Small-Molecule Loaded Biomimetic Biphasic Scaffold for Osteochondral Regeneration: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nano-Gelatin Fiber Embedded with Kartogenin (NGFK)
2.3. Preparation of Metformin Embedded Gelatin/Hydroxyapatite Scaffold (GHSM)
2.4. Synthesis of Biphasic Scaffold and Study of Morphology
2.5. Identification of Crystal Phase and Functional Group
2.6. In Vitro Experiment
2.7. Cell Viability
2.8. Cytotoxicity
2.9. Live/Dead Assay
2.10. Gene Expression
2.11. Western Blotting
2.12. Biochemical Analysis
2.13. Immunofluorescence Staining
2.14. In Vivo Experiment
2.15. Generation of Osteochondral Defect
2.16. Histological Staining
2.17. Statistical Analysis
3. Results
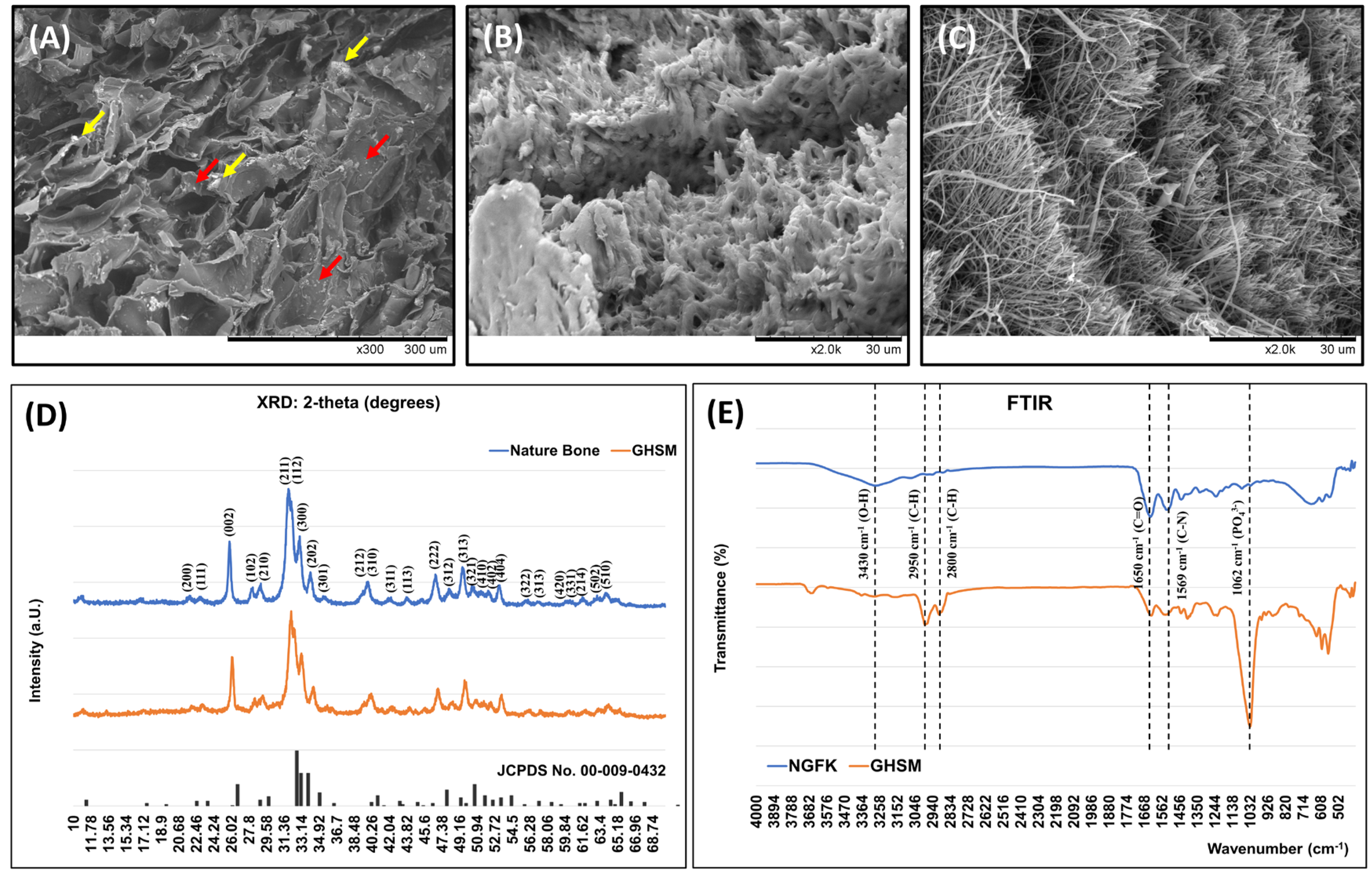
3.1. Characteristics of the Biomimetic Biphasic Scaffold (BPS)
3.1.1. Morphology of the Biphasic Scaffold (BPS)
3.1.2. Crystal Phase Identification
3.1.3. Functional Group Identification
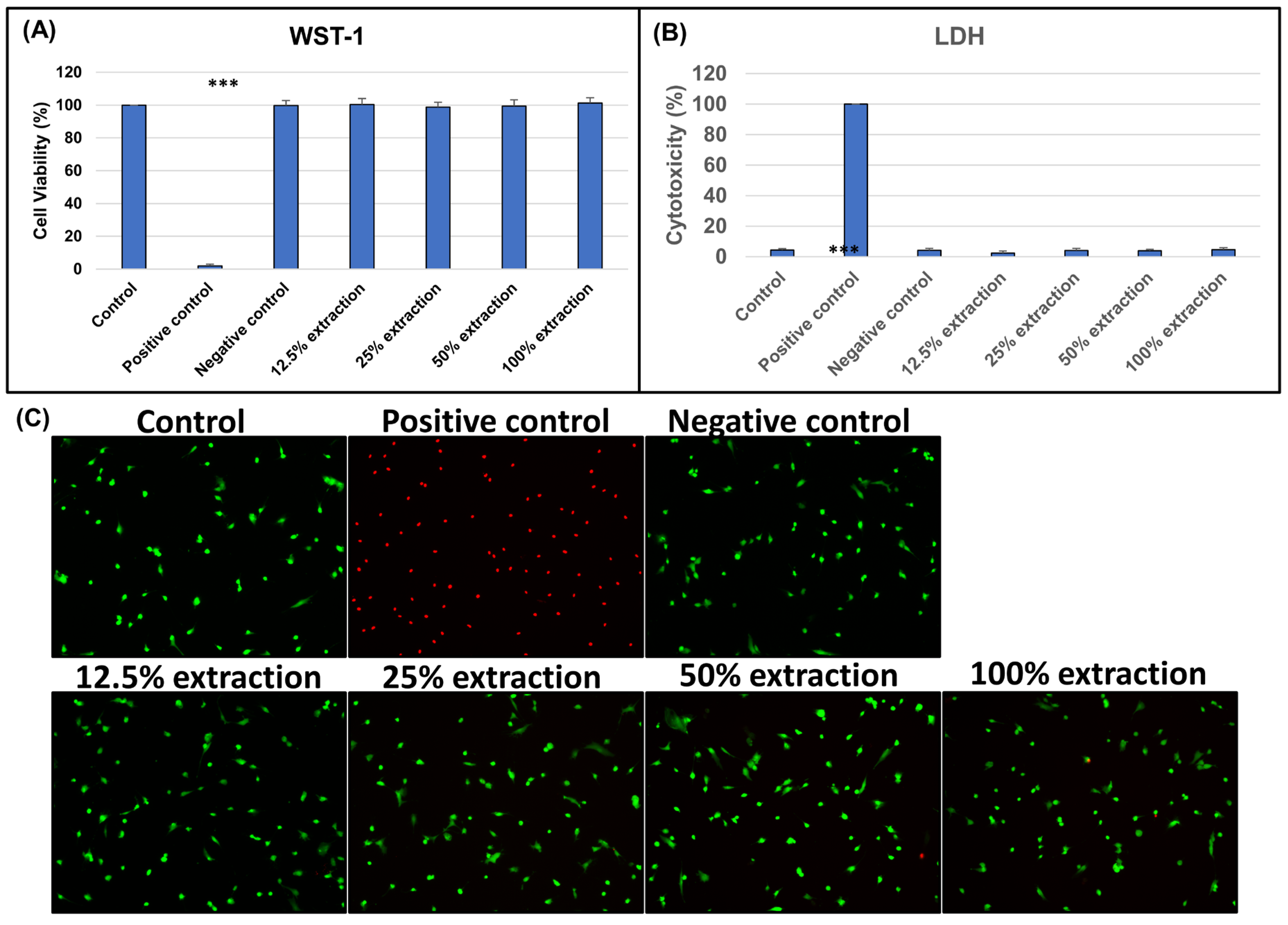
3.2. In Vitro Study
3.2.1. Biocompatibility of Biphasic Scaffold (BPS)
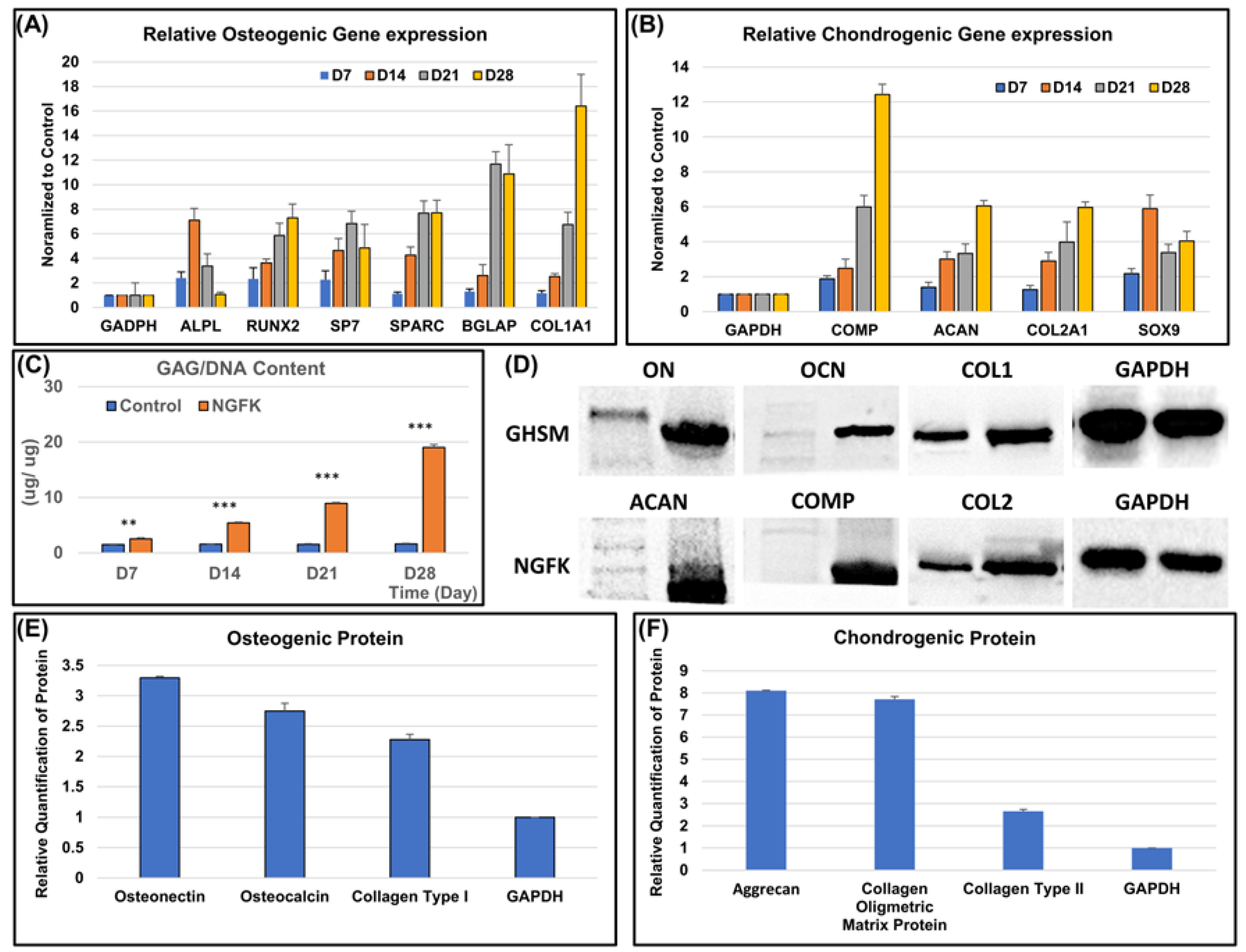
3.2.2. Osteo- and Chondrogenic Differentiation Potential of the Biomimetic Biphasic Scaffold
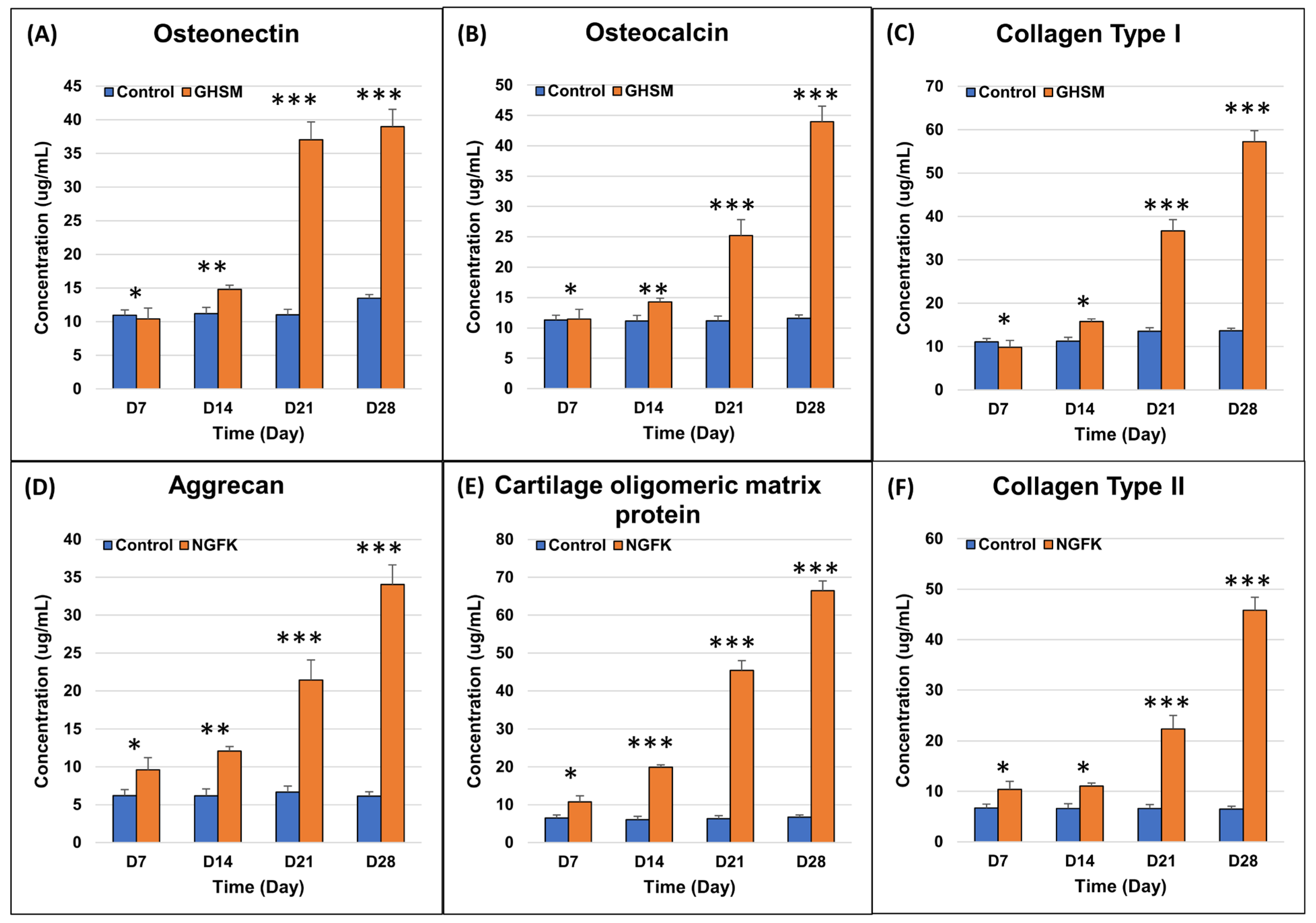
3.2.3. Biochemical Analysis
3.2.4. Immunofluorescence Analysis
3.3. In Vivo Histological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, B.; Lowe, J.S.; Stevens, A.; Heath, J.W. (Eds.) Wheater’s Functional Histology A Text and Colour Atlas, 5th ed.; Churchill Livingstone Elsevier: London, UK, 2006; pp. 65–79. [Google Scholar]
- Minzlaff, P.; Feucht, M.J.; Saier, T.; Cotic, M.; Plath, J.E.; Imhoff, A.B.; Hinterwimmer, S. Can young and active patients participate in sports after osteochondral autologous transfer combined with valgus high tibial osteotomy? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Oka, H.; Akune, T.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Suzuki, T.; Yoshida, H.; Ishibashi, H.; et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: The ROAD study. Osteoarthr. Cartil. 2009, 17, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Szerb, I.; Hangody, L.; Duska, Z.; Kaposi, N.P. Mosaicplasty. Bull. NYU Hosp. Jt. Dis. 2005, 63, 54–62. [Google Scholar]
- Lane, J.G.; Massie, J.B.; Ball, S.T.; Amiel, M.E.; Chen, A.C.; Bae, W.C.; Sah, R.L.; Amiel, D. Follow-up of osteochondral plug transfers in a goat model: A 6-month study. Am. J. Sports Med. 2004, 32, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, Y.; Yamamuro, T.; Hama, H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthrosc. J. Arthrosc. Relat. Surg. 1993, 9, 318–321. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Diaz-Romero, J.; Gaillard, J.P.; Grogan, S.P.; Nesic, D.; Trub, T.; Mainil-Varlet, P. Immunophenotypic analysis of human articular chondrocytes: Changes in surface markers associated with cell expansion in monolayer culture. J. Cell. Physiol. 2005, 202, 731–742. [Google Scholar] [CrossRef]
- Tatebe, M.; Nakamura, R.; Kagami, H.; Okada, K.; Ueda, M. Differentiation of transplanted mesenchymal stem cells in a large osteochondral defect in rabbit. Cytotherapy 2005, 7, 520–530. [Google Scholar] [CrossRef]
- Nakamura, T.; Sekiya, I.; Muneta, T.; Hatsushika, D.; Horie, M.; Tsuji, K.; Kawarasaki, T.; Watanabe, A.; Hishikawa, S.; Fujimoto, Y.; et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy 2012, 14, 327–338. [Google Scholar]
- Unterman, S.A.; Gibson, M.; Lee, J.H.; Crist, J.; Chansakul, T.; Yang, E.C.; Elisseeff, J.H. Hyaluronic acid-binding scaffold for articular cartilage repair. Tissue Eng. Part. A 2012, 18, 2497–2506. [Google Scholar] [CrossRef]
- Yoon, I.-S.; Chung, C.W.; Sung, J.-H.; Cho, H.-J.; Kim, J.S.; Shim, W.-S.; Shim, C.-K.; Chung, S.-J.; Kim, D.-D. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J. Biosci. Bioeng. 2011, 112, 402–408. [Google Scholar] [CrossRef]
- Cheuk, Y.C.; Wong, M.W.N.; Lee, K.M.; Fu, S.C. Use of allogeneic scaffold-free chondrocyte pellet in repair of osteochondral defect in a rabbit model. J. Orthop. Res. 2011, 29, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G.A.; Sweeney, S.M.; Korkko, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Yannas, I.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Ghassemi, Z.; Slaughter, G. Storage stability of electrospun pure gelatin stabilized with EDC/Sulfo-NHS. Biopolymers 2018, 109, e23232. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.R.; Coombes, A.G. Gravity spinning of polycaprolactone fibres for applications in tissue engineering. Biomaterials 2004, 25, 459–465. [Google Scholar] [CrossRef]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Med. Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef]
- Daugan, M.; Wojcicki, A.D.; d’Hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27. [Google Scholar]
- McCarthy, A.D.; Cortizo, A.M.; Sedlinsky, C. Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J. Diabetes 2016, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; D’Onofrio, L.; Eastell, R.; Schwartz, A.; Pozzilli, P.; Napoli, N. Oral anti-diabetic drugs and fracture risk, cut to the bone: Safe or dangerous? A narrative review. Osteoporos. Int. 2015, 26, 2073–2089. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Gillogly, S.D.; Voight, M.; Blackburn, T. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J. Orthop. Sports Phys. Ther. 1998, 28, 241–251. [Google Scholar] [PubMed]
- Martins, A.; Reis, R.L.; Neves, N.M. Micro/Nano Scaffolds for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 125–139. [Google Scholar]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [PubMed]
- Camp, C.L.; Stuart, M.J.; Krych, A.J. Current concepts of articular cartilage restoration techniques in the knee. Sports Health 2014, 6, 265–273. [Google Scholar] [CrossRef]
- Tamaddon, M.; Wang, L.; Liu, Z.; Liu, C. Osteochondral tissue repair in osteoarthritic joints: Clinical challenges and opportunities in tissue engineering. Biodes Manuf. 2018, 1, 101–114. [Google Scholar] [CrossRef]
- Martin, I.; Schaefer, D.; Dozin, B. Repair of Osteochondral Lesions. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2004; pp. 2000–2013. [Google Scholar]
- Seda Tigli, R.; Ghosh, S.; Laha, M.M.; Shevde, N.K.; Daheron, L.; Gimble, J.; Gümüşderelioğlu, M.; Kaplan, D.L. Comparative chondrogenesis of human cell sources in 3D scaffolds. J. Tissue Eng. Regen. Med. 2009, 3, 348–360. [Google Scholar]
- Kang, M.L.; Ko, J.Y.; Kim, J.E.; Im, G.I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef]
- Ortega, Z.; Alemán, M.E.; Donate, R. Nanofibers and Microfibers for Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 97–123. [Google Scholar] [PubMed]
- Rowland, C.R.; Glass, K.A.; Ettyreddy, A.R.; Gloss, C.C.; Matthews, J.R.L.; Huynh, N.P.T.; Guilak, F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018, 177, 161–175. [Google Scholar] [PubMed]
- Casanova, M.R.; Reis, R.L.; Martins, A.; Neves, N.M. The Use of Electrospinning Technique on Osteochondral Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1058, 247–263. [Google Scholar] [PubMed]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef]
- Mohan, G.; Magnitsky, S.; Melkus, G.; Subburaj, K.; Kazakia, G.; Burghardt, A.J.; Dang, A.; Lane, N.E.; Majumdar, S. Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: A pilot study. J. Orthop. Res. 2016, 34, 1780–1789. [Google Scholar] [CrossRef]
- Shi, D.; Xu, X.; Ye, Y.; Song, K.; Cheng, Y.; Di, J.; Hu, Q.; Li, J.; Ju, H.; Jiang, Q.; et al. Photo-Cross-Linked Scaffold with Kartogenin-Encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano 2016, 10, 1292–1299. [Google Scholar] [CrossRef]
- Lairson, L.L.; Lyssiotis, C.A.; Zhu, S.; Schultz, P.G. Small molecule-based approaches to adult stem cell therapies. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 107–125. [Google Scholar] [CrossRef]
- Stage, T.B.; Christensen, M.M.H.; Jorgensen, N.R.; Beck-Nielsen, H.; Brosen, K.; Gram, J.; Frost, M. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone 2018, 112, 35–41. [Google Scholar]
- Bahrambeigi, S.; Yousefi, B.; Rahimi, M.; Shafiei-Irannejad, V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed. Pharmacother. 2019, 109, 1593–1601. [Google Scholar]
- Zhang, C.S.; Li, M.Q.; Ma, T.; Zong, Y.; Cui, J.W.; Feng, J.W.; Wu, Y.-Q.; Lin, S.-Y.; Lin, S.-C. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016, 24, 521–522. [Google Scholar] [CrossRef]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. J. Tissue Eng. Regen. M. 2018, 12, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. RUNX genes in development and cancer: Regulation of viral gene expression and the discovery of RUNX family genes. Adv. Cancer Res. 2008, 99, 33–76. [Google Scholar] [PubMed]
- Chava, S.; Chennakesavulu, S.; Gayatri, M.B.; Reddy, A.B.M. A novel phosphorylation by AMP-activated kinase regulates RUNX2 from ubiquitination in osteogenesis over adipogenesis. Cell Death Dis. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.F.; Xue, J.; Jia, Y.Q.A.; Hu, J. Effect of the anti-diabetic drug metformin on bone mass in ovariectomized rats. Eur. J. Pharmacol. 2010, 635, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Mai, Q.G.; Zhang, Z.M.; Xu, S.; Lu, M.; Zhou, R.P.; Zhao, L.; Jia, C.-H.; Wen, Z.-H.; Jin, D.-D.; Bai, X.-C. Metformin Stimulates Osteoprotegerin and Reduces RANKL Expression in Osteoblasts and Ovariectomized Rats. J. Cell Biochem. 2011, 112, 2902–2909. [Google Scholar] [CrossRef]
- Di Martino, A.; Perdisa, F.; Filardo, G.; Busacca, M.; Kon, E.; Marcacci, M.; Zaffagnini, S. Cell-Free Biomimetic Osteochondral Scaffold for the Treatment of Knee Lesions: Clinical and Imaging Results at 10-Year Follow-up. Am. J. Sports Med. 2021, 49, 2645–2650. [Google Scholar] [CrossRef]



| Osteogenic Gene | ||
|---|---|---|
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
| ALP | GAGAAGCCGGGACACAGTTC | CCTCCTCAACTGGGATGATGC |
| RUNX2 | TAGGCGCATTTCAGGTGCTT | GGTGTGGTAGTGAGTGGTGG |
| SP7 | TAGGACTGTAGGACCGGAGC | CATAGTGAACTTCCTCCTGGGG |
| SPARC | ATTGACGGGTACCTCTCCCA | GAAAAAGCGGGTGGTGCAAT |
| BGLAP | CTCACACTCCTCGCCCTATTG | GCTTGGACACAAAGGCTGCAC |
| COL1A1 | AGAGGTCGCCCTGGAGC | CAGGAACACCCTGTTCACCA |
| GAPDH | AATGGGCAGCCGTTAGGAAA | GCCCAATACGACCAAATCAGAG |
| Chondrogenic Gene | ||
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
| SOX9 | GGCAAGCTCTGGAGACTTCTG | CCCGTTCTTCACCGACTTCC |
| ACAN | TAAAAAGGGCACAGCCACCAC | GTGAGCTCCGCTTCTGTAGTC |
| COMP | CAAGGCCAACAAGCAGGTTT | TATGTTGCCCGGTCTCACAC |
| COL2A1 | ATGAGGGCGCGGTAGAGA | GCCAGCCTCCTGGACATC |
| GAPDH | AATGGGCAGCCGTTAGGAAA | GCCCAATACGACCAAATCAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.-H.; Lin, Y.-W.; Sun, C.-K.; Sun, J.-S. Small-Molecule Loaded Biomimetic Biphasic Scaffold for Osteochondral Regeneration: An In Vitro and In Vivo Study. Bioengineering 2023, 10, 847. https://doi.org/10.3390/bioengineering10070847
Fang C-H, Lin Y-W, Sun C-K, Sun J-S. Small-Molecule Loaded Biomimetic Biphasic Scaffold for Osteochondral Regeneration: An In Vitro and In Vivo Study. Bioengineering. 2023; 10(7):847. https://doi.org/10.3390/bioengineering10070847
Chicago/Turabian StyleFang, Chih-Hsiang, Yi-Wen Lin, Chung-Kai Sun, and Jui-Sheng Sun. 2023. "Small-Molecule Loaded Biomimetic Biphasic Scaffold for Osteochondral Regeneration: An In Vitro and In Vivo Study" Bioengineering 10, no. 7: 847. https://doi.org/10.3390/bioengineering10070847
APA StyleFang, C.-H., Lin, Y.-W., Sun, C.-K., & Sun, J.-S. (2023). Small-Molecule Loaded Biomimetic Biphasic Scaffold for Osteochondral Regeneration: An In Vitro and In Vivo Study. Bioengineering, 10(7), 847. https://doi.org/10.3390/bioengineering10070847









