Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Vessel Geometries
2.1.1. Cylindrical Geometry
2.1.2. Image-Based Aorta Geometry
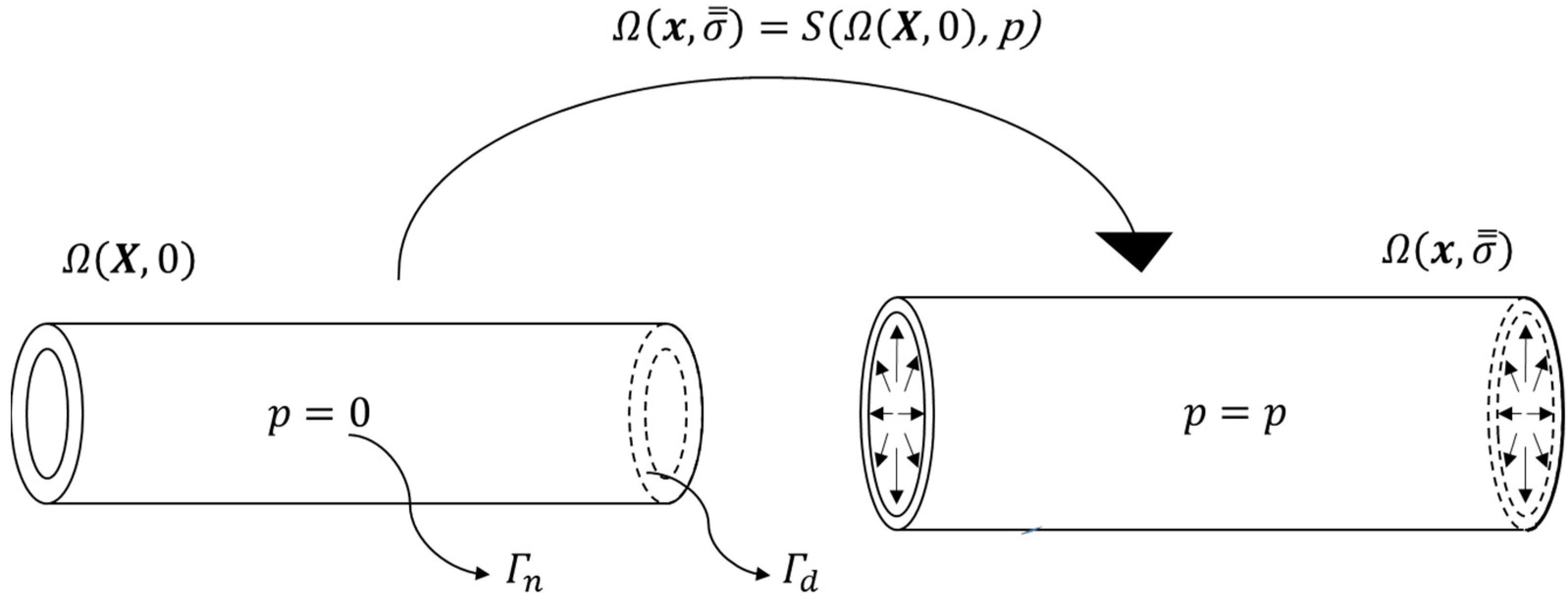
2.2. Finite Element Analysis
2.3. Mechanical Parameter Estimation
2.3.1. Experimental Data Used for Fitting
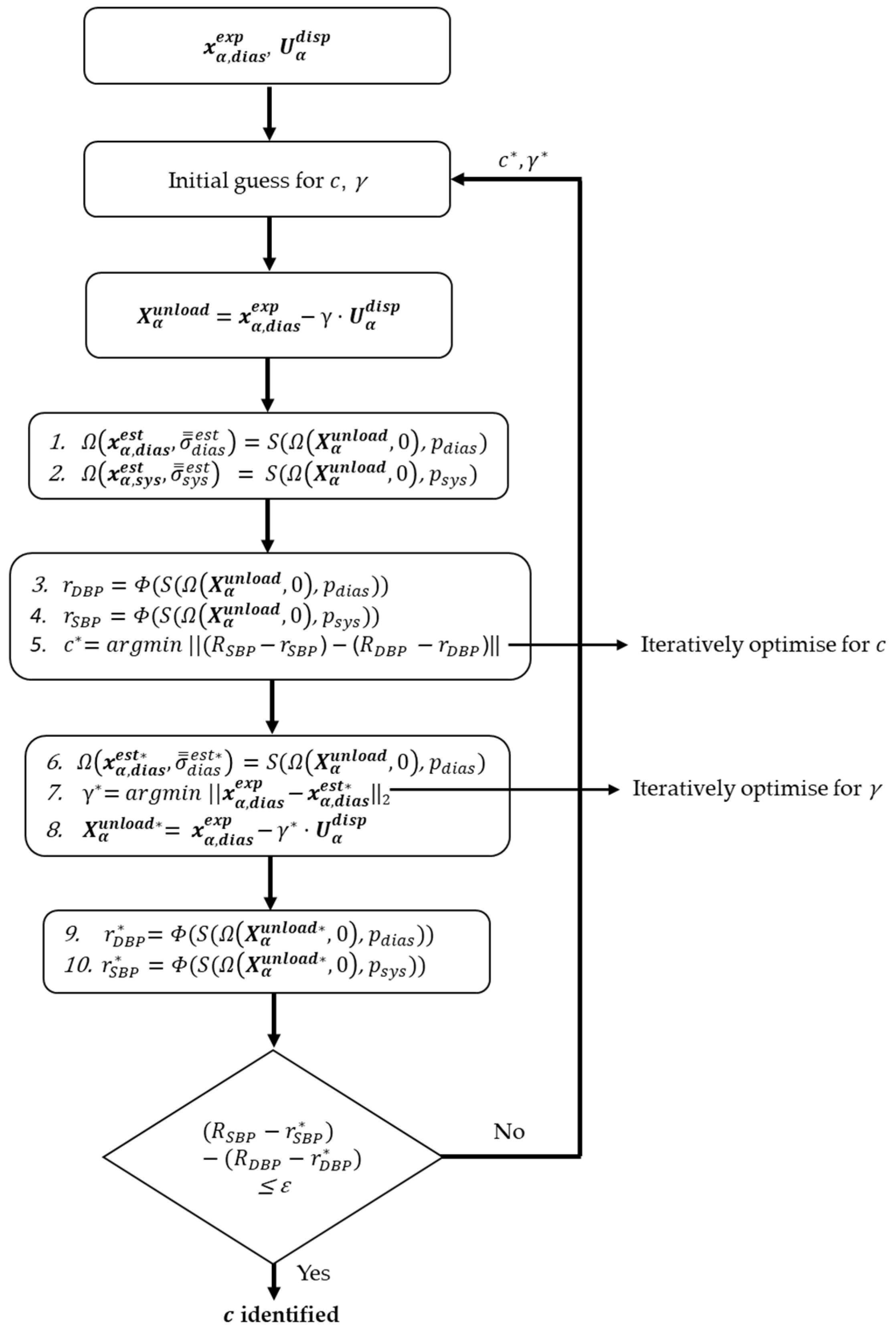
2.3.2. Algorithm Description
2.4. Method Validation
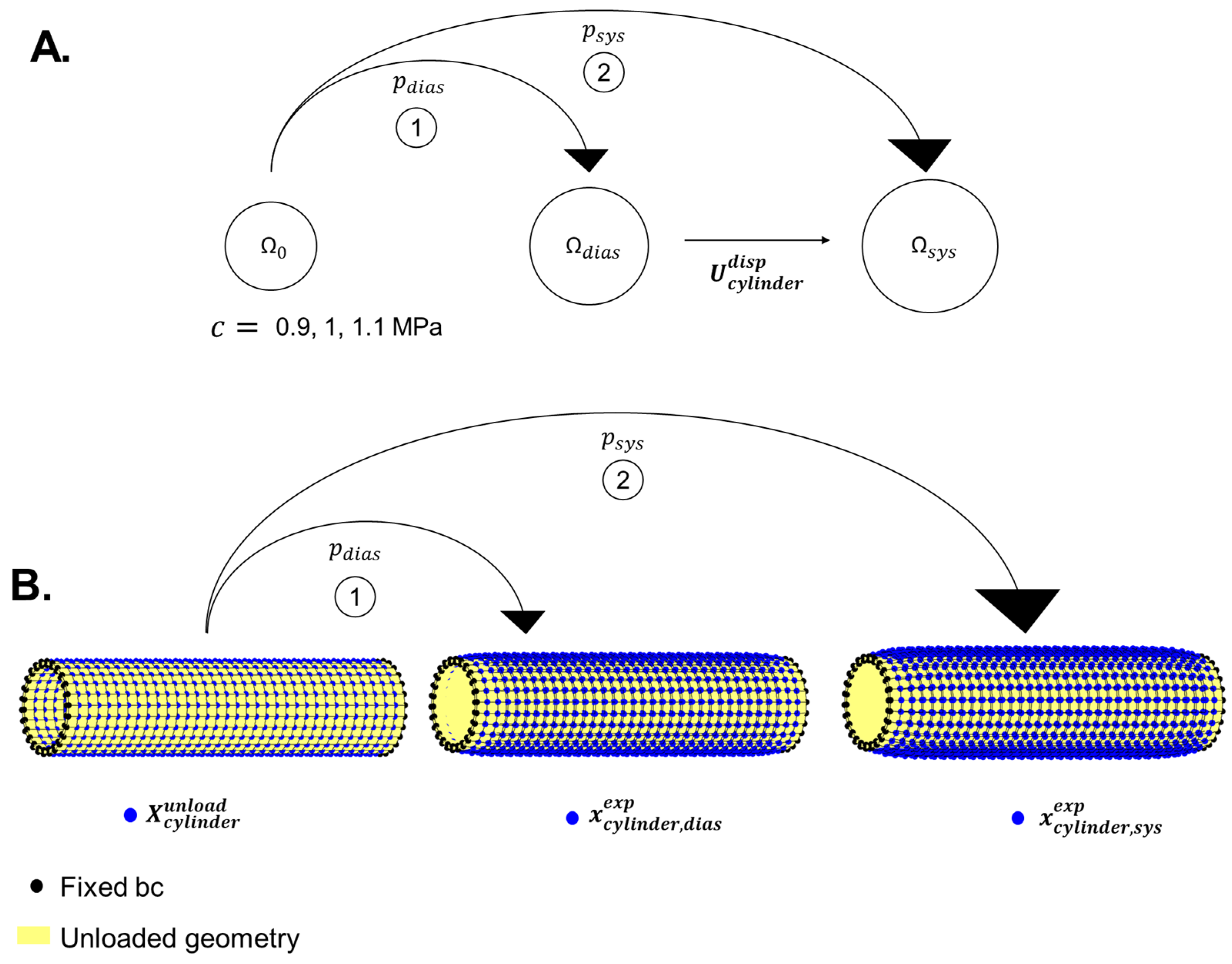
2.4.1. Synthetically Created Reference Data
2.4.2. Cylindrical Geometry Reference Data
2.4.3. Aortic Geometry Reference Data
2.5. Simulations and Analyses
2.5.1. Assessment of Algorithm Accuracy
2.5.2. Pre-Stresses
2.5.3. Effect of Different Initial Conditions
3. Results
4. Discussion
4.1. Methodological Differences with Respect to BI
4.2. Basic Numerical Validation
4.3. Residual Stresses
4.4. Fitting Procedure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef]
- Choudhury, N.; Bouchot, O.; Rouleau, L.; Tremblay, D.; Cartier, R.; Butany, J.; Mongrain, R.; Leask, R.L. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc. Pathol. 2009, 18, 83–91. [Google Scholar] [CrossRef]
- Deplano, V.; Boufi, M.; Gariboldi, V.; Loundou, A.D.; D’journo, X.B.; Cautela, J.; Djemli, A.; Alimi, Y.S. Mechanical characterisation of human ascending aorta dissection. J. Biomech. 2019, 94, 138–146. [Google Scholar] [CrossRef]
- Vorp, D.A.; Schiro, B.J.; Ehrlich, M.P.; Juvonen, T.S.; Ergin, M.A.; Griffith, B.P. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann. Thorac. Surg. 2003, 75, 1210–1214. [Google Scholar] [CrossRef]
- Humphrey, J.D. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bols, J.; Degroote, J.; Trachet, B.; Verhegghe, B.; Segers, P.; Vierendeels, J. A computational method to assess the in vivo stresses and unloaded configuration of patient-specific blood vessels. J. Comput. Appl. Math. 2013, 246, 10–17. [Google Scholar] [CrossRef]
- de Putter, S.; Wolters, B.; Rutten, M.; Breeuwer, M.; Gerritsen, F.; van de Vosse, F.N. Patient-specific initial wall stress in abdominal aortic aneurysms with a backward incremental method. J. Biomech. 2007, 40, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Gee, M.; Reeps, C.; Eckstein, H.; Wall, W. Prestressing in finite deformation abdominal aortic aneurysm simulation. J. Biomech. 2009, 42, 1732–1739. [Google Scholar] [CrossRef]
- Gee, M.W.; Förster, C.; Wall, W.A. A computational strategy for prestressing patient-specific biomechanical problems under finite deformation. Int. J. Numer. Methods Biomed. Eng. 2010, 26, 52–72. [Google Scholar] [CrossRef]
- Raghavan, M.L.; Ma, B.; Fillinger, M.F. Non-invasive determination of zero-pressure geometry of arterial aneurysms. Ann. Biomed. Eng. 2006, 34, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- van Disseldorp, E.M.J.; Petterson, N.; Rutten, M.; van De Vosse, F.N.; van Sambeek, M.; Lopata, R. Patient specific wall stress analysis and mechanical characterization of abdominal aortic aneurysms using 4D ultrasound. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Weisbecker, H.; Pierce, D.M.; Holzapfel, G.A. A generalized prestressing algorithm for finite element simulations of preloaded geometries with application to the aorta. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Agnese, V.; Raffa, G.M.; Gentile, G.; Bellavia, D.; Zingales, M.; Pilato, M.; Pasta, S. On the role of material properties in ascending thoracic aortic aneurysms. Comput. Biol. Med. 2019, 109, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liang, L.; Sun, W. A new inverse method for estimation of in vivo mechanical properties of the aortic wall. J. Mech. Behav. Biomed. Mater. 2017, 72, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Moerman, K.M. GIBBON: The geometry and image-based bioengineering add-on. J. Open Source Softw. 2018, 3, 506. [Google Scholar] [CrossRef]
- Moerman, K.M.; Simms, C.K.; Nagel, T. Control of tension–compression asymmetry in Ogden hyperelasticity with application to soft tissue modelling. J. Mech. Behav. Biomed. Mater. 2016, 56, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Wei, W.; Evin, M.; Rapacchi, S.; Kober, F.; Bernard, M.; Jacquier, A.; Kahn, C.J.F.; Behr, M. Investigating heartbeat-related in-plane motion and stress levels induced at the aortic root. BioMedical Eng. OnLine 2019, 18, 19. [Google Scholar] [CrossRef]
- Beller, C.J.; Labrosse, M.R.; Thubrikar, M.J.; Robicsek, F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation 2004, 109, 763–769. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Liu, H.; Zhang, M.; Martin, C.; Sun, W. On the computation of in vivo transmural mean stress of patient-specific aortic wall. Biomech. Model. Mechanobiol. 2019, 18, 387–398. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Chen, Q.; Li, Z. Numerical Determination of the Circumferential Residual Stress of Porcine Aorta by Pulling-Back Method. Acta Mech. Solida Sin. 2021, 34, 346–355. [Google Scholar] [CrossRef]
- Maes, L.; Fehervary, H.; Vastmans, J.; Mousavi, S.J.; Avril, S.; Famaey, N. Constrained mixture modeling affects material parameter identification from planar biaxial tests. J. Mech. Behav. Biomed. Mater. 2019, 95, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Sokolis, D.P. Effects of aneurysm on the directional, regional, and layer distribution of residual strains in ascending thoracic aorta. J. Mech. Behav. Biomed. Mater. 2015, 46, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Smoljkić, M.; Fehervary, H.; Van den Bergh, P.; Jorge-Peñas, A.; Kluyskens, L.; Dymarkowski, S.; Verbrugghe, P.; Meuris, B.; Vander Sloten, J.; Famaey, N. Biomechanical characterization of ascending aortic aneurysms. Biomech. Model. Mechanobiol. 2017, 16, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Stålhand, J. Determination of human arterial wall parameters from clinical data. Biomech. Model. Mechanobiol. 2009, 8, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Guala, A.; Teixidó-Tura, G.; Rodríguez-Palomares, J.; Ruiz-Muñoz, A.; Dux-Santoy, L.; Villalva, N.; Granato, C.; Galian, L.; Gutiérrez, L.; González-Alujas, T.; et al. Proximal aorta longitudinal strain predicts aortic root dilation rate and aortic events in Marfan syndrome. Eur. Heart J. 2019, 40, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.M.; Choi, G.; Zarins, C.K.; Taylor, C.A. Circumferential and longitudinal cyclic strain of the human thoracic aorta: Age-related changes. J. Vasc. Surg. 2009, 49, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Ganizada, B.; Debeij, G.; Natour, E.; Maessen, J.; Spronck, B.; Schurgers, L.; Delhaas, T.; Huberts, W.; Bidar, E.; et al. Intra-operative video-based measurement of biaxial strains of the ascending thoracic aorta. Biomedicines 2021, 9, 670. [Google Scholar] [CrossRef]
- Ferruzzi, J.; Di Achille, P.; Tellides, G.; Humphrey, J.D. Combining in vivo and in vitro biomechanical data reveals key roles of perivascular tethering in central artery function. PLoS ONE 2018, 13, e0201379. [Google Scholar] [CrossRef]


| Geometry | (MPa) | Initial Guesses | Estimated Values | (MPa) | |||
|---|---|---|---|---|---|---|---|
| (MPa) | (MPa) | ||||||
| 0.5 | 1 | 0.930 | 1.260 | 0.030 | |||
| 0.9 | 0.75 | 0.75 | 0.908 | 1.289 | 0.008 | 98.43% | |
| 1 | 0.5 | 0.911 | 1.285 | 0.011 | |||
| 1.5 | 0.25 | 0.914 | 1.281 | 0.014 | |||
| 0.5 | 1 | 1.032 | 1.293 | 0.032 | |||
| Cylinder | 1 | 0.75 | 0.75 | 1.008 | 1.322 | 0.008 | 98.50% |
| 1 | 0.5 | 1.010 | 1.320 | 0.010 | |||
| 1.5 | 0.25 | 1.010 | 1.317 | 0.010 | |||
| 0.5 | 1 | 1.134 | 1.320 | 0.034 | |||
| 1.1 | 0.75 | 0.75 | 1.107 | 1.350 | 0.007 | 98.50% | |
| 1 | 0.5 | 1.109 | 1.347 | 0.009 | |||
| 1.5 | 0.25 | 1.110 | 1.345 | 0.010 | |||
| 0.5 | 1 | 0.895 | 0.303 | 0.005 | |||
| 0.9 | 0.75 | 0.75 | 0.897 | 0.302 | 0.003 | 99.15% | |
| 1 | 0.5 | 0.879 | 0.313 | 0.021 | |||
| 1.5 | 0.25 | 0.905 | 0.297 | 0.005 | |||
| 0.5 | 1 | 0.996 | 0.302 | 0.004 | |||
| Aorta | 1 | 0.75 | 0.75 | 0.997 | 0.302 | 0.003 | 99.22% |
| 1 | 0.5 | 0.979 | 0.312 | 0.021 | |||
| 1.5 | 0.25 | 1.003 | 0.298 | 0.003 | |||
| 0.5 | 1 | 1.096 | 0.302 | 0.004 | |||
| 1.1 | 0.75 | 0.75 | 1.098 | 0.301 | 0.002 | 99.20% | |
| 1 | 0.5 | 1.079 | 0.311 | 0.021 | |||
| 1.5 | 0.25 | 1.105 | 0.297 | 0.005 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, S.; Moerman, K.M.; Ramaekers, M.J.F.G.; Schalla, S.; Bidar, E.; Delhaas, T.; Reesink, K.; Huberts, W. Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses. Bioengineering 2023, 10, 846. https://doi.org/10.3390/bioengineering10070846
Parikh S, Moerman KM, Ramaekers MJFG, Schalla S, Bidar E, Delhaas T, Reesink K, Huberts W. Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses. Bioengineering. 2023; 10(7):846. https://doi.org/10.3390/bioengineering10070846
Chicago/Turabian StyleParikh, Shaiv, Kevin M. Moerman, Mitch J. F. G. Ramaekers, Simon Schalla, Elham Bidar, Tammo Delhaas, Koen Reesink, and Wouter Huberts. 2023. "Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses" Bioengineering 10, no. 7: 846. https://doi.org/10.3390/bioengineering10070846
APA StyleParikh, S., Moerman, K. M., Ramaekers, M. J. F. G., Schalla, S., Bidar, E., Delhaas, T., Reesink, K., & Huberts, W. (2023). Biomechanical Characterisation of Thoracic Ascending Aorta with Preserved Pre-Stresses. Bioengineering, 10(7), 846. https://doi.org/10.3390/bioengineering10070846







