3.1. Continuous Time Model
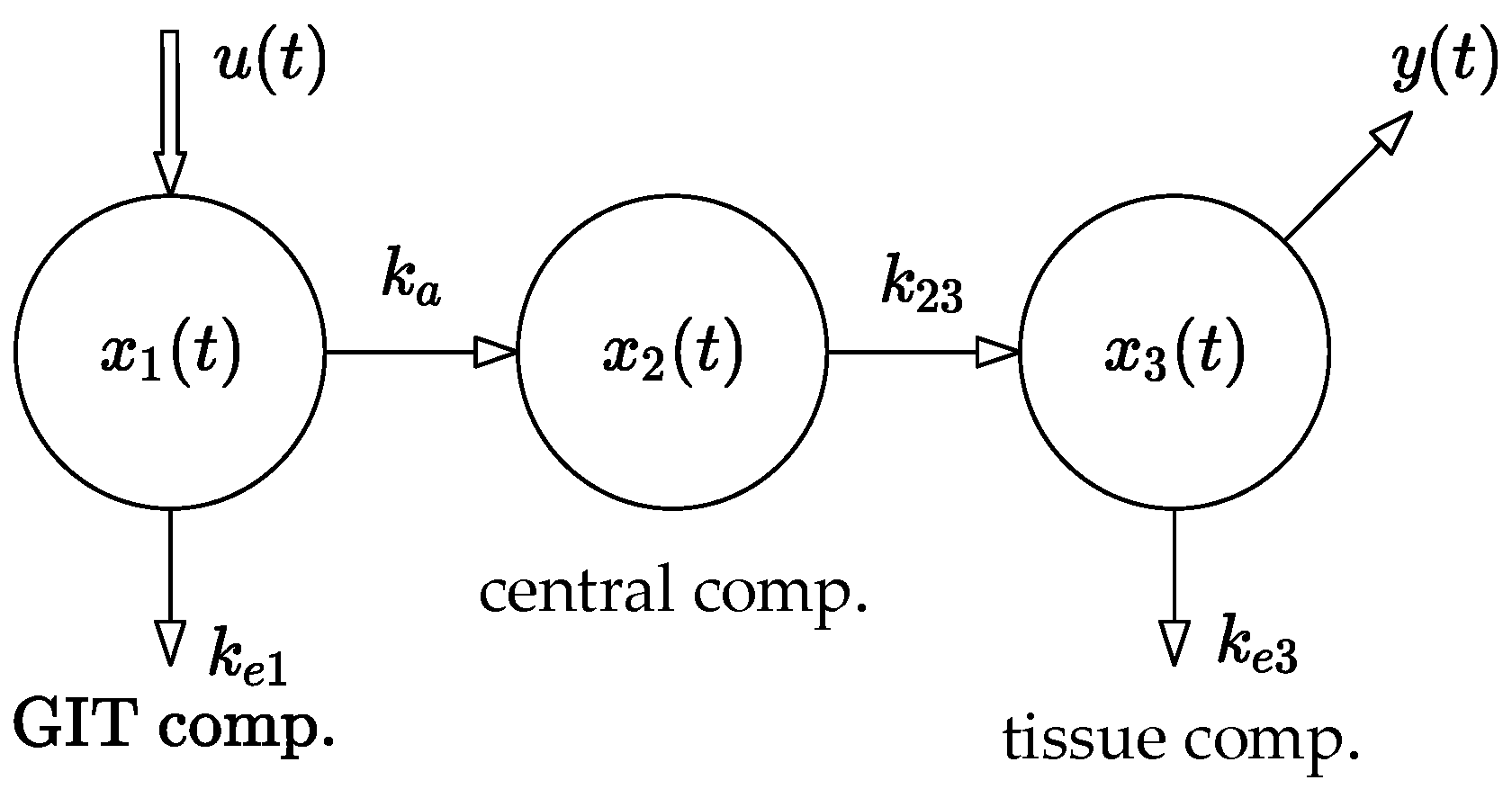
The method used in this paper is based on the compartmental model shown in
Figure 1. The model was parametrically identified using the least squares method.
Let us recall that the general form of the linear system with single input and single output (SISO) is described by the following system of
first-order differential equations:
where
are the state, control, and observation vectors, respectively, and
is the system matrix, while
and
are the output and input of the system, respectively. Generally, if
is a Metzler matrix and vector
, system (1) belongs to the category of linear positive systems. In addition to that, if
is Metzler and Hurwitz, then system (1) is positive and stable. Specifically, if a positive and stable system respects the rule of mass balance, it is called compartmental [
4]. Note that linear and nonlinear compartmental systems are essential for describing the transport of an administered drug throughout the body. In particular, the three-compartment system shown in
Figure 1 will be the subject of further analysis in this paper. Its mathematical model takes the following form [
5]:
Parameters , , , and ] are real positive constants. Parameter quantifies the rate of the drug absorption from the site of its administration. In this case, it is the gastrointestinal tract (abbr. GIT). The values of , are the rates of the drug elimination from the compartments and , and is a measure of the rate of the drug flow from to .
The relation between the system input
—the administered doses—and the system output
—the observed concentrations of the drug in the body—is given by the following transfer function [
4]:
where “
” indicates that
and
are the Laplace transforms of signals
and
[
15,
16]. The following nominal parameters were identified in [
5]:
.
Remark 1. After performing the mathematical operations indicated on the right side of (3), we obtain the so-called system transfer function ,
which defines the relation between the system input and output. It can be expressed in the form of a faction of two polynomial functions (4).
The polynomial in the denominator is the so-called characteristic polynomial and can be used to determine the system stability. Parameters ai and bi include the rate constants , , , and .
In contrast to the state (or internal) quantities
,
,
, which are hidden, the values of input
u(
t) and output
y(
t) can be directly measured. Therefore, while the parameters
ai, bi are often easily identifiable, the situation for the constants
,
,
, and
is more complicated. If we cannot determine them from the known values of
ai,
bi unambiguously, the system is considered “unidentifiable” and the values of the
,
,
,
must be identified directly from the in vivo samples while considering the system (2). The details can be found in [
17,
18] and the references therein.
A detailed explanation of the biological meanings of the system parameters, together with their method of identification from the in vivo samples, can be found in [
4,
5].
Clearly, if the off-diagonal entries of the system matrix
in (2) are non-negative (Metzler matrix), the system (2) is called a positive system. In addition to that, for the nominal parameters, if every column-wise sum is non-positive, the model (1) satisfies the mass balance condition, implying that the system (2) is a nominally stable compartmental system. However, the situation may change when the parameters are subject to uncertainties. Therefore, we check the system stability based on the characteristic polynomial (5).
The direct result of the above polynomial root decomposition shows that all three roots , , of this characteristic polynomial are negative for any , , , ; hence, the nominal system is stable. This directly implies that the system with uncertain parameters, namely , , , will remain stable if . The opposite case would be conditional to the existence of a negative parameter, which is biologically impossible.
3.4. Drug Dosing in the Open Loop
In this strategy, the steady-state value of
, i.e.,
for the unit input
, will be determined first. Then, based on
, an appropriate dose
will be adjusted such that
should be equal to the requested value
r. Formally, it can be described as follows:
Hence, the main idea behind the design of the open-loop dosing protocol is as follows: Consider the discrete time model of a general subject shown in
Figure 1 and described by the set of difference Equations (6) and (7). Its input
is a sequence of the administrated doses and the output
is the corresponding sequence of the drug concentrations in the body, as shown in
Figure 2.
Following the system theory [
17], the steady-state value
is given by relation (14).
Note that the expression for the steady-state gain (denoted by the brace in (14)) is nothing more than a scalar multiplier by which the constant unit sequence
, of the drug doses should be multiplied to obtain
. Therefore, for a given
and the steady-state gain defined using (14), the following constant drug doses should be repeatedly administered:
Note that we will consider the requested steady-state concentration
mg/mL and the sampling period
h. Therefore, the repeated constant doses
should be equal to the following:
For each of the experiments presented further, the area under curve (abbr. AUC) will be determined to quantify the total drug exposure across time considering the experiment length 144 h, nominal models, and using the trapezoidal rule.
The main drawback of this open-loop approach is that the doses are constant in size while presenting no feedback on the current drug concentration in the body. In other words, for a given the values of remain constant regardless of the current drug concentration . Therefore, at least four disadvantages of this approach can be highlighted as follows:
First, the doctor has no means through which they could speed up or slow down the transition process, i.e., the process of gradually approaching the steady-state value mg/mL. Second, the transition process of is unique as it cannot be modified by the constant input sequence . Therefore, there are cases where the transition process can last for an unacceptably long time. The third and most serious drawback is that the behavior of the system induced by the uncertainties cannot be automatically compensated for.
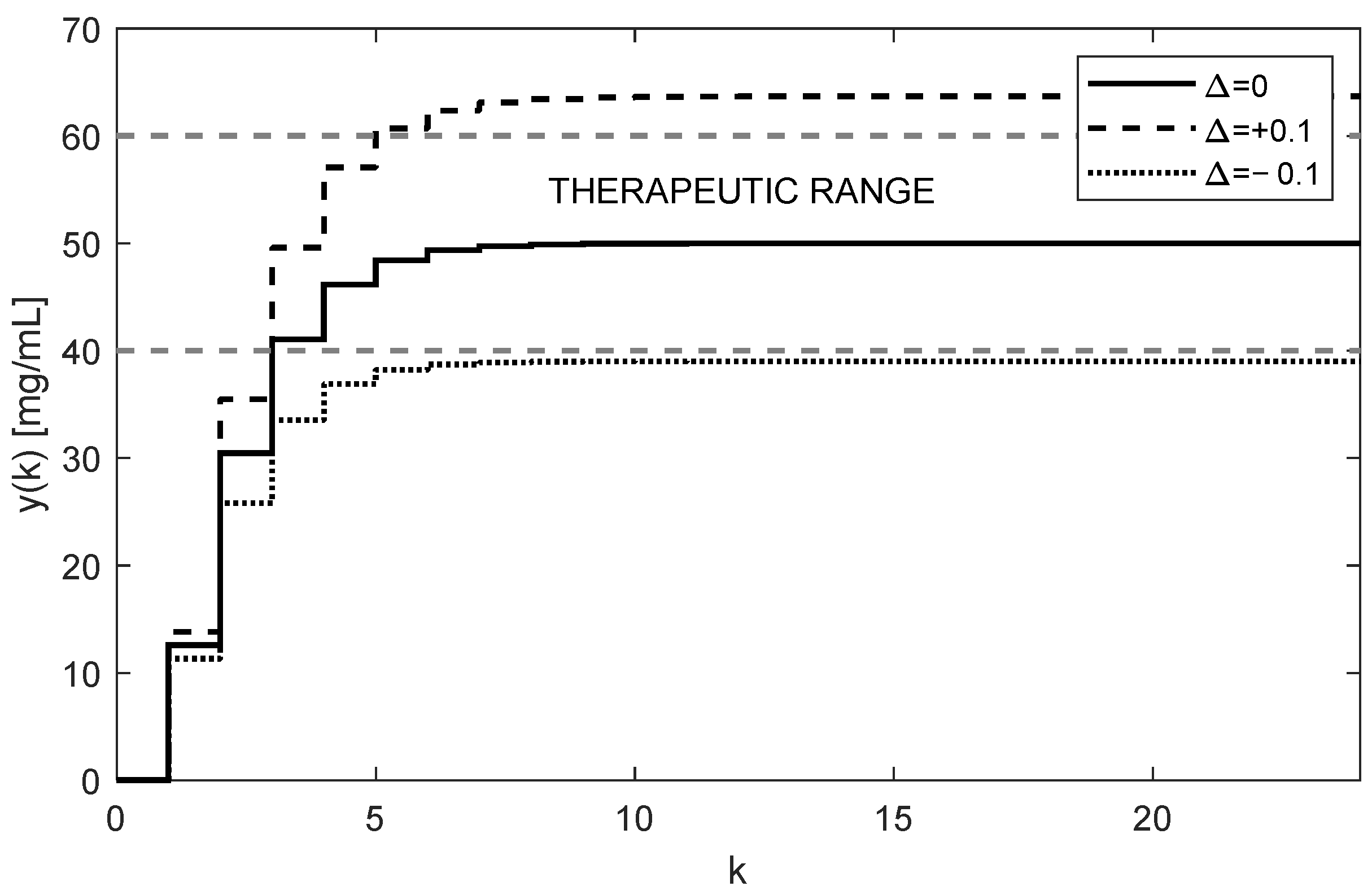
The trajectories of drug concentration
for the calculated constant doses
mg administered every 6 h during the period of 144 h are shown in
Figure 3. It can be clearly seen that the nominal trajectory of
(full curve) reaches the requested value
mg exactly and it is maintained within the therapeutic range. Contrary to that, assuming the effect of ±10% uncertainties of the nominal
and
, the steady-state value
leaves the therapeutic range (dotted and dashed curves). This means that for model (6), the parametric uncertainties ±10% are too large; hence, the open-loop therapy can significantly jeopardize the patient’s health. This is the fourth serious drawback of the open-loop dosing strategy.
Since the doctor is not aware of the extent of the parametric uncertainties, the aforementioned drawbacks naturally require a more sophisticated dosing. Much better management of the dosing protocol can be achieved using the closed-loop approach described in the
Section 3.5.
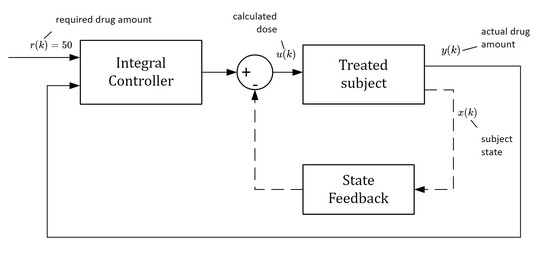
3.5. Drug Dosing in the Closed Loop
In this approach, doses
are not constant as they will depend on the current samples of the drug concentrations
, for
…. As shown by the closed-loop arrangement, the concentrations
will always converge towards the requested value
regardless of the uncertainties. Due to this property, one can claim that this control strategy is robust with respect to parametric uncertainties [
19,
20,
21]. The main idea is illustrated in the scheme shown in
Figure 4.
The block “subject” represents an ill body, which should be treated by repeatedly administrating non-constant doses
. To this end, the control algorithm (17) is based on the so-called integral controller. The output of this controller is the sequence of doses
, which are to be administered consecutively at the time instants
for
[
16]. As mentioned above, the role of
is to force the drug concentration
to converge towards the desired steady-state value
and to maintain it on this level despite the presence of parametric uncertainties.
From the physical and biological nature of the problem, it follows that the quantities
,
,
are equal to zero. The integral controller works according to algorithm (17).
The whole process works as follows: At the beginning, the tunable parameter
(the so-called integral gain) [
16] is set to some value, for example,
. This value defines not only the rate of increasing the subsequent doses
, but also the number of doses required to reach the steady-state concentration
. Then, for
the initial dose is computed as
mg and the doctor will administrate it. At the end of the first dosing interval (
h), the doctor takes the first sample
and, together with the required steady-state value
, passes this information to the controller (algorithm (17)), which determines the next dose as
. The process is repeated for the next indices
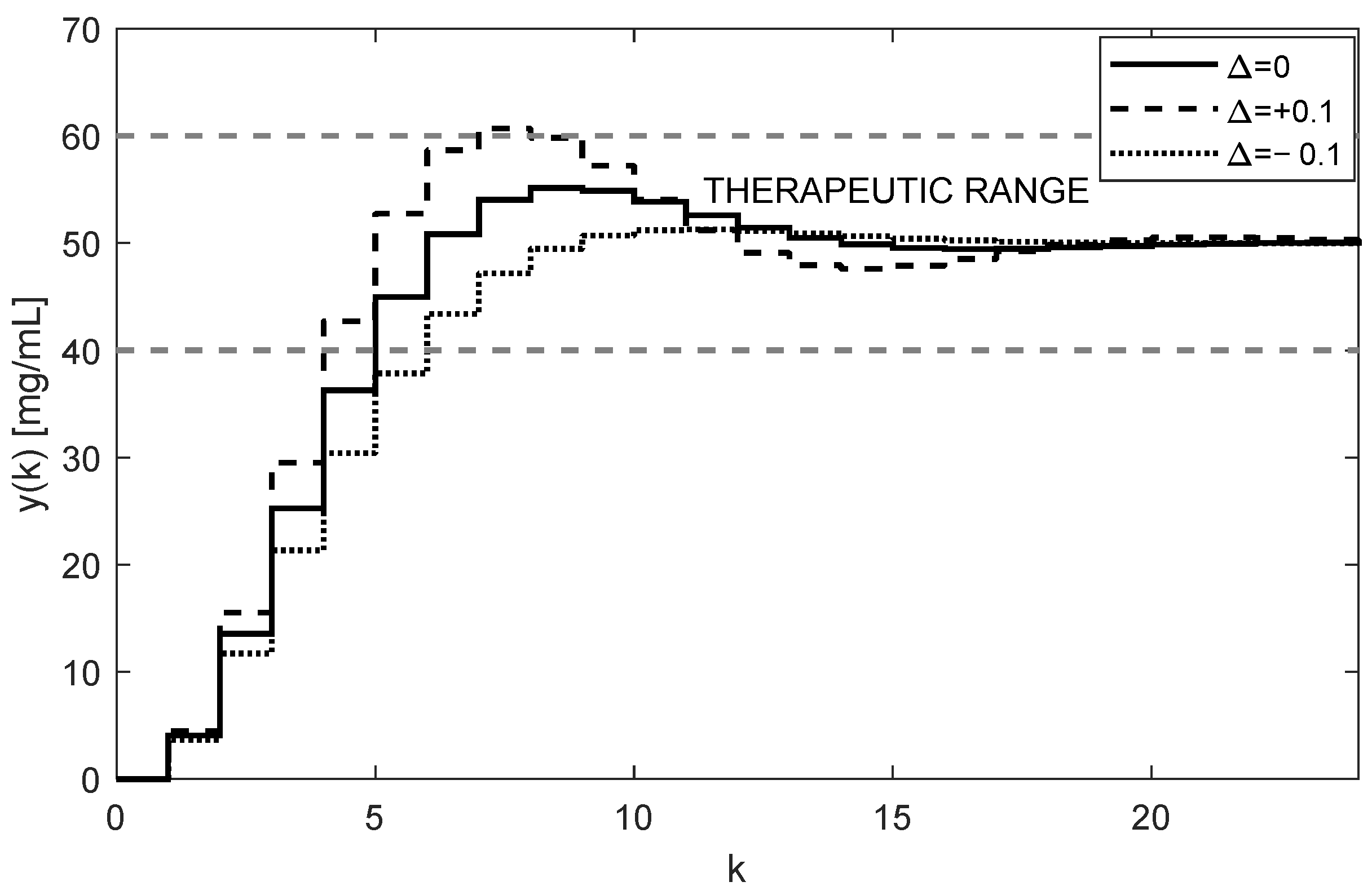
. The simulated trajectory of
, for
is shown in
Figure 5.
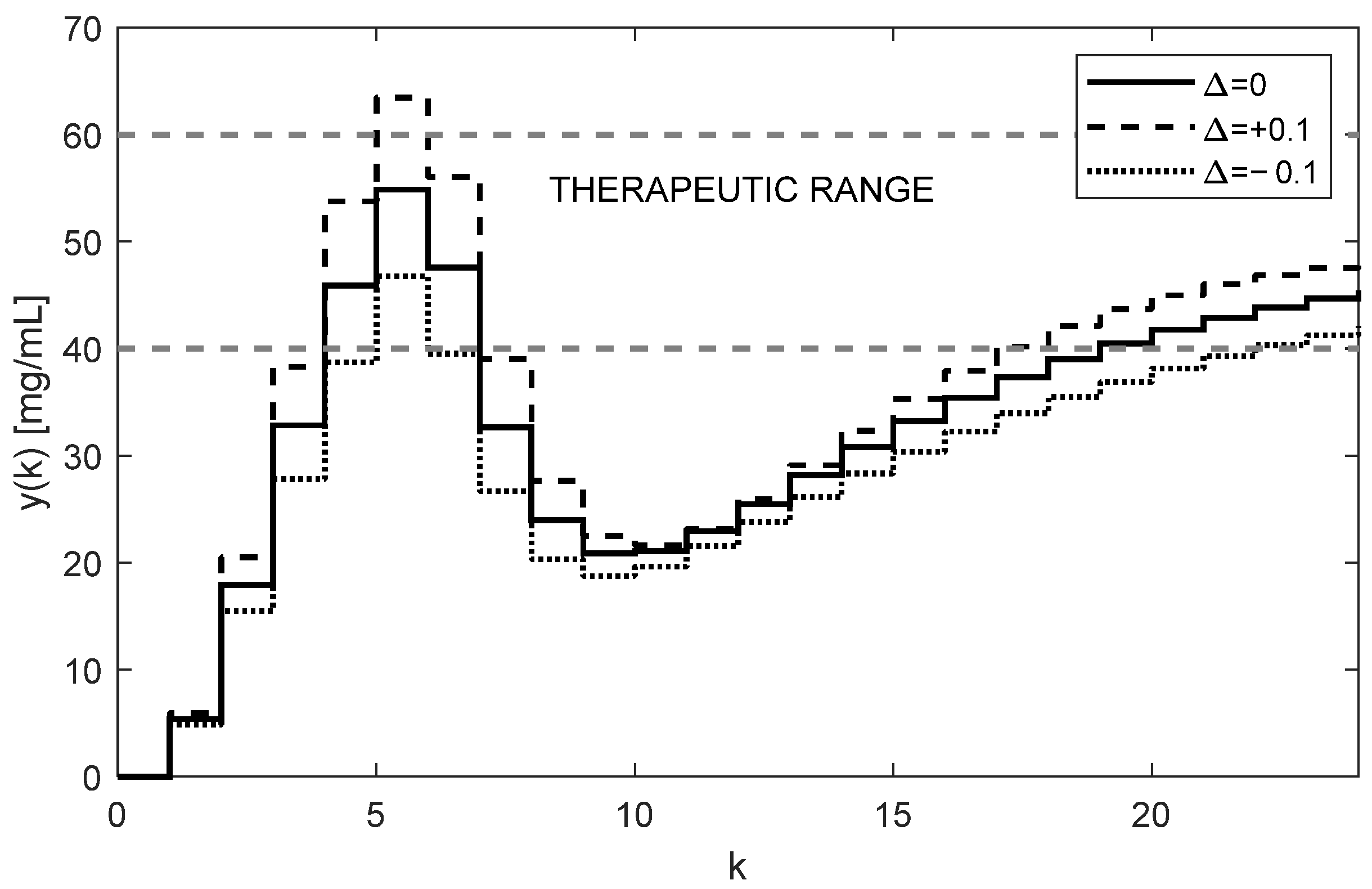
The corresponding trajectory of the drug
in the site where samples are taken (i.e., from the third compartment) is shown in
Figure 6.
For illustration purposes, the first ten values of the computed doses
and the corresponding trajectory of the drug concentrations
for the nominal case
) are given in
Table 2.
As
Figure 5 and
Figure 6 illustrate, both trajectories
and
exhibit some overshoots above their steady-state values. This is caused by choosing an integration gain
that is too high. Threfore, it is recommended to decrease its value to
. After carrying this out, more appropriate trajectories are obtained, as shown in
Figure 7 and
Figure 8.
The nominal trajectory is presented by the full line. The trajectory for positive uncertainties (11) is presented by the dashed curve, and the dot trajectory corresponds to the negative uncertainties (12).
It is even possible to speed up the transition process by intentionally applying a higher loading dose, such as
mg; by carrying this out, we achieved the desired results, as demonstrated in
Figure 9.
We can artificially disturb the control configuration and the experiment described above, first, by applying underestimated and, second, by applying overestimated initial doses to make the control algorithm properly correct the dosing. The corresponding response to drug concentration with the first five doses scaled by a factor of 0.1 can be seen in
Figure 10, where we can essentially observe a delayed start of the treatment. On the contrary, the response of drug concentration with the first five doses scaled by a factor of 5 can be seen in
Figure 11, demonstrating that overestimation can lead to deteriorated performance or even toxic treatment; nevertheless, the control algorithm could ultimately manage this scenario.
It is worth mentioning that from the aspect of system theory, the processes of the drug distribution through the body belong to the category of dynamic processes [
15]. Therefore, they can be stable or unstable depending on the ways in which the subsystems are mutually connected, as well as the model parameters. The only source of instability of the control structure shown in
Figure 4 can arise from an inappropriate (usually too high) integration gain
. This was documented by the overshoots in
Figure 5 and
Figure 6, indicating that the value
is too high and its further increase may lead to the instability of the closed loop. For that reason,
should be chosen to prevent the system from destabilization.
As an illustration, we will check the stability for the considered integral gain
. The transfer function form of the nominal discrete time model (6) and (7) obtains the following:
Then, we can derive the closed-loop transfer function as follows:
For the nominal model with
and
given by (9), we have the following transfer function:
The roots of its characteristic polynomial are .
For
with
and
given by (11), we have the following:
The roots of its characteristic polynomial are .
For
with
and
given by (12), we have the following:
The roots of its characteristic polynomial are . Since the roots of all characteristic polynomials (20)–(22) lie in the unit circle, the closed-loop control is stable.
The optimal value of
should be a trade-off between the speed of increasing the control response of
and the system stability. It is also important to note that, contrary to the open-loop case, the individual doses
are not constant; rather, they gradually increase while their increments decrease as
and
approach their steady-state values. This is evident from
Figure 5 and
Figure 7. In addition to that, these figures illustrate that at the time instant
the dose is
mg, which is virtually equivalent to the value determined for the open-loop control, i.e.,
mg, from (16).
Another important observation is that
Figure 5,
Figure 6,
Figure 7 and
Figure 8 convincingly demonstrate the robustness of the closed-loop control, meaning that if the parametric uncertainties do not exceed their limits
, the system remains stable. Hence, contrary to the open loop, the closed-loop approach with the integral controller ensures that both in the nominal process and in the process disturbed by the parametric uncertainties, the drug concentration
converges to the requested steady-state value
mg/mL, which implies that the control system is robust.
In addition to the advantages of closed-loop dosing mentioned earlier, another practical advantage is the possibility of changing the rate of increasing the trajectory
by changing the controller gain
. Clearly, the change in the rate of
is related to the changes in the size of its increments, which, in turn, influence the number of doses needed to reach the requested level
. To see this, compare
Figure 5 and
Figure 7.
3.6. Drug Dosing in the Case of an Unstable Subject
It was shown that the discrete time system (6) is stable for all positive parameters. On the other hand, some special models of bioprocesses, such as physiology-based pharmacokinetic, economic, and ecological models, may not share this feature. Even if their states of equilibria are stable, the region of stability around them can be very small. Therefore, even negligibly small parametric changes or exogenous factors affecting the living subject may render the system unstable. This is manifested by the limitless increase in some characteristic quantities, in particular the system states.
For example, imagine a model of tumor growth, which is typically defined by a set of differential equations [
16]. The normally stable tumor-free state equilibrium, i.e., the one computed from the nominal model, can become unstable due to the deviation of the parameters from their nominal values. The instability of the equilibrium will manifest itself through some (or all) variables, e.g., the tumor volume will grow beyond all limits.
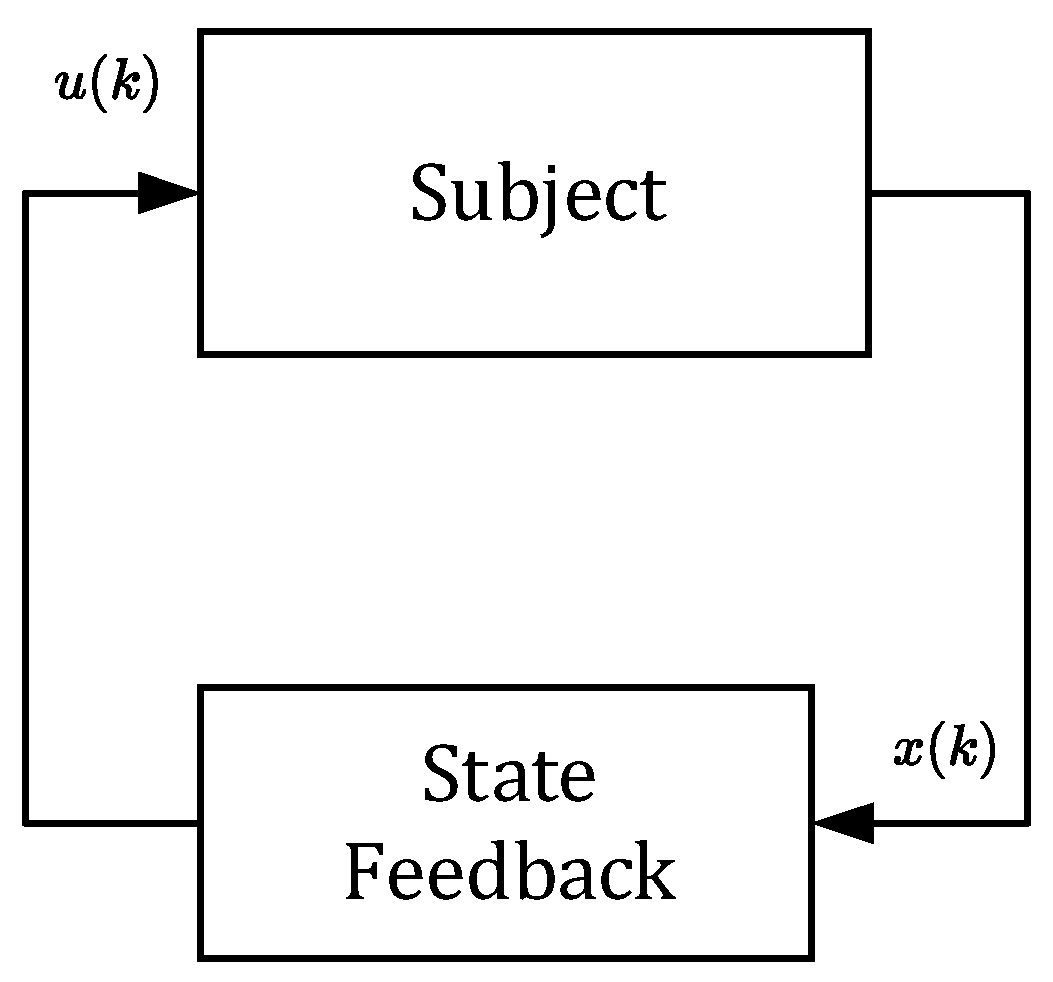
However, in this section, we will not deal with any specific model of tumor growth. Instead, we will artificially destabilize the pharmacokinetic model described by (6) and (7). It will be shown that the closed-loop control structure can also be used to control an unstable subject. Consider the feedback scheme shown in
Figure 12.
The principle of stabilization via state feedback generally assumes all three components of the state vector
. Therefore, the vector
should contain information on the drug concentrations in all three compartments. As shown in
Figure 12, the state vector
is repeatedly sensed and passed to the state feedback where it is converted to the sequence of stabilizing doses
.
Obviously, taking drug samples from all three compartments is biologically infeasible; however, their values can be obtained without the need for direct measurement. The states can be generated by the state observer (we have synthesized it in [
5], which requires taking only the samples of the output variable
To illustrate this, consider the stable discrete time system described in Equations (6) and (7). To make it artificially unstable, we will intentionally modify the lower uncertainty matrix
by adding a destabilizing matrix, as in (23).
The eigenvalues of matrix (23) are as follows:
Because the eigenvalue 1.0479 is larger than one, it indicates that the dynamic system (6) with matrix (23) in (9) is unstable [
15,
17]. The trajectories
obtained using (17) are shown in
Figure 13.
It can be observed that for the parametric uncertainty
, the dotted trajectory
decreases and, at a certain time, becomes negative. Clearly, it can be concluded that the system does not work properly. Therefore, the system should be stabilized via state feedback as proposed. Stabilization was performed by coupling the subject with the stabilizing state feedback
. To determine
K, we suppose the interval uncertainties included in (2) and apply Theorem 5 from [
22]. To satisfy the conditions of this theorem, we resolved the corresponding problem (25) of linear programming [
23].
The obtained feedback gain vector
is as follows:
The state feedback with the gain
stabilizes the closed-loop system in
Figure 12 robustly, that is, under the conditions of uncertain entries of
and
. Actually, by applying the state feedback
all eigenvalues of the closed-loop system shown in
Figure 12 are smaller than one, including the following:
Although all components of vector
are positive, it can be deduced from (26) that one component of the vector
is negative. This means that for some special values of the components of
the product
can result in negative doses
which is infeasible. Therefore, the scheme in
Figure 12 cannot be used alone. It must be part of the closed loop in
Figure 4, as shown in
Figure 14. Then, the requested steady-state value
can be flexibly set up to an arbitrary positive value.
Although the original subject (without state feedback) is unstable, the inner closed-loop system shown in
Figure 14 is stable. The resulting drug doses
are given by the sum of the controller output
and the feedback signal
is as follows from (28).
The first ten values of the feedback signal
and the drug doses
for
h and
are given in
Table 3 and
Table 4. While the samples of the feedback signals
are negative, the doses
are positive for any positive value of
. The corresponding trajectories of
are shown in
Figure 15.
Although the trajectories
displayed in
Figure 6 and
Figure 15 correspond to the same dosing period and the controller gain (
h,
), they are not quite equivalent. This was expected because, in the previous case, algorithm (16) controlled the stable subject alone, whereas now it controls the subject augmented by the stabilizing state feedback; therefore, it has different dynamics. Regardless of this, by suitably adjusting the integral gain
the shape of the trajectory
can be affected as before.