryopreservation, dehydration, or lyophilization [63]. It is important to note that all methods of preservation compromise the tissue’s integrity to varying degrees.
Cryopreservation or freezing is the most widely used method of tissue preservation. Cryopreservation is a process in which the structure and function of cells, tissues, or organs are preserved by cooling the samples to very low temperatures [102,103]. As part of the cryopreservation process, cryoprotectants are mixed with cells and tissues to reduce ice crystal formation. Various cryoprotectants have been used to preserve placental-derived biomaterials, including glycerol, dimethylsulfoxide, and ethylene glycol. However, the cryopreservation method is criticized for impairing the viability and proliferative capacity of cells and requires the tissue to be shipped and stored at −80 °C [104].
Dehydration is an alternate method of tissue preservation that removes the water from the tissue sample. This process helps to preserve the structural and biochemical activity of placental tissue and prevent the growth of microorganisms and the breakdown of the tissue. Dehydration is achieved using air or heat to dehydrate the tissue [61]. Unlike cryopreservation, dehydration preserves the tissue without the need for freezers, dry ice, or liquid nitrogen and can be shipped and stored at room temperature with a 5+ year shelf-life. The dehydration of tissue provides an additional advantage of allowing allografts to be terminally sterilized, thus reducing the risk of infectious disease transmission from the donor tissue.
Lyophilization or freeze drying involves cooling the tissue to −80 °C and using a sublimation process to remove the water by vacuum desiccation [105]. Like cryopreservation, the freezing step in this process can cause ice crystal formation and damage to the tissues. To reduce tissue damage, sugars can be used as a cryoprotectant to stabilize the proteins [106]. Similar to dehydration, lyophilization allows tissues to be shipped and stored at room temperature without the need for freezers or liquid nitrogen [105].
3.5. Decellularization
Decellularization is a process by which the cellular components of a tissue or organ (e.g., endogenous cells, cell debris, and genetic materials) are removed, while the structural and regulatory proteins of the ECM are preserved (Figure 6).
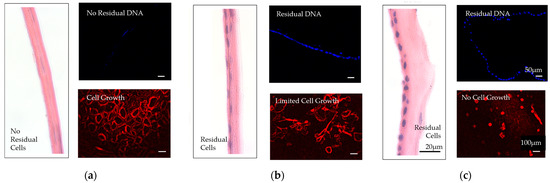
Figure 6.
Comparison of decellularized and nondecellularized amniotic membranes. (a) A proprietarily processed decellularized dehydrated amniotic membrane (BIOVANCE®, Celularity Inc., Florham Park, NJ) is shown. The decellularization process completely removes the residual cellular components, cells, cells debris, and DNA, as well as growth factors and cytokines. The collagen framework remains intact in its native three-dimensional form with essential extracellular matrix molecules. (b) Nondecellularized dehydrated amniotic membrane. (c) Nondecellularized cryopreserved amniotic membranes are shown. These membranes retain residual cellular components, cells, cell debris, and DNA, as well as growth factors and cytokines. The images are used with permission from the original publisher [6]. Images were originally published by and used with permission from John Wiley and Sons.
This process is used to create acellular tissues and organs for use in regenerative medicine and tissue engineering applications. The elimination of cellular content from natural tissue-derived matrices has the potential to promote healing, integration with host tissues, and limit a foreign body reaction [107]. The decellularization process typically involves the use of chemicals, enzymes, physical forces, and/or a combination of these methods to remove cellular components, while preserving the ECM [108,109]. Methods of decellularization affect the structure of the ECM, the structure of the tissue, and the biomechanical behavior. Therefore, it is important to find methods that balance the removal of cellular content with the retention of the structures and entities within the ECM. Several methods have been used in the decellularization of placental tissues [9,109,110,111,112]. Table 3 summarizes the methods and agents used to achieve an acellular scaffold.

Table 3.
Methods used to decellularize placental tissues.
As indicated, tissue preservation and decellularization methods have specific advantages and disadvantages. The processing of placental tissues aims to remove any hazardous materials while preserving the structural and biochemical activity of the tissue to optimize healing.
3.6. Clinical Application
The clinical application of placental-derived biomaterials also varies in form, administration, and delivery system. Placental-derived biomaterials are available in many forms, including sheet scaffolds, injectables, extracts, and cells (Figure 7) [56]. Reported administrations include topical application, intradermal/subcutaneous injection, and intravenous or intraperitoneal injection [49]. Delivery systems include hydrogels, synthetic or natural biomaterials as carriers for transplanted cells, and extracts or secretomes [49].
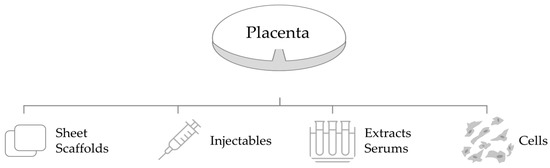
Figure 7.
Forms of placental-derived biomaterials. Various forms of biomaterials are extracted from the placenta, including (1) sheet scaffolds; (2) injectables; (3) extracts/serums; (4) cells.
Several cell types exist within the placenta and have different mechanisms of action, which are source dependent [100]. While the therapeutic benefit of placental-derived mesenchymal stem cells in wound healing has been described throughout the literature [125,126,127], it is not a subject of this review. Rather, this review focuses on the use of human placental-derived biomaterials as scaffolds for wound healing. Placental-derived biomaterials are available as scaffolds in two principal forms: sheets and injectables [128].
The human AM is one of the most widely used and studied placental-derived biomaterials. Human AM sheets are processed to retain the native ECM structure with its high collagen content and key bioactive molecules, such as fibronectin, laminin, glycosaminoglycans, and elastin [99]. Some scaffolds undergo proprietary processing procedures to retain the native growth factors and cytokines [99], while others are decellularized to remove all residual cells, cell debris, growth factors, and cytokines [129]. Although sheet scaffolds are commonly used as a wound covering, they can also be secured to the wound bed, permitting application to wounds of varying severity [117]. Sheet scaffold can be composed of amnion alone [130,131], amnion and chorion [132,133,134,135,136], umbilical cord alone [136,137,138,139,140,141], or umbilical cord and amnion [142]. The sheet ECMs possess the properties associated with their tissue of origin [56]. For example, AM sheet scaffolds are thought to promote healing via epithelialization [143,144,145], reduction of inflammation [6,79,80,81], inhibition of scar tissue formation [85,86], and the ability to act as an antimicrobial agent [82,83,84]. In addition, sheet scaffolds are modified to improve the mechanical properties of the tissue and are available in many configurations, including single layer and multilayer [6], full-thickness, and composite grafts. For example, lamination of sheet scaffolds is performed to create biomaterials with improved handleability and tensile strength [6,146].
Placental-derived biomaterials are also available in an injectable form as suspension allografts [147] and particulates/micronized powders [128,148,149]. The micronized powders can be applied directly to the wound or can be rehydrated and injected through a syringe directly into the wound until approximately one- to two-thirds of the wound is filled [128,148]. Commercially available products are sourced from the amnion, chorion, amniotic fluid, umbilical cord, and placental disc or a combination of these sources. Similar to the sheet scaffolds, the micronized form provides a connective tissue matrix complete with regulatory proteins, which stimulate cell migration [5], proliferation [5], and epithelialization [150,151,152]. Unlike the sheet scaffolds, the injectable form offers the added benefits of filling irregularly shaped and deep tunneling wounds and is reportedly easier to handle intraoperatively [128,149,153].
3.7. Commercial Products
Placental-derived allografts are processed from human tissue according to the American Association of Tissue Banks (AATB) standards and are regulated as a Human Cell, Tissue, or Cellular or Tissue-Based Product (HCT/P) by the US FDA under section 361 of the Public Health Service act as HCT/P (21 CFR, Part 127.10a). According to these guidelines, at a minimum, these products must be minimally manipulated, not combined with drugs or devices, and not reliant on cell metabolic activity as a primary function. In 2017, the company AmnioChor provided a list of placental-derived tissue products sold under section 361 [154]. In total, 116 products were listed. Table 4 and Table 5 provide an updated list of the commercially available placental-derived scaffolds, intended for wound healing applications.

Table 4.
Placental-derived sheet scaffolds for wound healing.

Table 5.
Placental-derived injectable scaffolds for wound healing.
This list is not exhaustive and, therefore, does not represent all commercially available HCT/Ps under section 361. However, it does provide a scope of the available products and variations in source, preservation methods, decellularization status, and unique design characteristics, where applicable.
4. Placental-Derived Biomaterials in Wound Healing: Clinical Results
Given the innate healing properties of the placenta, placental-derived biomaterials have been investigated as advanced wound care therapies for more than 100 years. The first clinical application of placental-derived biomaterials was reported in 1910, when Davis used AM as a substrate for skin transplantation [179]. At that time, only fresh AM was available, which was difficult to procure and carried a significant risk of disease transmission. With advancements in tissue processing and preservation, the use of these biomaterials has expanded considerably and now includes applications in tissue engineering, regenerative medicine, and cell-based therapies [3,148].
4.1. Outcomes
Randomized controlled trials (RCTs) provide the most reliable evidence for determining the effectiveness of a treatment/intervention. A comprehensive literature search was performed to identify RCTs published in the last ten years that evaluated the application of placental-derived scaffolds to treat nonhealing wounds. The PubMed database was queried for the terms “placenta matrix wound healing”, “amnion wound healing”, “chorion wound healing”, “umbilical cord wound healing”, and “amniotic fluid wound healing”. Inclusion criteria included randomized controlled trials reporting on the use of placental-derived scaffolds to treat nonhealing wounds, clinical outcomes, and human subjects. Exclusion criteria included animal data, basic science studies, review articles, articles with inadequate sample sizes for statistical analysis, studies evaluating skin grafts, burn wound healing, punch biopsy wounds, and non-English language literature. The search was limited to the previous 10 years. A summary of the results is provided in Table 6.

Table 6.
Randomized controlled trials evaluating placental-derived biomaterials in wound healing.
Although a large percentage of the randomized controlled trials published within the last 10 years focused on the application of dehydrated human amnion/chorion membranes (dHACMs), the literature also includes studies evaluating the application of dehydrated amnion powder, dried human AM, hypothermically stored AM, and umbilical cord allografts in the treatment of hard-to-heal ulcers. The research consistently demonstrates the effectiveness of placental-derived biomaterials to treat diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs). Several of these high-level studies compared the application of placental-derived biomaterials with standard wound care and demonstrated that the application of placental-derived biomaterials significantly improves the proportion of healed ulcers [132,135,137,171,175,181,184,185,186,187,189,190], time to healing and rates of healing [132,135,137,175,184,185,186,187,191], and ulcer size [171,180,182,189,191]. In addition, Selena and colleagues [190] reported that 79.5% of patients treated with dHACM and multilayer compression therapy reported reduced VLU pain, compared with 52.4% patients who were treated with multilayer compression therapy alone. This finding suggests that the application of placental-derived biomaterials may also alleviate the pain associated with VLUs.
To better understand the ideal application strategy, two studies evaluated the frequency of placental-derived biomaterial application [189,190]. In 2014, Serena and colleagues [189] conducted a multicenter study evaluating the use of dHACM and multilayer compression therapy versus multilayer compression therapy alone in the treatment of VLUs. As a secondary aim, the study compared the proportion of VLUs demonstrating ≥40% closure at 4 weeks in patients receiving one application of dHACM versus two applications of dHACM. After 4 weeks, the proportion of wounds demonstrating a ≥40% closure was similar with one or two applications (62% and 63%, respectively). However, the lack of a significant difference in dHACM application frequency may be attributable to the short study period of 4 weeks. In 2014, Zelen and colleagues [190] conducted a similar study over 12 weeks to determine if the weekly application of dHACMs reduces the time to healing more effectively than biweekly applications for the management of DFUs. Although the proportion of DFUs that achieved complete healing were similar between the two groups, DFUs receiving weekly application of dHACM healed significantly faster than those receiving biweekly dHACM applications. These results suggest that more frequent application reduces the time to healing. However, additional studies are needed to determine the optimal application frequency for placental-derived biomaterials.
Given the significant economic burden associated with treating DFUs and VLUs, investigators have analyzed the economic impact of treating ulcers with placental-derived products [183,185,186,187]. In each of these analyses, the results demonstrate that the application of placental-derived biomaterials is a cost-effective treatment for ulcers. For example, in 2015, Zelen and colleagues [187] conducted an interim analysis of 60 patients and compared the application of Apligraf® (Organogenesis, Inc., Canton, MA, USA) and EpiFix® (MiMedx Group Inc.,Marietta, GA, USA) for the treatment of DFUs. The study found that the application of dHACM significantly reduced the median number of grafts (2.15 vs. 6.2 grafts), as well as the median graft cost per healed wound (USD 1669 vs. USD 9216). The study was continued, expanding the cohort to 100 patients and, again, dHACM was found to significantly reduce the median number of grafts (2.5 vs. 6 grafts) and the median graft cost per healed wound (USD 1517 vs. USD 8918) [186]. Using the data from a previously published study by DiDomenico and colleagues [185], Carter [183] conducted an economics health study to estimate the cost-utility of an aseptically processed dehydrated human amnion and chorion allograft (dHACA) (AmnioBand®, Musculoskeletal Transplant Foundation (MTF), Edison, NJ, USA) plus standard wound care versus standard wound care alone. This data modeling study demonstrated that the use of a dHACA combined with standard wound care compared with standard wound care alone is a cost-effective treatment for DFUs. When collectively considered, these results confirm the superior resource utilization with the application of placental-derived biomaterials.
RCTs have been criticized for excluding a large percentage of the population because of strict inclusion and exclusion criteria and, therefore, not generalizing to the population at large [130]. Although not randomized or controlled, in 2015, Smiell and colleagues [130] conducted a real-world multicenter trial, evaluating wound closure, following treatment of uninfected full- and partial-thickness wounds with a decellularized dehydrated human amniotic membrane (DDHAM). Chronic wounds included venous ulcers, diabetic ulcers, pressure ulcers, arterial ulcers, and collagen vascular ulcers of varying severity and duration. In total, 179 wounds in 165 patients were included. The two most common ulcer types were venous ulcers (50%) and diabetic ulcers (26%). The median time to closure in the Good Wound Care group, a subset of compliant patients, was 6.3 weeks. Most notably, 50% of patients who had failed treatment with one or more advanced biologic therapies achieved complete closure after treatment with DDHAM. No serious or unexpected adverse events were considered related to DDHAM application. The results from this real-world population are compelling and, in many instances, may be considered more useful than “controlled” studies.
4.2. Safety
Several of the recently published RCTs evaluated the safety profile of placental-derived biomaterials and concluded that placental-derived biomaterials are safe in the management of hard-to-heal ulcers [132,137,175,180,181,182,184,185,187,190]. Although most investigators did not attribute any adverse events (AEs) to the application of placental-derived biomaterials [132,137,181,184,185,186,187,190], a few were unable to rule out the possibility that an AE was product related [135,189]. In a 2019 study by Tettelbach and colleagues [135], there were 230 AEs reported during the study period. Of these 230, 3 were deemed possibly product related. There was one case of wound maceration and two positive wound cultures. Similarly, in a 2014 study by Serena and colleagues [189], there were 14 AEs reported. Nine AEs occurred in patients treated with dHACM. Five of the nine AEs were unrelated to treatment, but the remaining four were considered potentially product related. There were two cases of cellulitis on the affected extremity: one wound infection and one wound with increased drainage and abscess. In addition, in 2014, Lavery and colleagues conducted a multicenter study comparing the efficacy of a human viable wound matrix (HVWM, Grafix®, Osiris Therapeutics, Inc., Columbia, MD) with standard wound care in the treatment of DFUs. Outcome variables included the proportion of AEs and wound related infections. The study found that patients treated with the HVWM had significantly fewer AEs (44% versus 66%) and wound-related infections (18% versus 36%), allowing the authors to conclude that HVWM is a safe treatment for DFUs. The results from RCTs in the past 10 years provide reliable evidence that the placental membrane biomaterials are safe for the management of DFUs and VLUs.
In summary, the available evidence demonstrates that placental-derived biomaterials are a safe and effective means to increase the rate of wound closure compared with conventional wound care alone [192,193]. An increased frequency of application appears to accelerate wound closure [190]; however, additional work is needed to determine the optimal application frequency. Additionally, it is important to note that these study results are generalized. Although the products are correctly classified as placental-derived biomaterials, there are many notable differences, namely, in source, form, preservation, decellularization status, and design. These specific differences have the potential to influence the morphological, physical–chemical, and biological properties of the ECM [6,194] and, perhaps, the clinical effectiveness of the product. Additional RCTs are needed to directly compare the efficacy of different commercially available products in the treatment of complex, hard-to-heal ulcers, considering differences in patient (e.g., age, comorbidities, activity level, and ability to comply with protocol) and wound characteristics (e.g., wound etiology, duration, depth, surface area, exudate, bacterial burden, and location) [116].
5. Discussion
Placental-derived biomaterials represent a promising new class of materials for wound healing applications. Placental derivatives have the unique capability of modulating and suppressing innate and adaptive immunities [87,89]. Moreover, placental tissues are a rich source of ECM components, which can be used to create a variety of scaffolds for wound healing.
This review article focused on the use of placental-derived biomaterials as scaffolds in the management of complex, hard-to-heal wounds, such as DFUs and VLUs. Within the last 10 years, reliable evidence has been published demonstrating the safety and effectiveness of placental-derived biomaterials to improve the time to healing. However, there are significant gaps in the literature that require further investigation. Despite the increased market availability of placental-derived scaffolds, it remains unclear how differences in source, preservation techniques, decellularization status, design, and clinical application influence clinical outcomes.
In vitro and clinical evidence supports the application of decellularized placental-derived biomaterials scaffolds in healing [4,5,6,32,148,158]. However, no RCTs have been published comparing decellularized and nondecellularized products in the management of complex wounds. Decellularization of placental-derived biomaterials is performed to limit the immune reaction and inflammatory response induced by the cells, cell debris, and genetic material in the implanted tissue [195]. Decellularized placental tissues have been shown to retain the ECM with a high collagen content (types I, III, and IV) along with key bioactive ECM molecules, such as fibronectin, laminin, glycosaminoglycans, and elastin [99], mimicking the native ECM and creating a reparative environment to promote ECM remodeling, cell migration, proliferation, and differentiation.
Using an in vitro wound healing model, DDHAM (BIOVANCE®, Celularity, Florham Park, NJ, USA) was shown to actively direct macrophage polarization into the M2 phenotype [4], mediating a regenerative response and the resolution of wound healing. Biomaterials, capable of directing macrophage polarization, have the potential to restore a timely transition through the phases of wound healing [185,186,187]. Other in vitro models have also demonstrated that decellularized placental-derived biomaterials attenuate the inflammatory response to a greater extent than other nondecellularized placental-derived scaffolds [5,6]. The more pronounced inflammatory response in nondecellularized products may be attributable to the presence of nonviable cells, growth factors, and cytokines [5]. Moreover, the decellularized products appear to promote the activity of various cell types to a greater extent than their nondecellularized counterparts [5,6]. These in vitro reports suggest that decellularized placental-derived biomaterials may more effectively improve healing in clinical applications.
As reviewed Section 4.1, Smiell and colleagues [130] conducted a real-world, multicenter trial to observe the outcomes associated with the use of DDHAM for the treatment of uninfected partial- and full-thickness wounds. Wound management with DDHAM resulted in wound closure after 6.3 weeks, and no DDHAM-related adverse events were reported. DDHAM also achieved wound closure in patients that previously failed one or more advanced biologic therapies. The results from this real-world population demonstrate the effectiveness of DDHAM in treating several wound types.
In 2009, Letendre and colleagues [131] conducted an open-label study to determine the healing rates for partial- and full-thickness DFUs treated with DDHAM. The secondary objective was to determine a safety profile. Of the 14 patients enrolled in the study, 9 patients completed the 12-week study without deviation. All but one wound responded to treatment with DDHAM. A total of 56% of the patients achieved complete wound closure (Group 1), 33% achieved 50–80% wound closure (Group 2), and 11% achieved less than 50% wound closure (Group 3). No adverse events were associated with DDHAM application. These findings demonstrate that DDHAM promotes wound healing and is a safe treatment for partial- and full-thickness DFUs. While the preliminary results evaluating decellularized placental-derived biomaterials show promise, comparative investigations are needed to assess the effect of decellularization status on clinical outcomes.
Moreover, there is a paucity of evidence evaluating placental-derived injectable scaffolds. None of the identified RCTs evaluated placental-derived injectable scaffolds for wound healing. Like sheet scaffolds, placental-derived injectable scaffolds provide structural and biochemical ECM components, but they also have the added benefits of conforming to the contours of irregularly shaped wounds [148,149,153]. This is highly desirable in the treatment of deep tunneling wounds [128]. To date, clinical research is limited. Although case studies have reported the complete epithelialization of complex wounds following treatment with a decellularized flowable placental-derived biomaterial [128,196], RCTs are warranted.
6. Next Steps
The use of placental-derived biomaterials in wound healing is a rapidly growing field of research. The existing research establishes placental-derived biomaterials as a safe and effective treatment for the management of complex ulcers. However, additional research is needed to fully understand how the differences in placental-derived biomaterial source, preservation techniques, decellularization methods, design, forms, frequency of application, and methods of administration influence clinical outcomes. Future studies are needed to compare the clinical outcomes associated with the application of decellularized and nondecellularized placental-derived biomaterials in wound management.
Author Contributions
Conceptualization, N.M.P., Y.M., R.S. and A.G.; resources, D.L., R.S., A.G., S.A.B. and R.J.H.; writing—original draft preparation, N.M.P.; writing—review and editing, N.M.P., Y.M., R.S. and A.G.; visualization, N.M.P., Y.M., R.S. and A.G.; project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Desiree Long, Raja Sivalenka, Anna Gosiewska, Stephen A. Brigido, and Robert J. Hariri are salaried employees at Celularity Inc. Nicole M. Protzman serves as an independent contractor for Celularity Inc. and reports personal fees from Celularity Inc. during the study. Yong Mao has nothing to disclose.
References
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory m1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, J.D.; Yoon, H.S.; Cho, Y.W. Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng. Part A 2013, 19, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Gleason, J.; Guo, X.; Protzman, N.M.; Mao, Y.; Kuehn, A.; Sivalenka, R.; Gosiewska, A.; Hariri, R.; Brigido, S.A. Decellularized and dehydrated human amniotic membrane in wound management: Modulation of macrophage differentiation and activation. J. Biotechnol. Biomater. 2022, 12, 1000288. [Google Scholar]
- Mao, Y.; John, N.; Protzman, N.M.; Kuehn, A.; Long, D.; Sivalenka, R.; Junka, R.A.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. A decellularized flowable placental connective tissue matrix supports cellular functions of human tenocytes in vitro. J. Exp. Orthop. 2022, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Protzman, N.M.; John, N.; Kuehn, A.; Long, D.; Sivalenka, R.; Junka, R.A.; Shah, A.U.; Gosiewska, A.; Hariri, R.J.; et al. An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 111, 684–700. [Google Scholar] [CrossRef]
- Thompson, P.; Hanson, D.S.; Langemo, D.; Anderson, J. Comparing human amniotic allograft and standard wound care when using total contact casting in the treatment of patients with diabetic foot ulcers. Adv. Skin. Wound Care 2019, 32, 272–277. [Google Scholar] [CrossRef]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Solarte David, V.A.; Guiza-Arguello, V.R.; Arango-Rodriguez, M.L.; Sossa, C.L.; Becerra-Bayona, S.M. Decellularized tissues for wound healing: Towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front. Bioeng. Biotechnol. 2022, 10, 821852. [Google Scholar] [CrossRef]
- Ozgok Kangal, M.K.; Regan, J.P. Wound healing. In Statpearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Suttles, J. Immunosenescence and macrophage functional plasticity: Dysregulation of macrophage function by age-associated microenvironmental changes. Immunol. Rev. 2005, 205, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Spiller, K.L.; Koh, T.J. Macrophage-based therapeutic strategies in regenerative medicine. Adv. Drug Deliv. Rev. 2017, 122, 74–83. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Risser, G.E.; Spiller, K.L. Sequential drug delivery to modulate macrophage behavior and enhance implant integration. Adv. Drug Deliv. Rev. 2019, 149–150, 85–94. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1, Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35s–41s. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that wounds impose on the national health service in the uk. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. Idf diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. Idf diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world--a growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef]
- Guo, X.; Kaplunovsky, A.; Zaka, R.; Wang, C.; Rana, H.; Turner, J.; Ye, Q.; Djuretic, I.; Gleason, J.; Jankovic, V.; et al. Modulation of cell attachment, proliferation, and angiogenesis by decellularized, dehydrated human amniotic membrane in in vitro models. Wounds 2017, 29, 28–38. [Google Scholar] [PubMed]
- O’Meara, S.; Cullum, N.A.; Nelson, E.A. Compression for venous leg ulcers. Cochrane Database Syst. Rev. 2009, 11, Cd000265. [Google Scholar] [CrossRef]
- Lavery, L.A.; Higgins, K.R.; La Fontaine, J.; Zamorano, R.G.; Constantinides, G.P.; Kim, P.J. Randomised clinical trial to compare total contact casts, healing sandals and a shear-reducing removable boot to heal diabetic foot ulcers. Int. Wound J. 2015, 12, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Lipp, A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2013, Cd002302. [Google Scholar] [CrossRef] [PubMed]
- Kottner, J.; Haesler, E. The dissemination of the prevention and treatment of pressure ulcers clinical practice guideline 2014 in the academic literature. Wound Repair Regen. 2020, 28, 580–583. [Google Scholar] [CrossRef]
- Gould, L.; Stuntz, M.; Giovannelli, M.; Ahmad, A.; Aslam, R.; Mullen-Fortino, M.; Whitney, J.D.; Calhoun, J.; Kirsner, R.S.; Gordillo, G.M. Wound healing society 2015 update on guidelines for pressure ulcers. Wound Repair Regen. 2016, 24, 145–162. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Cen, S.; Huang, F. Negative pressure wound therapy versus conventional wound dressings in treatment of open fractures: A systematic review and meta-analysis. Int. J. Surg. 2018, 53, 72–79. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Da, J.; Wu, W.; Ma, F.; Tang, C.; Li, G.; Zhong, D.; Liao, B. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in the treatment of diabetic foot ulcer. Ann. Palliat. Med. 2021, 10, 10830–10839. [Google Scholar] [CrossRef]
- Andrade, S.M.; Santos, I.C. Hyperbaric oxygen therapy for wound care. Rev. Gaucha Enferm. 2016, 37, e59257. [Google Scholar] [CrossRef]
- Nik Hisamuddin, N.A.R.; Wan Mohd Zahiruddin, W.N.; Mohd Yazid, B.; Rahmah, S. Use of hyperbaric oxygen therapy (hbot) in chronic diabetic wound—A randomised trial. Med. J. Malaysia 2019, 74, 418–424. [Google Scholar]
- Thistlethwaite, K.R.; Finlayson, K.J.; Cooper, P.D.; Brown, B.; Bennett, M.H.; Kay, G.; O’Reilly, M.T.; Edwards, H.E. The effectiveness of hyperbaric oxygen therapy for healing chronic venous leg ulcers: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2018, 26, 324–331. [Google Scholar] [CrossRef]
- Salama, S.E.; Eldeeb, A.E.; Elbarbary, A.H.; Abdelghany, S.E. Adjuvant hyperbaric oxygen therapy enhances healing of nonischemic diabetic foot ulcers compared with standard wound care alone. Int. J. Low. Extrem. Wounds 2019, 18, 75–80. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Hunt, C.; Morrow, A.S.; Urtecho, M.; Amin, M.; Shah, S.; Hasan, B.; Abd-Rabu, R.; Ashmore, Z.; et al. The effectiveness and safety of platelet-rich plasma for chronic wounds: A systematic review and meta-analysis. Mayo Clin. Proc. 2021, 96, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Hu, Z.; Zhang, Y.; Wang, P.; Li, S.; Huang, S.; Dong, Y.; Xu, H.; Rong, Y.; Zhu, W.; et al. Clinical studies on platelet-rich plasma therapy for chronic cutaneous ulcers: A systematic review and meta-analysis of randomized controlled trials. Adv. Wound Care (New Rochelle) 2022, 11, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, M.H.; Phillips, S.A.; Stacey, M.C. Growth factors for treating chronic venous leg ulcers: A systematic review and meta-analysis. Wound Repair Regen. 2022, 30, 117–125. [Google Scholar] [CrossRef]
- Brown, G.L.; Nanney, L.B.; Griffen, J.; Cramer, A.B.; Yancey, J.M.; Curtsinger, L.J., 3rd; Holtzin, L.; Schultz, G.S.; Jurkiewicz, M.J.; Lynch, J.B. Enhancement of wound healing by topical treatment with epidermal growth factor. N. Engl. J. Med. 1989, 321, 76–79. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Q.; Sun, X. Comprehensive analysis of cell therapy on chronic skin wound healing: A meta-analysis. Hum. Gene Ther. 2021, 32, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Pichlsberger, M.; Jerman, U.D.; Obradović, H.; Tratnjek, L.; Macedo, A.S.; Mendes, F.; Fonte, P.; Hoegler, A.; Sundl, M.; Fuchs, J.; et al. Systematic review of the application of perinatal derivatives in animal models on cutaneous wound healing. Front. Bioeng. Biotechnol. 2021, 9, 742858. [Google Scholar] [CrossRef]
- Jones, J.E.; Nelson, E.A.; Al-Hity, A. Skin grafting for venous leg ulcers. Cochrane Database Syst. Rev. 2013, 2013, Cd001737. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv. Wound Care (New Rochelle) 2015, 4, 490–500. [Google Scholar] [CrossRef]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatolog. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.A.; Rush, E.W.; Richardson, M.K.; Williams, C.L. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (apligraf) with living, cryopreserved, human skin allograft (theraskin). Clin. Podiatr. Med. Surg. 2018, 35, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental tissues as biomaterials in regenerative medicine. Biomed. Res. Int. 2022, 2022, 6751456. [Google Scholar] [CrossRef]
- Silini, A.R.; Di Pietro, R.; Lang-Olip, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaite, V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal derivatives: Where do we stand? A roadmap of the human placenta and consensus for tissue and cell nomenclature. Front. Bioeng. Biotechnol. 2020, 8, 610544. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and placental derivatives in regenerative therapies: Experimental studies, history, and prospects. Stem Cells Int. 2018, 2018, 4837930. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef]
- Tong, X.L.; Wang, L.; Gao, T.B.; Qin, Y.G.; Qi, Y.Q.; Xu, Y.P. Potential function of amniotic fluid in fetal development---novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J. Chin. Med. Assoc. 2009, 72, 368–373. [Google Scholar] [CrossRef]
- Suliburska, J.; Kocyłowski, R.; Komorowicz, I.; Grzesiak, M.; Bogdański, P.; Barałkiewicz, D. Concentrations of mineral in amniotic fluid and their relations to selected maternal and fetal parameters. Biol. Trace Elem. Res. 2016, 172, 37–45. [Google Scholar] [CrossRef]
- Nyman, E.; Huss, F.; Nyman, T.; Junker, J.; Kratz, G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: In vitro studies of re-epithelialisation in human skin wounds. J. Plast. Surg. Hand Surg. 2013, 47, 89–92. [Google Scholar] [CrossRef]
- Lim, J.J.; Koob, T.J. Placental cells and tissues: The transformative rise in advanced wound care. In Worldwide wound Healing-Innovation in Natural and Conventional Methods; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Bourne, G. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad. Med. J. 1962, 38, 193–201. [Google Scholar] [CrossRef]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef]
- Parry, S.; Strauss, J.F., 3rd. Premature rupture of the fetal membranes. N. Engl. J. Med. 1998, 338, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Aplin, J.D. Placental extracellular matrix: Gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta 2003, 24, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S. Integrated systems physiology: From molecules to function to disease. In Vascular Biology of the Placenta; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Ferguson, V.L.; Dodson, R.B. Bioengineering aspects of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Spurway, J.; Logan, P.; Pak, S. The development, structure and blood flow within the umbilical cord with particular reference to the venous system. Australas. J. Ultrasound Med. 2012, 15, 97–102. [Google Scholar] [CrossRef]
- Meyer, F.A.; Laver-Rudich, Z.; Tanenbaum, R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in wharton’s jelly. Biochim. Biophys. Acta 1983, 755, 376–387. [Google Scholar] [CrossRef]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of wharton’s jelly. Biol. Neonate 1997, 71, 11–21. [Google Scholar] [CrossRef]
- Franc, S.; Rousseau, J.C.; Garrone, R.; van der Rest, M.; Moradi-Améli, M. Microfibrillar composition of umbilical cord matrix: Characterization of fibrillin, collagen vi and intact collagen v. Placenta 1998, 19, 95–104. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef]
- Sobolewski, K.; Małkowski, A.; Bańkowski, E.; Jaworski, S. Wharton’s jelly as a reservoir of peptide growth factors. Placenta 2005, 26, 747–752. [Google Scholar] [CrossRef]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H. Isolation, culture and characterisation of fibroblast-like cells derived from the wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29s. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kubota, T.; Aso, T. Study on myofibroblast differentiation in the stromal cells of wharton’s jelly: Expression and localization of alpha-smooth muscle actin. Early Hum. Dev. 1998, 51, 223–233. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of human amniotic membrane for tissue engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Rennert, R.; Gurtner, G.; Li, W.W. Angiogenic properties of dehydrated human amnion/chorion allografts: Therapeutic potential for soft tissue repair and regeneration. Vasc. Cell 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Shimazaki, J.; Ohashi, Y.; Tsubota, K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 2001, 20, 408–413. [Google Scholar] [CrossRef]
- Moreno, S.E.; Massee, M.; Koob, T.J. Dehydrated human amniotic membrane regulates tenocyte expression and angiogenesis in vitro: Implications for a therapeutic treatment of tendinopathy. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 731–742. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000, 19, 348–352. [Google Scholar] [CrossRef]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Mencucci, R.; Paladini, I.; Menchini, U.; Gicquel, J.J.; Dei, R. Inhibition of viral replication in vitro by antiviral-treated amniotic membrane. Possible use of amniotic membrane as drug-delivering tool. Br. J. Ophthalmol. 2011, 95, 28–31. [Google Scholar] [CrossRef]
- Ramuta, T.; Šket, T.; Starčič Erjavec, M.; Kreft, M.E. Antimicrobial activity of human fetal membranes: From biological function to clinical use. Front. Bioeng. Biotechnol. 2021, 9, 691522. [Google Scholar] [CrossRef]
- Mamede, K.M.; Sant’anna, L.B. Antifibrotic effects of total or partial application of amniotic membrane in hepatic fibrosis. An. Acad. Bras. Cienc. 2019, 91, e20190220. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Deihim, T.; Solati-Hashjin, M.; Peirovi, H. The effects of preservation procedures on amniotic membrane’s ability to serve as a substrate for cultivation of endothelial cells. Cryobiology 2011, 63, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.H.; Berishvili, E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr. Diab Rep. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Perucca Orfei, C.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of ev-free fraction, evs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Papait, A.; Cargnoni, A.; Vertua, E.; Romele, P.; Bonassi Signoroni, P.; Magatti, M.; De Munari, S.; Masserdotti, A.; Pasotti, A.; et al. Cm from intact ham: An easily obtained product with relevant implications for translation in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 540. [Google Scholar] [CrossRef]
- Pozzobon, M.; D’Agostino, S.; Roubelakis, M.G.; Cargnoni, A.; Gramignoli, R.; Wolbank, S.; Gindraux, F.; Bollini, S.; Kerdjoudj, H.; Fenelon, M.; et al. General consensus on multimodal functions and validation analysis of perinatal derivatives for regenerative medicine applications. Front. Bioeng. Biotechnol. 2022, 10, 961987. [Google Scholar] [CrossRef]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Derby, A.; Abd Elkodous, M.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Uchida, S.; Inanaga, Y.; Kobayashi, M.; Hurukawa, S.; Araie, M.; Sakuragawa, N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J. Neurosci. Res. 2000, 62, 585–590. [Google Scholar] [CrossRef]
- Moorefield, E.C.; McKee, E.E.; Solchaga, L.; Orlando, G.; Yoo, J.J.; Walker, S.; Furth, M.E.; Bishop, C.E. Cloned, cd117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS ONE 2011, 6, e26535. [Google Scholar] [CrossRef]
- Kamiya, K.; Wang, M.; Uchida, S.; Amano, S.; Oshika, T.; Sakuragawa, N.; Hori, J. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp. Eye Res. 2005, 80, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Blach-Olszewska, Z.; Gejdel, E. Constitutive and induced cytokine production by human placenta and amniotic membrane at term. Placenta 1997, 18, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Williams, J.D.; Fraser, D.J.; Phillips, A.O. Hyaluronan regulates transforming growth factor-beta1 receptor compartmentalization. J. Biol. Chem. 2004, 279, 25326–25332. [Google Scholar] [CrossRef] [PubMed]
- Grzywocz, Z.; Pius-Sadowska, E.; Klos, P.; Gryzik, M.; Wasilewska, D.; Aleksandrowicz, B.; Dworczynska, M.; Sabalinska, S.; Hoser, G.; Machalinski, B.; et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem. Cytobiol. 2014, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Pereira, M.; Rana, H.; Stout, B.; Lewis, C.; Abramson, S.; Labazzo, K.; Ray, C.; Liu, Q.; Hofgartner, W.; et al. The mechanism of cell interaction and response on decellularized human amniotic membrane: Implications in wound healing. Wounds 2007, 19, 207–217. [Google Scholar]
- Cheng, H.Y. The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. J. Stem Cell Res. Therapy 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Hopkinson, A.; McIntosh, R.S.; Tighe, P.J.; James, D.K.; Dua, H.S. Amniotic membrane for ocular surface reconstruction: Donor variations and the effect of handling on tgf-beta content. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4316–4322. [Google Scholar] [CrossRef]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation: Vitrification and controlled rate cooling. Methods Mol. Biol. 2017, 1590, 41–77. [Google Scholar] [CrossRef]
- Kruse, F.E.; Joussen, A.M.; Rohrschneider, K.; You, L.; Sinn, B.; Baumann, J.; Völcker, H.E. Cryopreserved human amniotic membrane for ocular surface reconstruction. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 68–75. [Google Scholar] [CrossRef]
- Rodríguez-Ares, M.T.; López-Valladares, M.J.; Touriño, R.; Vieites, B.; Gude, F.; Silva, M.T.; Couceiro, J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009, 87, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Cesur, N.P.; Yalman, V.; LaÇİNTÜRkoĞLu, N. Decellularization of tissues and organs. Cumhur. Med. J 2020, 42, 192–197. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization strategies for regenerative medicine: From processing techniques to applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.M.; Majidi Zolbin, M. Decellularization in tissue engineering and regenerative medicine: Evaluation, modification, and application methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Leonel, L.; Miranda, C.; Coelho, T.M.; Ferreira, G.A.S.; Caãada, R.R.; Miglino, M.A.; Lobo, S.E. Decellularization of placentas: Establishing a protocol. Braz. J. Med. Biol. Res. 2017, 51, e6382. [Google Scholar] [CrossRef]
- Schneider, K.H.; Enayati, M.; Grasl, C.; Walter, I.; Budinsky, L.; Zebic, G.; Kaun, C.; Wagner, A.; Kratochwill, K.; Redl, H.; et al. Acellular vascular matrix grafts from human placenta chorion: Impact of ecm preservation on graft characteristics, protein composition and in vivo performance. Biomaterials 2018, 177, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Asgari, H.R.; Najafi, M.; Eftekhari, B.S.; Vardiani, M.; Gholipourmalekabadi, M.; Koruji, M. Optimization of decellularized human placental macroporous scaffolds for spermatogonial stem cells homing. J. Mater. Sci. Mater. Med. 2021, 32, 47. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.J.D.; Delrosario, L.; Fang, J.; Wong, S.Y.; Fang, Q.; Sievers, R.; Kotha, S.; Wang, A.; Farmer, D.; Janaswamy, P.; et al. Development of injectable amniotic membrane matrix for postmyocardial infarction tissue repair. Adv. Healthc. Mater. 2020, 9, e1900544. [Google Scholar] [CrossRef]
- Wilshaw, S.P.; Kearney, J.; Fisher, J.; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.H.; Aigner, P.; Holnthoner, W.; Monforte, X.; Nürnberger, S.; Rünzler, D.; Redl, H.; Teuschl, A.H. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016, 29, 125–134. [Google Scholar] [CrossRef]
- Flynn, L.; Semple, J.L.; Woodhouse, K.A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. A 2006, 79, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Bandehpour, M.; Mozafari, M.; Hashemi, A.; Ghanbarian, H.; Sameni, M.; Salimi, M.; Gholami, M.; Samadikuchaksaraei, A. Decellularized human amniotic membrane: More is needed for an efficient dressing for protection of burns against antibiotic-resistant bacteria isolated from burn patients. Burns 2015, 41, 1488–1497. [Google Scholar] [CrossRef]
- Funamoto, S.; Nam, K.; Kimura, T.; Murakoshi, A.; Hashimoto, Y.; Niwaya, K.; Kitamura, S.; Fujisato, T.; Kishida, A. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010, 31, 3590–3595. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Basok, Y.B.; Grigoriev, A.M.; Nemets, E.A.; Kirillova, A.D.; Kirsanova, L.A.; Lazhko, A.E.; Subbot, A.; Kravchik, M.V.; Khesuani, Y.D.; et al. Decellularization of cartilage microparticles: Effects of temperature, supercritical carbon dioxide and ultrasound on biochemical, mechanical, and biological properties. J. Biomed. Mater. Res. A 2023, 111, 543–555. [Google Scholar] [CrossRef]
- Nonaka, P.N.; Campillo, N.; Uriarte, J.J.; Garreta, E.; Melo, E.; de Oliveira, L.V.; Navajas, D.; Farré, R. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J. Biomed. Mater. Res. A 2014, 102, 413–419. [Google Scholar] [CrossRef]
- Shevtsov, A.; Leybovich, B.; Artyuhov, I.; Maleev, Y.; Peregudov, A. Production of organ extracellular matrix using a freeze-thaw cycle employing extracellular cryoprotectants. Cryo Lett. 2014, 35, 400–406. [Google Scholar]
- Luo, G.; Cheng, W.; He, W.; Wang, X.; Tan, J.; Fitzgerald, M.; Li, X.; Wu, J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010, 18, 506–513. [Google Scholar] [CrossRef]
- Abd-Allah, S.H.; El-Shal, A.S.; Shalaby, S.M.; Abd-Elbary, E.; Mazen, N.F.; Abdel Kader, R.R. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life 2015, 67, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Ghasemi-Mobarakeh, L.; Norouzi, M.R.; Siavash, M.; Hamblin, M.R.; Fesharaki, M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int. Immunopharmacol. 2021, 101, 108282. [Google Scholar] [CrossRef]
- Protzman, N.M.; Brigido, S.A. Recent advances in acellular regenerative tissue scaffolds. Clin. Podiatr. Med. Surg. 2015, 32, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denoziere, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Smiell, J.M.; Treadwell, T.; Hahn, H.D.; Hermans, M.H. Real-world experience with a decellularized dehydrated human amniotic membrane allograft. Wounds 2015, 27, 158–169. [Google Scholar] [PubMed]
- Letendre, S.; LaPorta, G.; O’Donnell, E.; Dempsey, J.; Leonard, K. Pilot trial of biovance collagen-based wound covering for diabetic ulcers. Adv. Skin. Wound Care 2009, 22, 161–166. [Google Scholar] [CrossRef]
- Bianchi, C.; Cazzell, S.; Vayser, D.; Reyzelman, A.M.; Dosluoglu, H.; Tovmassian, G. A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (epifix(®)) allograft for the treatment of venous leg ulcers. Int. Wound J. 2018, 15, 114–122. [Google Scholar] [CrossRef]
- Mrugala, A.; Sui, A.; Plummer, M.; Altman, I.; Papineau, E.; Frandsen, D.; Hill, D.; Ennis, W.J. Amniotic membrane is a potential regenerative option for chronic non-healing wounds: A report of five cases receiving dehydrated human amnion/chorion membrane allograft. Int. Wound J. 2016, 13, 485–492. [Google Scholar] [CrossRef]
- Caporusso, J.; Abdo, R.; Karr, J.; Smith, M.; Anaim, A. Clinical experience using a dehydrated amnion/chorion membrane construct for the management of wounds. Wounds 2019, 31, S19–S27. [Google Scholar] [PubMed]
- Tettelbach, W.; Cazzell, S.; Reyzelman, A.M.; Sigal, F.; Caporusso, J.M.; Agnew, P.S. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dhacm allograft in the management of diabetic foot ulcers: A prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int. Wound J. 2019, 16, 19–29. [Google Scholar] [CrossRef]
- Tettelbach, W.H.; Cazzell, S.M.; Hubbs, B.; Jong, J.L.; Forsyth, R.A.; Reyzelman, A.M. The influence of adequate debridement and placental-derived allografts on diabetic foot ulcers. J. Wound Care 2022, 31, S16–S26. [Google Scholar] [CrossRef]
- Tettelbach, W.; Cazzell, S.; Sigal, F.; Caporusso, J.M.; Agnew, P.S.; Hanft, J.; Dove, C. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (epicord) allograft for the treatment of diabetic foot ulcers. Int. Wound J. 2019, 16, 122–130. [Google Scholar] [CrossRef]
- Garoufalis, M.; Tettelbach, W. Expandable dehydrated human umbilical cord allograft for the management of nonhealing lower extremity wounds in patients with diabetes: A case series. Wounds 2022, 34, E91–E95. [Google Scholar] [CrossRef]
- Couture, M. A single-center, retrospective study of cryopreserved umbilical cord for wound healing in patients suffering from chronic wounds of the foot and ankle. Wounds 2016, 28, 217–225. [Google Scholar]
- Raphael, A. A single-centre, retrospective study of cryopreserved umbilical cord/amniotic membrane tissue for the treatment of diabetic foot ulcers. J. Wound Care 2016, 25, S10–S17. [Google Scholar] [CrossRef]
- Raphael, A.; Grimes, L. Implantation of cryopreserved umbilical cord allograft in hard-to-heal foot wounds: A retrospective study. J. Wound Care 2020, 29, S12–S17. [Google Scholar] [CrossRef]
- Bemenderfer, T.B.; Anderson, R.B.; Odum, S.M.; Davis, W.H. Effects of cryopreserved amniotic membrane-umbilical cord allograft on total ankle arthroplasty wound healing. J. Foot Ankle Surg. 2019, 58, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.J.; Inatomi, T.J.; Sotozono, C.J.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mrna and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mermet, I.; Pottier, N.; Sainthillier, J.M.; Malugani, C.; Cairey-Remonnay, S.; Maddens, S.; Riethmuller, D.; Tiberghien, P.; Humbert, P.; Aubin, F. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007, 15, 459–464. [Google Scholar] [CrossRef]
- Mohajeri-Tehrani, M.R.; Variji, Z.; Mohseni, S.; Firuz, A.; Annabestani, Z.; Zartab, H.; Rad, M.A.; Tootee, A.; Dowlati, Y.; Larijani, B. Comparison of a bioimplant dressing with a wet dressing for the treatment of diabetic foot ulcers: A randomized, controlled clinical trial. Wounds 2016, 28, 248–254. [Google Scholar]
- Moore, M.C.; Van De Walle, A.; Chang, J.; Juran, C.; McFetridge, P.S. Human perinatal-derived biomaterials. Adv. Healthc. Mater. 2017, 6, 21700345. [Google Scholar] [CrossRef]
- Irvin, J.; Danchik, C.; Rall, J.; Babcock, A.; Pine, M.; Barnaby, D.; Pathakamuri, J.; Kuebler, D. Bioactivity and composition of a preserved connective tissue matrix derived from human placental tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2731–2740. [Google Scholar] [CrossRef]
- Brigido, S.A.; Carrington, S.C.; Protzman, N.M. The use of decellularized human placenta in full-thickness wound repair and periarticular soft tissue reconstruction: An update on regenerative healing. Clin. Podiatr. Med. Surg. 2018, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Protzman, N.M.; Mao, Y.; Sivalenka, R.; Long, D.; Gosiewska, A.; Hariri, R.; Brigido, S.A. Flowable placental connective tissue matrices for tendon repair: A review. J. Biol. Med. 2022, 6, 010–020. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, S.; Wu, H.; Tian, S.; Zhang, Y.; Wang, L.; Fang, H.; Luo, P.; Wang, X.; Hu, X.; et al. Topical administration of cryopreserved living micronized amnion accelerates wound healing in diabetic mice by modulating local microenvironment. Biomaterials 2017, 113, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Garoufalis, M.; Nagesh, D.; Sanchez, P.J.; Lenz, R.; Park, S.J.; Ruff, J.G.; Tien, A.; Goldsmith, J.; Seat, A. Use of dehydrated human amnion/chorion membrane allografts in more than 100 patients with six major types of refractory nonhealing wounds. J. Am. Podiatr. Med. Assoc. 2018, 108, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B. The use of micronized dehydrated human amnion/chorion membrane allograft for the treatment of diabetic foot ulcers: A case series. Wounds 2016, 28, 152–157. [Google Scholar] [PubMed]
- Brigido, S.A.; Schwartz, E.; McCarroll, R.; Hardin-Young, J. Use of an acellular flowable dermal replacement scaffold on lower extremity sinus tract wounds: A retrospective series. Foot Ankle Spec. 2009, 2, 67–72. [Google Scholar] [CrossRef]
- Amniochor. Available online: https://celltrials.org/sites/default/files/report_files/Amniotic-Membrane-Tissue-Allograft-Products_AmnioChor20170529.pdf (accessed on 21 May 2023).
- Duarte, I.G.; Duval-Araujo, I. Amniotic membrane as a biological dressing in infected wound healing in rabbits. Acta Cir. Bras. 2014, 29, 334–339. [Google Scholar] [CrossRef]
- Caputo, W.J.; Vaquero, C.; Monterosa, A.; Monterosa, P.; Johnson, E.; Beggs, D.; Fahoury, G.J. A retrospective study of cryopreserved umbilical cord as an adjunctive therapy to promote the healing of chronic, complex foot ulcers with underlying osteomyelitis. Wound Repair Regen. 2016, 24, 885–893. [Google Scholar] [CrossRef]
- Cooke, M.; Tan, E.K.; Mandrycky, C.; He, H.; O’Connell, J.; Tseng, S.C. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J. Wound Care 2014, 23, 465–474, 476. [Google Scholar] [CrossRef] [PubMed]
- Brigido, S.A.; Riniker, M.L.; Protzman, N.M.; Constant, D.D. Application of acellular amniotic scaffold following total ankle replacement: A retrospective comparison. Clin. Res. Foot Ankle 2018, 6. [Google Scholar] [CrossRef]
- Snyder, R.J.; Shimozaki, K.; Tallis, A.; Kerzner, M.; Reyzelman, A.; Lintzeris, D.; Bell, D.; Rutan, R.L.; Rosenblum, B. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds 2016, 28, 70–77. [Google Scholar]
- Lintzeris, D.; Yarrow, K.; Johnson, L.; White, A.; Hampton, A.; Strickland, A.; Albert, K.; Cook, A. Use of a dehydrated amniotic membrane allograft on lower extremity ulcers in patients with challenging wounds: A retrospective case series. Ostomy Wound Manag. 2015, 61, 30–36. [Google Scholar]
- Barr, S.M. Dehydrated amniotic membrane allograft for treatment of chronic leg ulcers in patients with multiple comorbidities: A case series. J. Am. Coll. Clin. Wound Spec. 2014, 6, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abdo, R.J. Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: A prospective case series. J. Wound Care 2016, 25, S4–S9. [Google Scholar] [CrossRef]
- Rosenblum, B.I. A retrospective case series of a dehydrated amniotic membrane allograft for treatment of unresolved diabetic foot ulcers. J. Am. Podiatr. Med. Assoc. 2016, 106, 328–337. [Google Scholar] [CrossRef]
- Doucette, M.; Payne, K.M.; Lough, W.; Beck, A.; Wayment, K.; Huffman, J.; Bond, L.; Thomas-Vogel, A.; Langley, S. Early advanced therapy for diabetic foot ulcers in high amputation risk veterans: A cohort study. Int. J. Low Extrem. Wounds 2022, 21, 111–119. [Google Scholar] [CrossRef]
- Tsai, G.L.; Zilberbrand, D.; Liao, W.J.; Horl, L.P. Healing hard-to-heal diabetic foot ulcers: The role of dehydrated amniotic allograft with cross-linked bovine-tendon collagen and glycosaminoglycan matrix. J. Wound Care 2021, 30, S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Bonvallet, P.P.; Damaraju, S.M.; Modi, H.N.; Gandhi, A.; McFetridge, P.S. Biological characterization of dehydrated amniotic membrane allograft: Mechanisms of action and implications for wound care. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3076–3083. [Google Scholar] [CrossRef]
- Boyar, V.; Galiczewski, C. Efficacy of dehydrated human amniotic membrane allograft for the treatment of severe extravasation injuries in preterm neonates. Wounds 2018, 30, 224–228. [Google Scholar] [PubMed]
- Lei, J.; Priddy, L.B.; Lim, J.J.; Massee, M.; Koob, T.J. Identification of extracellular matrix components and biological factors in micronized dehydrated human amnion/chorion membrane. Adv. Wound Care (New Rochelle) 2017, 6, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bullard, J.D.; Lei, J.; Lim, J.J.; Massee, M.; Fallon, A.M.; Koob, T.J. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1035–1046. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Vines, J.B.; Mowry, K.C. In vitro assessment of a novel, hypothermically stored amniotic membrane for use in a chronic wound environment. Int. Wound J. 2017, 14, 993–1005. [Google Scholar] [CrossRef]
- Serena, T.E.; Yaakov, R.; Moore, S.; Cole, W.; Coe, S.; Snyder, R.; Patel, K.; Doner, B.; Kasper, M.A.; Hamil, R.; et al. A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J. Comp. Eff. Res. 2020, 9, 23–34. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Vines, J.B.; Kimmerling, K.A.; Mowry, K.C. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds 2017, 29, E36–E40. [Google Scholar]
- McQuilling, J.P.; Sanders, M.; Poland, L.; Sanders, M.; Basadonna, G.; Waldrop, N.E.; Mowry, K.C. Dehydrated amnion/chorion improves achilles tendon repair in a diabetic animal model. Wounds 2019, 31, 19–25. [Google Scholar]
- McQuilling, J.P.; Burnette, M.; Kimmerling, K.A.; Kammer, M.; Mowry, K.C. A mechanistic evaluation of the angiogenic properties of a dehydrated amnion chorion membrane in vitro and in vivo. Wound Repair Regen. 2019, 27, 609–621. [Google Scholar] [CrossRef]
- Lavery, L.A.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J. The efficacy and safety of grafix® for the treatment of chronic diabetic foot ulcers: Results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Farivar, B.S.; Toursavadkohi, S.; Monahan, T.S.; Sharma, J.; Ucuzian, A.A.; Kundi, R.; Sarkar, R.; Lal, B.K. Prospective study of cryopreserved placental tissue wound matrix in the management of chronic venous leg ulcers. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 228–233. [Google Scholar] [CrossRef]
- Gibbons, G.W. Grafix(®), a cryopreserved placental membrane, for the treatment of chronic/stalled wounds. Adv. Wound Care (New Rochelle) 2015, 4, 534–544. [Google Scholar] [CrossRef]
- Cole, A.; Lee, M.; Koloff, Z.; Ghani, K.R. Complex re-do robotic pyeloplasty using cryopreserved placental tissue: An adjunct for success. Int. Braz. J. Urol. 2021, 47, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Davis, J. Skin transplantation with a review of 550 cases at the johns hopkins hospital. Johns Hopkins Med. 1910, 15, 307. [Google Scholar]
- Mohammadi Tofigh, A.; Tajik, M. Comparing the standard surgical dressing with dehydrated amnion and platelet-derived growth factor dressings in the healing rate of diabetic foot ulcer: A randomized clinical trial. Diabetes Res. Clin. Pract. 2022, 185, 109775. [Google Scholar] [CrossRef]
- Serena, T.E.; Orgill, D.P.; Armstrong, D.G.; Galiano, R.D.; Glat, P.M.; Carter, M.J.; Kaufman, J.P.; Li, W.W.; Zelen, C.M. A multicenter, randomized, controlled, clinical trial evaluating dehydrated human amniotic membrane in the treatment of venous leg ulcers. Plast. Reconstr. Surg. 2022, 150, 1128–1136. [Google Scholar] [CrossRef]
- Game, F.; Gray, K.; Davis, D.; Sherman, R.; Chokkalingam, K.; Connan, Z.; Fakis, A.; Jones, M. The effectiveness of a new dried human amnion derived membrane in addition to standard care in treating diabetic foot ulcers: A patient and assessor blind, randomised controlled pilot study. Int. Wound J. 2021, 18, 692–700. [Google Scholar] [CrossRef]
- Carter, M.J. Dehydrated human amnion and chorion allograft versus standard of care alone in treatment of wagner 1 diabetic foot ulcers: A trial-based health economics study. J. Med. Econ. 2020, 23, 1273–1283. [Google Scholar] [CrossRef]
- Bianchi, C.; Tettelbach, W.; Istwan, N.; Hubbs, B.; Kot, K.; Harris, S.; Fetterolf, D. Variations in study outcomes relative to intention-to-treat and per-protocol data analysis techniques in the evaluation of efficacy for treatment of venous leg ulcers with dehydrated human amnion/chorion membrane allograft. Int. Wound J. 2019, 16, 761–767. [Google Scholar] [CrossRef]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Jacobs, A.M.; Zelen, C.M. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomised, multi-centre clinical trial in 80 patients. Int. Wound J. 2018, 15, 950–957. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Gould, L.; Le, L.; Carter, M.J.; Keller, J.; Li, W.W. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: A prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int. Wound J. 2016, 13, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.J.; Keller, J.; Li, W.W. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int. Wound J. 2015, 12, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Yaakov, R.; DiMarco, D.; Le, L.; Taffe, E.; Donaldson, M.; Miller, M. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: Correlation between 4-week and 24-week outcomes. J. Wound Care 2015, 24, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Carter, M.J.; Le, L.T.; Sabo, M.J.; DiMarco, D.T. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. Multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014, 22, 688–693. [Google Scholar] [CrossRef]
- Zelen, C.M.; Serena, T.E.; Snyder, R.J. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int. Wound J. 2014, 11, 122–128. [Google Scholar] [CrossRef]
- Zelen, C.M.; Poka, A.; Andrews, J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis—A feasibility study. Foot Ankle Int. 2013, 34, 1332–1339. [Google Scholar] [CrossRef]
- Lakmal, K.; Basnayake, O.; Hettiarachchi, D. Systematic review on the rational use of amniotic membrane allografts in diabetic foot ulcer treatment. BMC Surg. 2021, 21, 87. [Google Scholar] [CrossRef]
- Paggiaro, A.O.; Menezes, A.G.; Ferrassi, A.D.; De Carvalho, V.F.; Gemperli, R. Biological effects of amniotic membrane on diabetic foot wounds: A systematic review. J. Wound Care 2018, 27, S19–S25. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Farhadihosseinabadi, B.; Faraji, M.; Nourani, M.R. How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns 2020, 46, 1254–1271. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-specific decellularization methods: Rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Mrdjenovich, D.E. Human placental connective tissue matrix (hctm) a pathway toward improved outcomes. In Proceedings of the Symposium on Advanced Wound Care & Wound Healing Society, San Diego, CA, USA, 5 April 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).