Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method
Abstract
1. Introduction
2. Materials and Methods
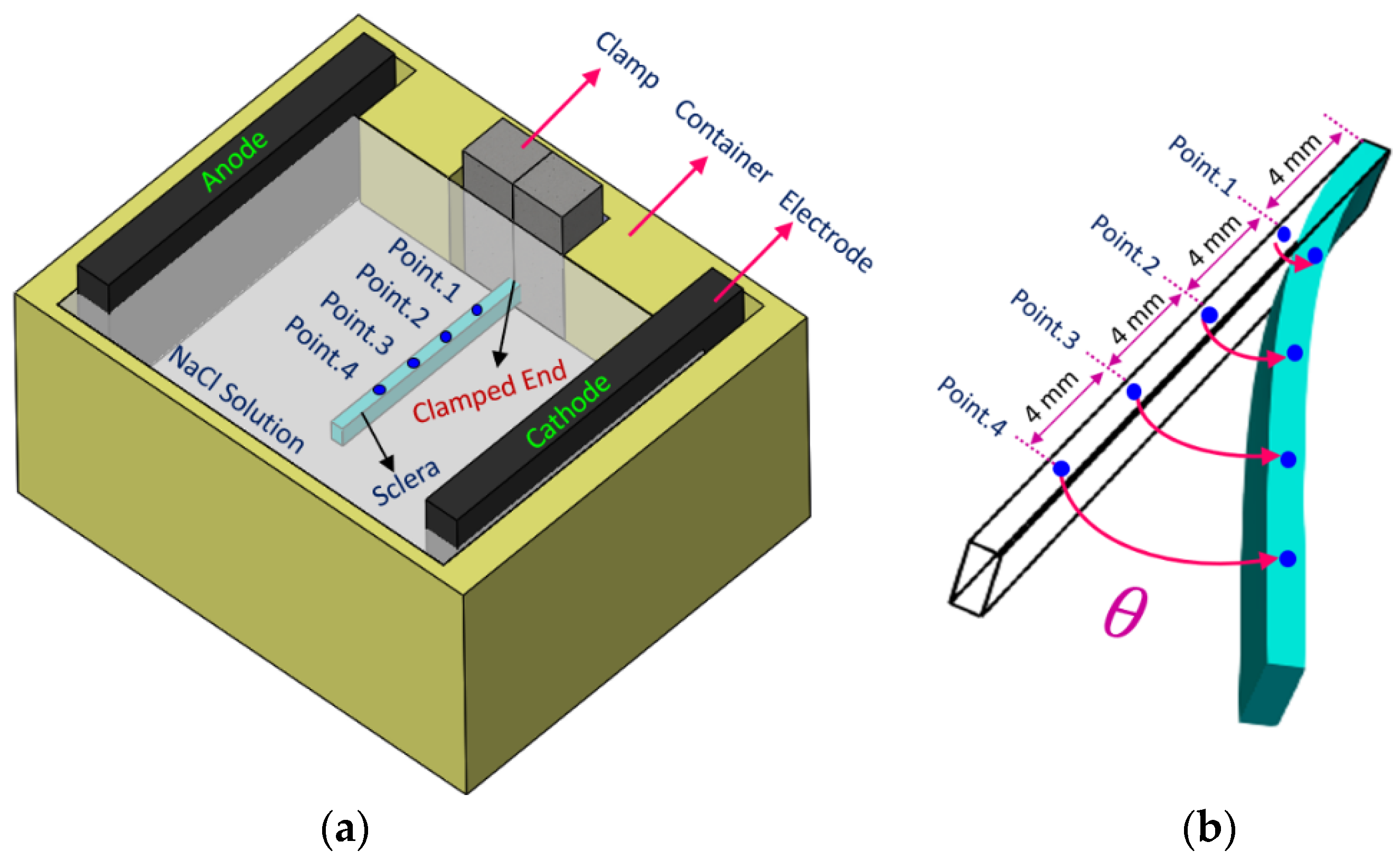
2.1. Experiments
2.2. Numerical Model
2.2.1. Calculation Domain
2.2.2. Constitutive Equations
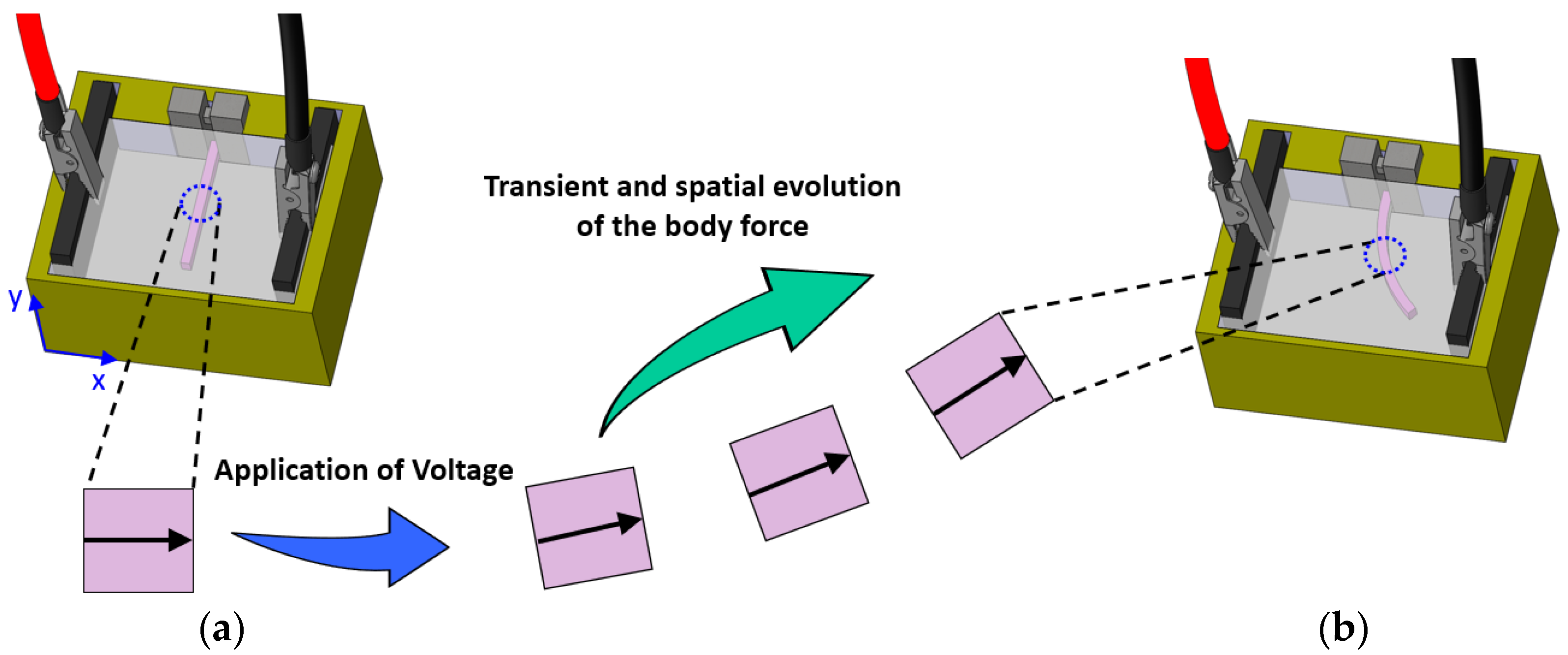
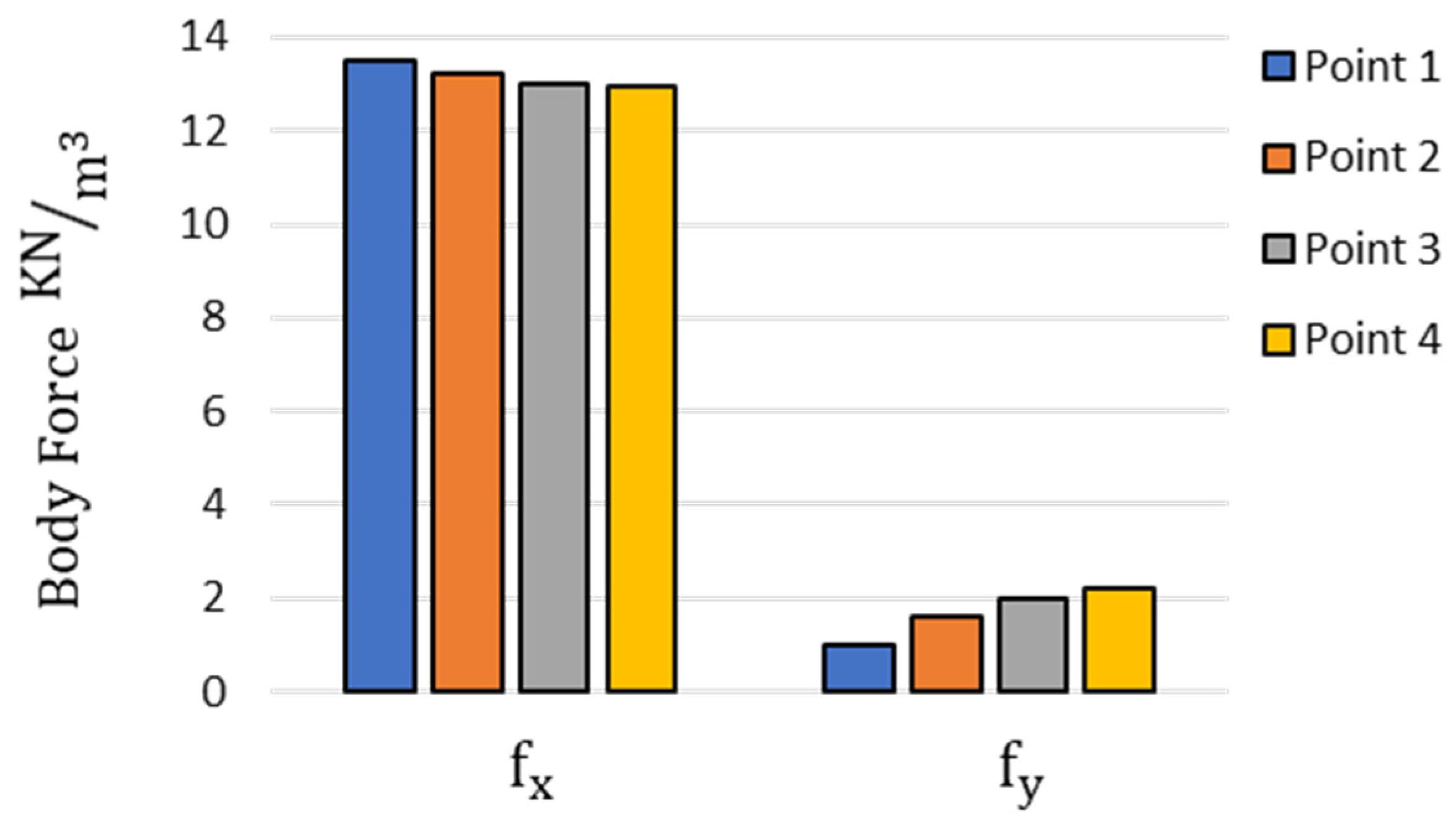
2.2.3. Body Force
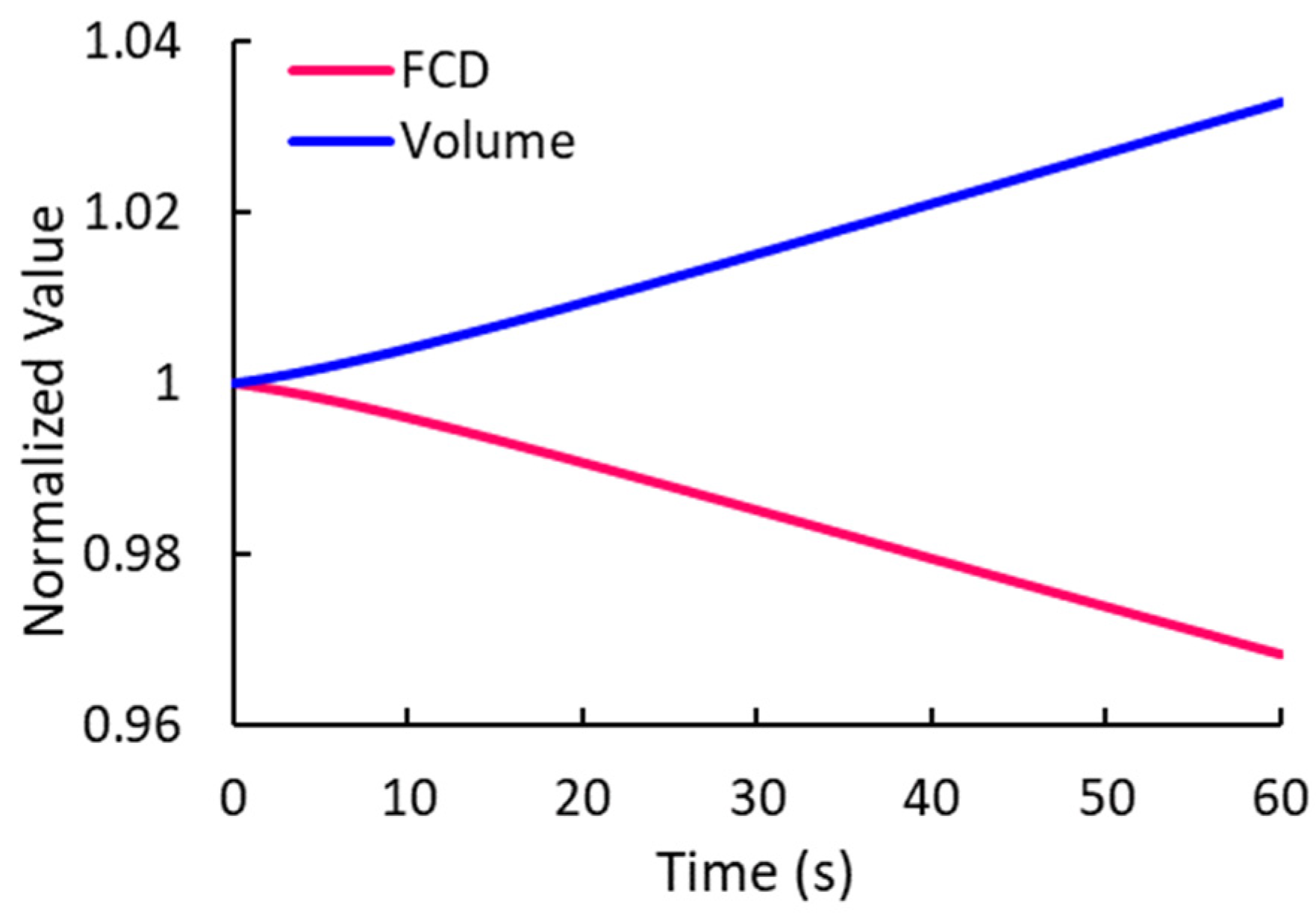
2.2.4. Transient Change in Fixed Charge Density (FCD)
2.2.5. Moving Mesh Implementation
2.2.6. Constants and Initial and Boundary Conditions
2.2.7. Fitting the Numerical Model
3. Results
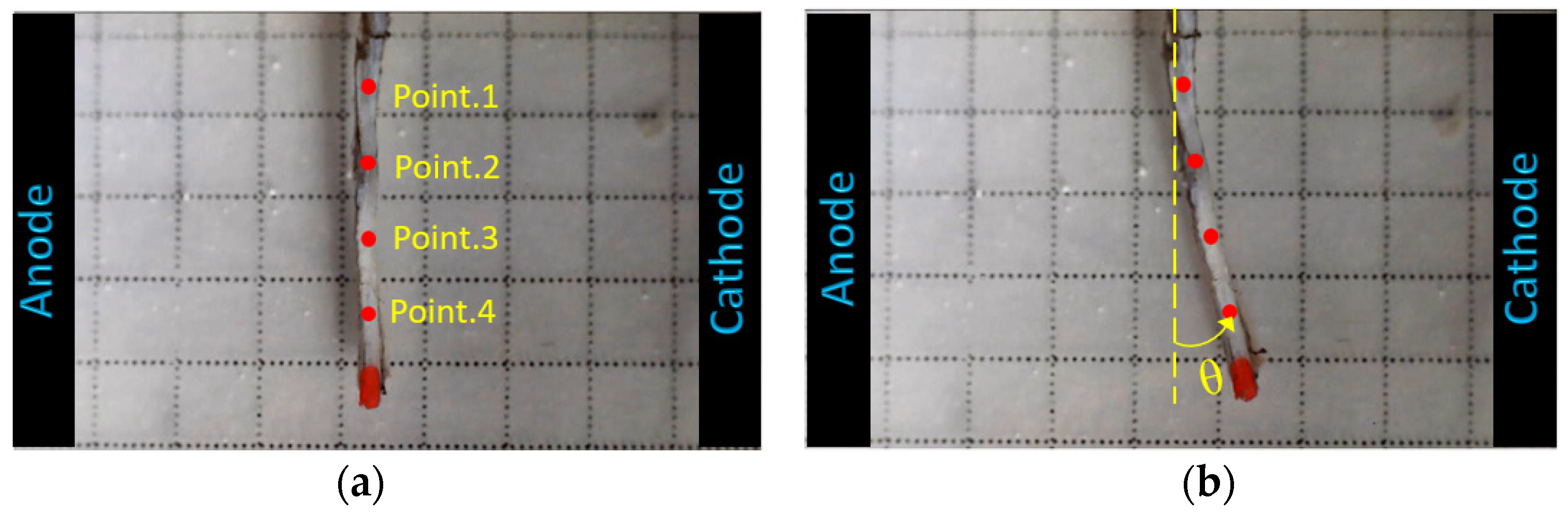
3.1. Experimental Results
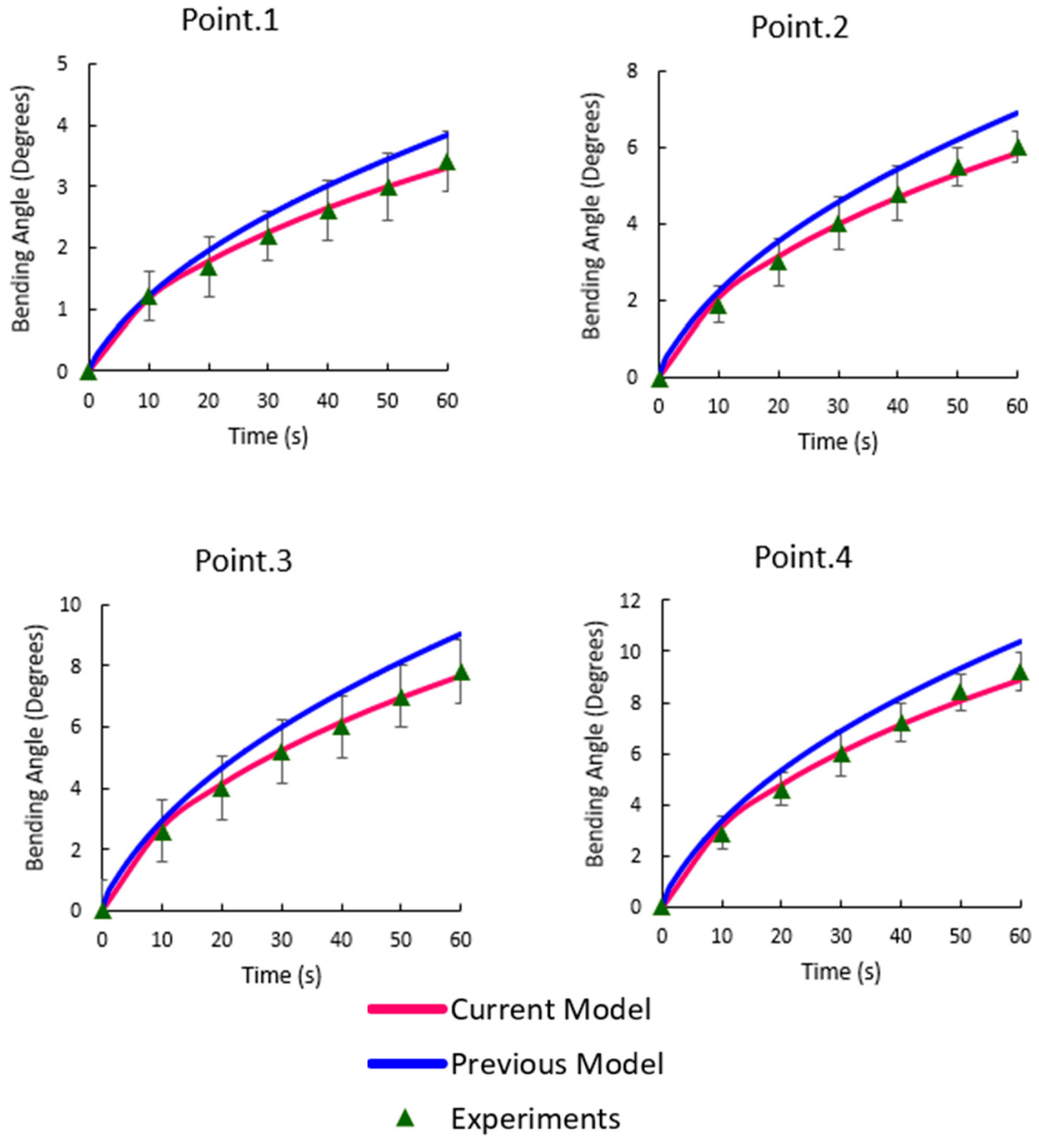
3.2. Bending Angles at the Target Points
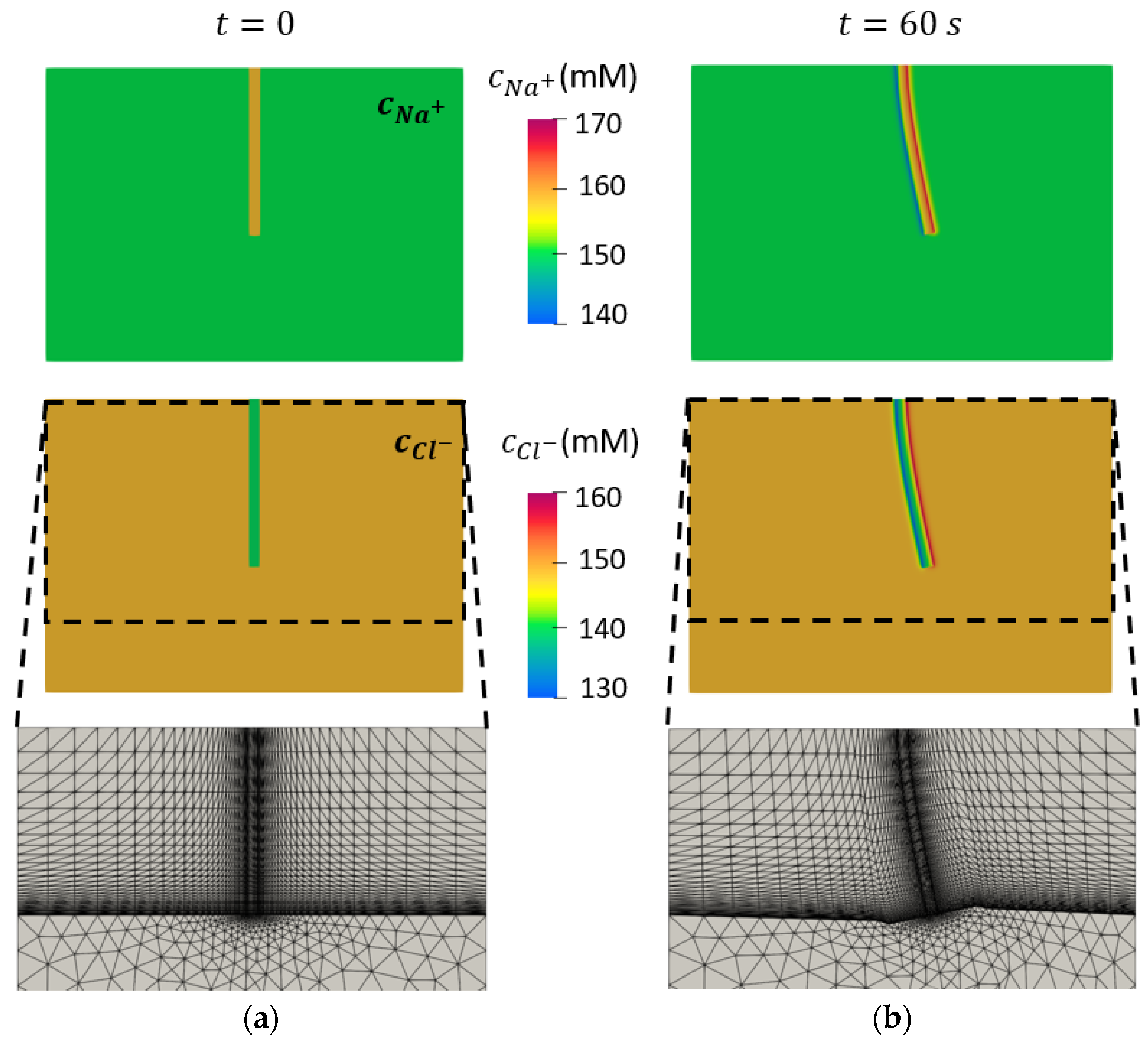
3.3. Concentrations of Mobile Ions
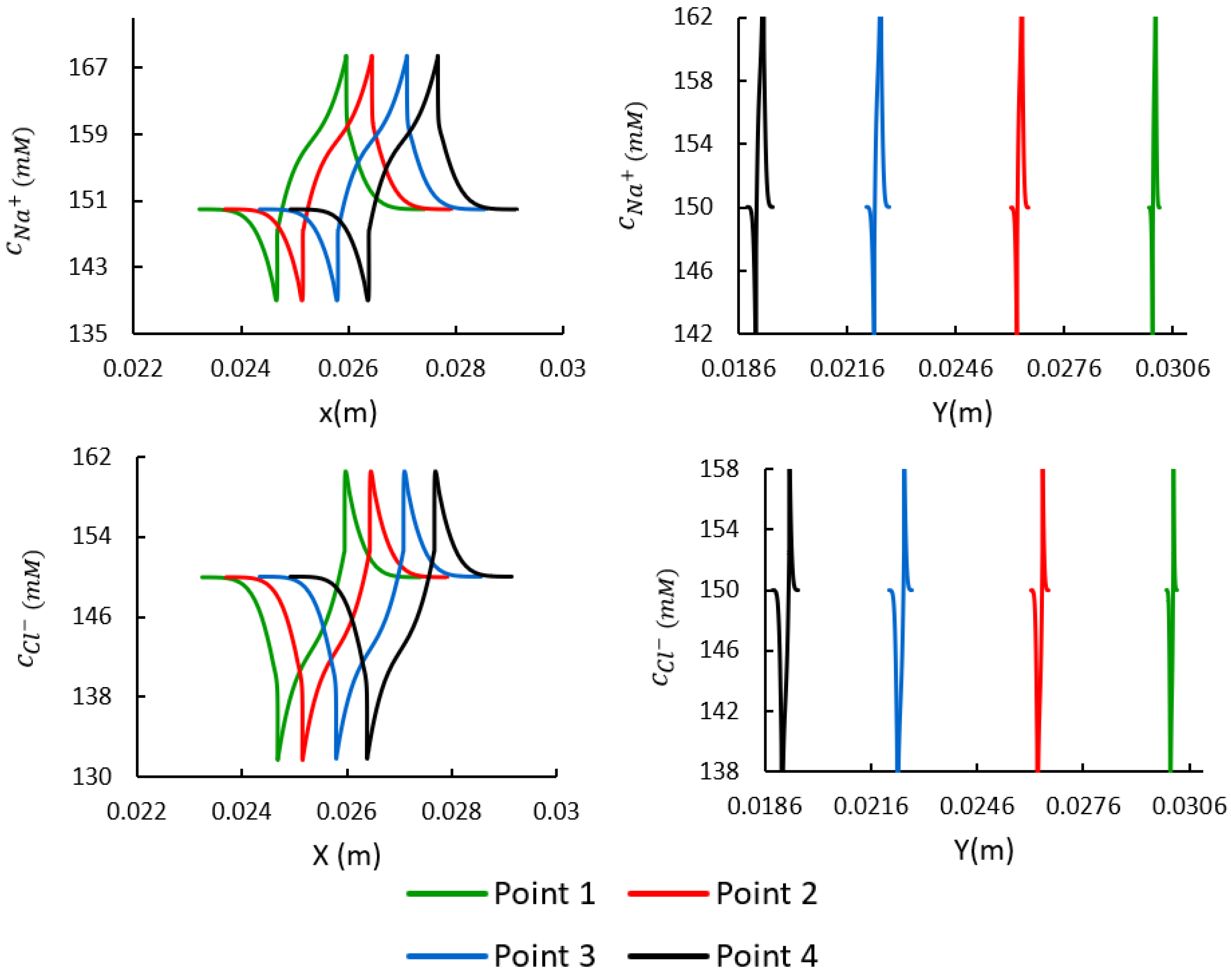
3.4. Spatial Distributions of the Mobile Ions at the Target Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Meek, K. The cornea and sclera. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 359–396. [Google Scholar]
- Hatami-Marbini, H.; Pachenari, M. Tensile viscoelastic properties of the sclera after glycosaminoglycan depletion. Curr. Eye Res. 2021, 46, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Pachenari, M.; Hatami-Marbini, H. Regional Differences in the Glycosaminoglycan Role in Porcine Scleral Hydration and Mechanical Behavior. Investig. Opthalmology Vis. Sci. 2021, 62, 28. [Google Scholar] [CrossRef]
- Dunlevy, J.R.; Rada, J.A.S. Interaction of lumican with aggrecan in the aging human sclera. Investig. Opthalmology Vis. Sci. 2004, 45, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Hatami-Marbini, H.; Etebu, E.; Rahimi, A. Swelling pressure and hydration behavior of porcine corneal stroma. Curr. Eye Res. 2013, 38, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Hatami-Marbini, H. Hydration dependent viscoelastic tensile behavior of cornea. Ann. Biomed. Eng. 2014, 42, 1740–1748. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Etebu, E. Hydration dependent biomechanical properties of the corneal stroma. Exp. Eye Res. 2013, 116, 47–54. [Google Scholar] [CrossRef]
- Rada, J.A.; Achen, V.R.; Penugonda, S.; Schmidt, R.W.; Mount, B.A. Proteoglycan composition in the human sclera during growth and aging. Investig. Opthalmology Vis. Sci. 2000, 41, 1639–1648. [Google Scholar]
- Loret, B.; Simões, F.M. Effects of the pH on the mechanical behavior of articular cartilage and corneal stroma. Int. J. Solids Struct. 2010, 47, 2201–2214. [Google Scholar] [CrossRef]
- Elliott, G.F.; Hodson, S.A. Cornea, and the swelling of polyelectrolyte gels of biological interest. Rep. Prog. Phys. 1998, 61, 1325. [Google Scholar] [CrossRef]
- Huang, Y.; Meek, K.M. Swelling studies on the cornea and sclera: The effects of ph and ionic strength. Biophys. J. 1999, 77, 1655–1665. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Mehr, J.A. Modeling and experimental investigation of electromechanical properties of scleral tissue; a CEM model using an anisotropic hyperelastic constitutive relation. Biomech. Model. Mechanobiol. 2022, 21, 1325–1337. [Google Scholar] [CrossRef]
- Mehr, J.A.; Hatami-Marbini, H. Experimental and numerical analysis of electroactive characteristics of scleral tissue. Acta Biomater. 2022, 143, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Palleau, E.; Dickey, M.D.; Velev, O.D. Electro-actuated hydrogel walkers with dual responsive legs. Soft Matter 2013, 10, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, J. Zirconium Hydroxide Cross-linked Nanocomposite Hydrogel with High Mechanical Strength and Fast Electro-Response. ACS Appl. Polym. Mater. 2020, 2, 3821–3827. [Google Scholar] [CrossRef]
- Jin, S.; Gu, J.; Shi, Y.; Shao, K.; Yu, X.; Yue, G. Preparation and electrical sensitive behavior of poly (N-vinylpyrrolidone-co-acrylic acid) hydrogel with flexible chain nature. Eur. Polym. J. 2013, 49, 1871–1880. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.; Chen, X. Electrical behavior of a natural polyelectrolyte hydrogel: Chitosan/carboxymethylcellulose hydrogel. Biomacromolecules 2008, 9, 1208–1213. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Xiao, Y.; Gao, G.; Liu, S.; Zhang, J.; Fu, J. Electric field actuation of tough electroactive hydrogels cross-linked by functional triblock copolymer micelles. ACS Appl. Mater. Interfaces 2016, 8, 26326–26331. [Google Scholar] [CrossRef]
- Fei, J.; Zhang, Z.; Gu, L. Bending behaviour of electroresponsive poly (vinyl alcohol)/poly (acrylic acid) semi-interpenetrating network hydrogel fibres under an electric stimulus. Polym. Int. 2002, 51, 502–509. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, H.S.; Lee, Y.M.; Jeong, C.N. Properties of electroresponsive poly (vinyl alcohol)/poly (acrylic acid) IPN hydrogels under an electric stimulus. J. Appl. Polym. Sci. 1999, 73, 1675–1683. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Du, F.-P.; Chen, L.; Yeung, K.-W.; Dong, Y.; Law, W.-C.; Tsui, G.C.-P.; Tang, C.-Y. Supramolecular ionic polymer/carbon nanotube composite hydrogels with enhanced electromechanical performance. Nanotechnol. Rev. 2020, 9, 478–488. [Google Scholar] [CrossRef]
- Attaran, A.; Brummund, J.; Wallmersperger, T. Modeling and simulation of the bending behavior of electrically-stimulated cantilevered hydrogels. Smart Mater. Struct. 2015, 24, 035021. [Google Scholar] [CrossRef]
- Luo, R.; Li, H.; Lam, K.Y. Coupled chemo-electro-mechanical simulation for smart hydrogels that are responsive to an external electric field. Smart Mater. Struct. 2007, 16, 1185. [Google Scholar] [CrossRef]
- Hatami-Marbini, H. Mechano-electrochemical mixture theories for the multiphase fluid infiltrated poroelastic media. In Handbook of Micromechanics and Nanomechanics; Li, S., Gao, X.-L., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Luo, R.; Li, H. A modeling study of the effect of environmental ionic valence on the mechanical characteristics of pH–electrosensitive hydrogel. Acta Biomater. 2009, 5, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Wallmersperger, T.; Ballhause, D. Coupled chemo-electro-mechanical finite element simulation of hydrogels: II. Electrical stimulation. Smart Mater. Struct. 2008, 17, 045012. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Pachenari, M. Hydration related changes in tensile response of posterior porcine sclera. J. Mech. Behav. Biomed. Mater. 2019, 104, 103562. [Google Scholar] [CrossRef]
- Wallmersperger, T.; Attaran, A.; Keller, K.; Brummund, J.; Guenther, M.; Gerlach, G. Modeling and simulation of hydrogels for the application as bending actuators. In Intelligent Hydrogels; Springer: Berlin/Heidelberg, Germany, 2013; pp. 189–204. [Google Scholar] [CrossRef]
- Wallmersperger, T.; Kröplin, B.; Gülch, R.W. Coupled chemo-electro-mechanical formulation for ionic polymer gels––numerical and experimental investigations. Mech. Mater. 2004, 36, 411–420. [Google Scholar] [CrossRef]
- Storn, R.; Price, K. Differential evolution—A simple and efficient heuristic for global optimization over continuous spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Brannon, R. Kinematics: The mathematics of deformation. Course Notes ME EN 2008, 6530, 10. [Google Scholar]
- Takizawa, K.; Tezduyar, T.E.; Avsar, R. A low-distortion mesh moving method based on fiber-reinforced hyperelasticity and optimized zero-stress state. Comput. Mech. 2020, 65, 1567–1591. [Google Scholar] [CrossRef]
- Geuzaine, C.; Remacle, J.-F. Gmsh: A 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int. J. Numer. Methods Eng. 2009, 79, 1309–1331. [Google Scholar] [CrossRef]
- Logg, A.; Mardal, K.A.; Wells, G. Automated Solution of Differential Equations by the Finite Element Method: The FEniCS Book; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 84. [Google Scholar]
- Gu, W.Y.; Yao, H.; Vega, A.L.; Flagler, D. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Ann. Biomed. Eng. 2004, 32, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Lanir, Y.; Seybold, J.; Schneiderman, R.; Huyghe, J.M. Partition and diffusion of sodium and chloride ions in soft charged foam: The effect of external salt concentration and mechanical deformation. Tissue Eng. 1998, 4, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.J.A.; Suh, J.-K.F.; Bottlang, M.; Burgoyne, C.F.; Downs, J.C. Scleral Biomechanics in the aging monkey eye. Investig. Opthalmology Vis. Sci. 2009, 50, 5226–5237. [Google Scholar] [CrossRef]
- Girard, M.J.A.; Downs, J.C.; Bottlang, M.; Burgoyne, C.F.; Suh, J.-K.F. Peripapillary and posterior scleral mechanics—Part II: Experimental and inverse finite element characterization. J. Biomech. Eng. 2009, 131, 051012. [Google Scholar] [CrossRef]
- Qiao, K.; Guo, S.; Zheng, Y.; Xu, X.; Meng, H.; Peng, J.; Fang, Z.; Xie, Y. Effects of graphene on the structure, properties, electro-response behaviors of GO/PAA composite hydrogels and influence of electro-mechanical coupling on BMSC differentiation. Mater. Sci. Eng. C 2018, 93, 853–863. [Google Scholar] [CrossRef]
- Attaran, A.; Keller, K.; Wallmersperger, T. Modeling and simulation of hydrogels for the application as finger grippers. J. Intell. Mater. Syst. Struct. 2017, 29, 371–387. [Google Scholar] [CrossRef]
- Wallmersperger, T.; Wittel, F.K.; D’Ottavio, M.; Kröplin, B. Multiscale modeling of polymer gels—Chemo-electric model versus discrete element model. Mech. Adv. Mater. Struct. 2008, 15, 228–234. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Lam, K.; Lee, H.; Chen, J.; Hanes, J.; Fu, J. Model development and numerical simulation of electric-stimulus-responsive hydrogels subject to an externally applied electric field. Biosens. Bioelectron. 2004, 19, 1097–1107. [Google Scholar] [CrossRef]
- Mehr, J.A.; Moss, H.E.; Hatami-Marbini, H. Numerical Investigation on the role of mechanical factors contributing to globe flattening in states of elevated intracranial pressure. Life 2020, 10, 316. [Google Scholar] [CrossRef]
- Nicoli, S.; Ferrari, G.; Quarta, M.; Macaluso, C.; Govoni, P.; Dallatana, D.; Santi, P. Porcine sclera as a model of human sclera for in vitro transport experiments: Histology, SEM, and comparative permeability. Mol. Vis. 2009, 15, 259. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehr, J.A.; Hatami-Marbini, H. Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method. Bioengineering 2023, 10, 920. https://doi.org/10.3390/bioengineering10080920
Mehr JA, Hatami-Marbini H. Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method. Bioengineering. 2023; 10(8):920. https://doi.org/10.3390/bioengineering10080920
Chicago/Turabian StyleMehr, Jafar Arash, and Hamed Hatami-Marbini. 2023. "Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method" Bioengineering 10, no. 8: 920. https://doi.org/10.3390/bioengineering10080920
APA StyleMehr, J. A., & Hatami-Marbini, H. (2023). Finite Deformation of Scleral Tissue under Electrical Stimulation: An Arbitrary Lagrangian-Eulerian Finite Element Method. Bioengineering, 10(8), 920. https://doi.org/10.3390/bioengineering10080920




