In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Human OA-Cartilage Explants and Isolated OA Chondrocytes
2.2. Synthesis of MPC and SBMA Hydrogels
2.3. Preparation of Cartilage–Hydrogel and Chondrocyte–Hydrogel Composites
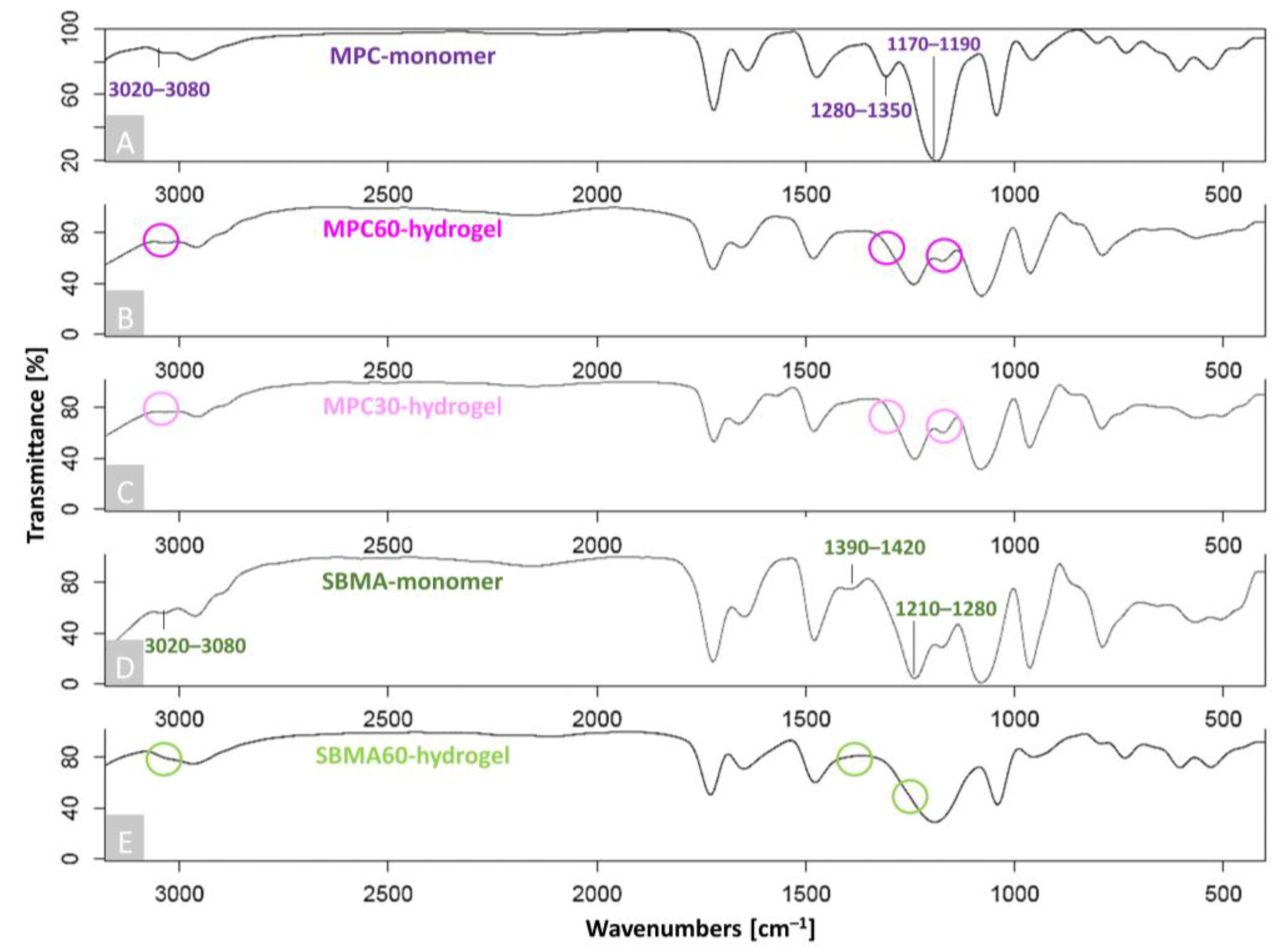
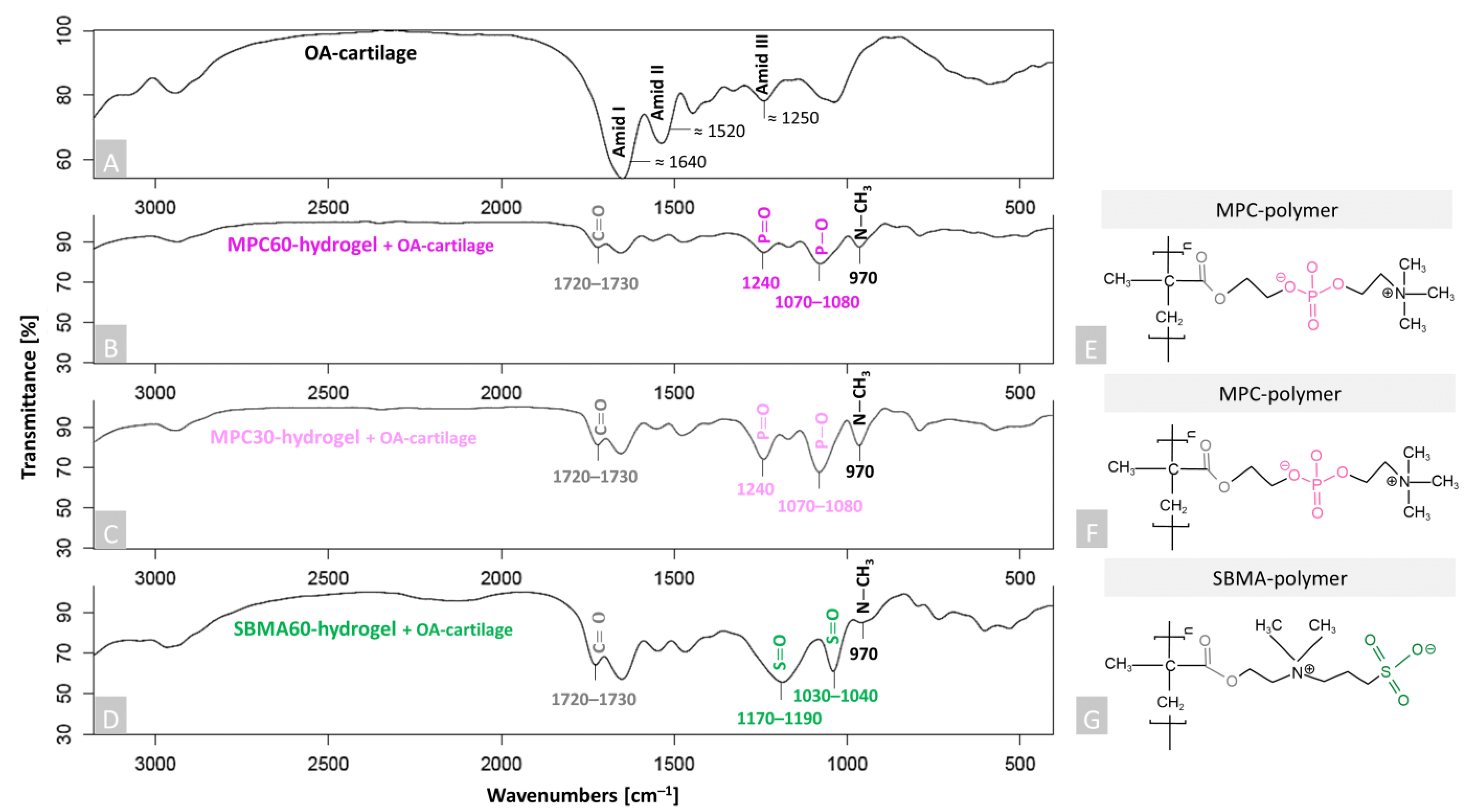
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
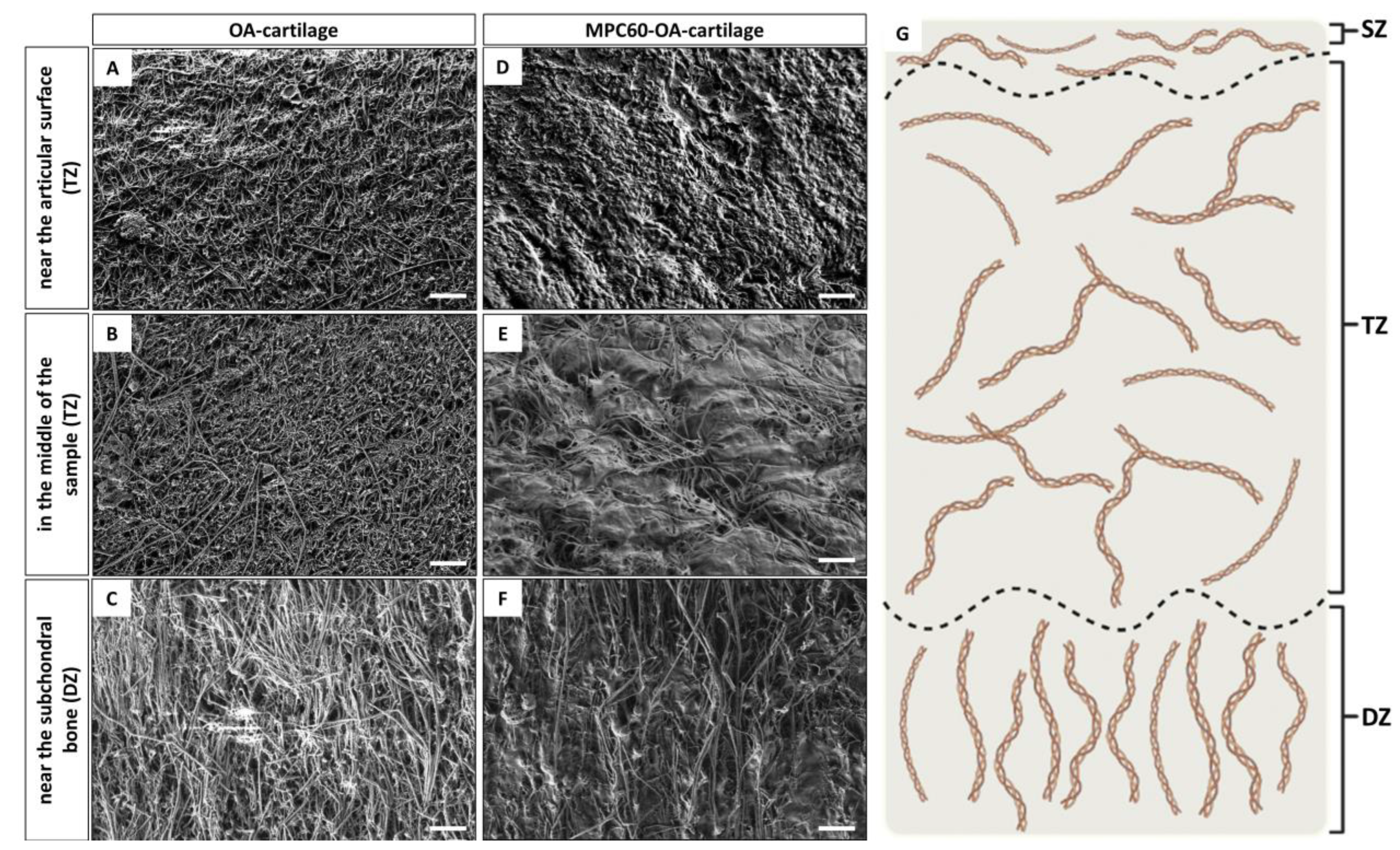
2.5. Scanning Electron Microscopy (SEM)
2.6. Fluorescence Microscopy
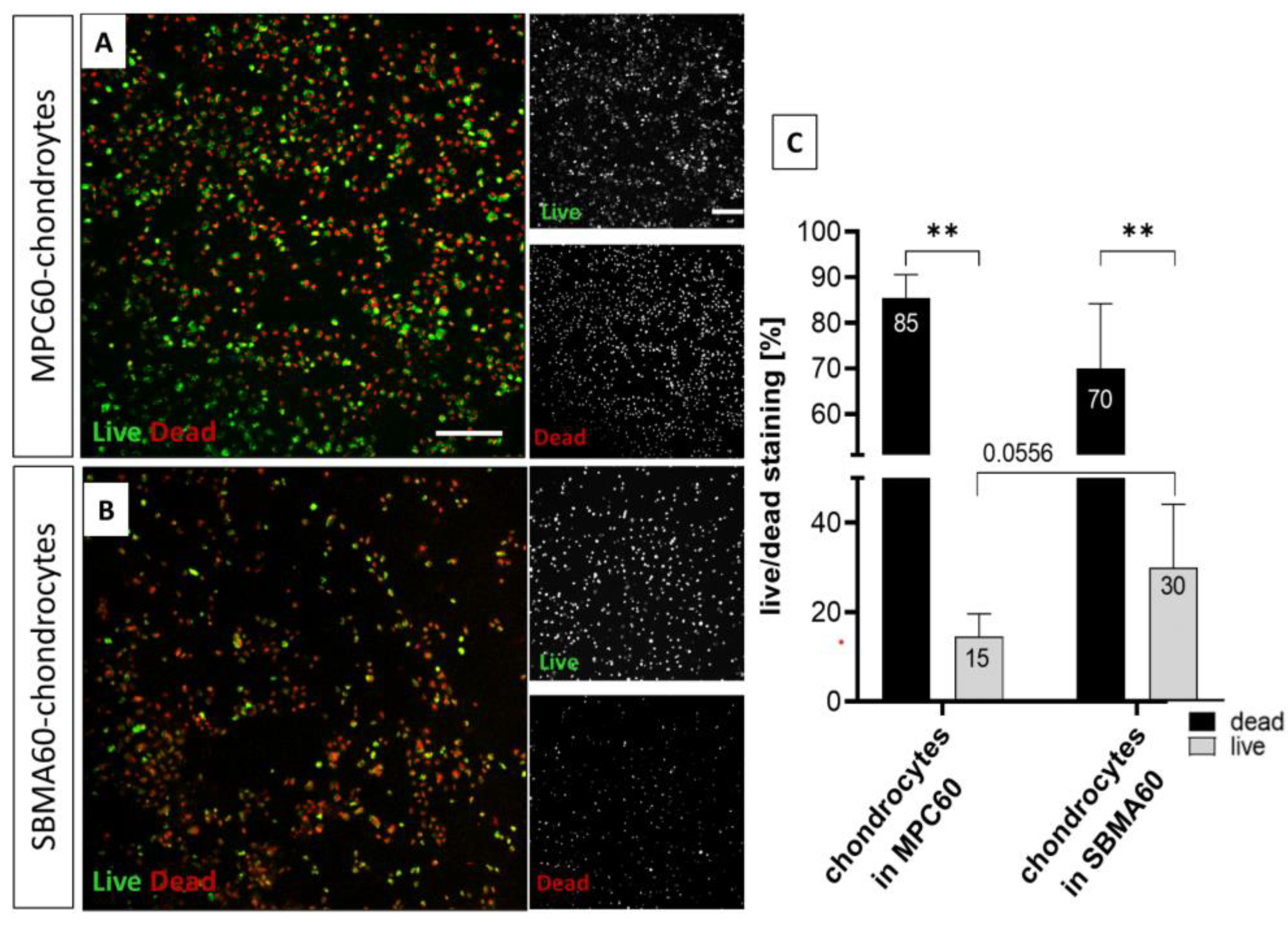
2.7. Live/Dead Cell Staining of Encapsulated Chondrocytes in Hydrogels and Cartilage–Hydrogel/Component Composites
2.8. CellTiter-Blue (CTB) Viability Assay
2.9. Statistical Analysis
3. Results
3.1. Successful Polymerization and Infiltration of Monomer Solutions into Human OA-Cartilage Plugs
3.2. Increased Toxicity of Monomer Solutions for Chondrocytes in 24 h Infiltrated OA-Cartilage Explants Compared to Embedded Isolated Chondrocytes
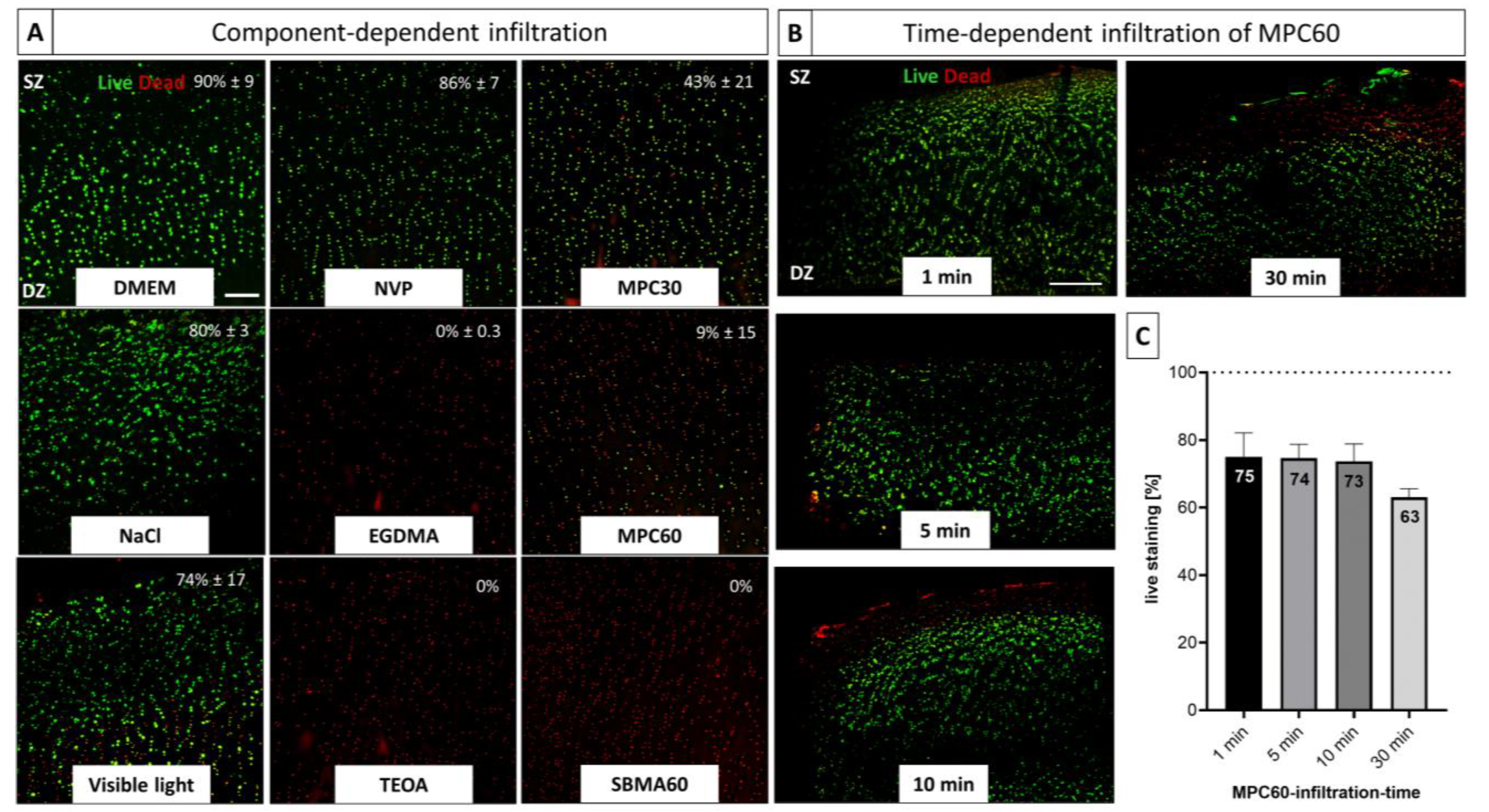
3.3. Time-Dependent and Component-Dependent Increase in Monomer Solution Cytotoxicity in Human OA-Cartilage Explants
4. Discussion
4.1. Evidence of Polymerization and Infiltration
4.2. Biocompatibility
4.3. Translational Aspects
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthrits. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Hunziker, E.B. Articular cartilage repair: Are the intrinsic biological constraints undermining this process insuperable? Osteoarthr. Cartil. 1999, 7, 15–28. [Google Scholar] [CrossRef]
- Kraus, V.; Blanco, F.; Englund, M.; Karsdal, M.; Lohmander, L. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Driban, J.B.; Harkey, M.S.; Barbe, M.F.; Ward, R.J.; MacKay, J.; Davis, J.E.; Lu, B.; Price, L.L.; Eaton, C.B.; Lo, G.H.; et al. Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskelet. Disord. 2020, 21, 332. [Google Scholar] [CrossRef]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Thromb. Haemost. 2009, 11, 224. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9, 325. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Jain, K.B.; Ravikumar, P. Recent advances in treatments of cartilage regeneration for knee osteoarthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 102014. [Google Scholar] [CrossRef]
- Reid, M.C.; Eccleston, C.; Pillemer, K. Management of chronic pain in older adults. BMJ 2015, 350, h532. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Martín, A.R.; Patel, J.M.; Zlotnick, H.M.; Carey, J.L.; Mauck, R.L. Emerging therapies for cartilage regeneration in currently excluded ‘red knee’ populations. Npj Regen. Med. 2019, 4, 12. [Google Scholar] [CrossRef]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Loo, S.J.Q.; Wong, N.K. Advantages and challenges of stem cell therapy for osteoarthritis (Review). Biomed. Rep. 2021, 15, 67. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, W.; Wan, R.; Wang, J.; Zhou, Q.; Qiu, S.; Singh, S. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur. Cells Mater. 2012, 23, 1–12. [Google Scholar] [CrossRef]
- Malda, J.; Groll, J.; van Weeren, P.R. Rethinking articular cartilage regeneration based on a 250-year-old statement. Nat. Rev. Rheumatol. 2019, 15, 571–572. [Google Scholar] [CrossRef]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019, 93, 222–238. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Qu, L.; Wang, Q.; Zhou, Q. Hydrogels for Treatment of Different Degrees of Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 858656. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Velasco-Salgado, C.; Pontes-Quero, G.M.; García-Fernández, L.; Aguilar, M.R.; de Wit, K.; Vázquez-Lasa, B.; Rojo, L.; Abradelo, C. The Role of Polymeric Biomaterials in the Treatment of Articular Osteoarthritis. Pharmaceutics 2022, 14, 1644. [Google Scholar] [CrossRef]
- Lin, X.; Tsao, C.T.; Kyomoto, M.; Zhang, M. Injectable Natural Polymer Hydrogels for Treatment of Knee Osteoarthritis. Adv. Healthc. Mater. 2021, 11, 2101479. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2020, 6, 998–1011. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef]
- Songkroh, T.; Xie, H.; Yu, W.; Liu, X.; Sun, G.; Xu, X.; Ma, X. Injectable in situ forming chitosan-based hydrogels for curcumin delivery. Macromol. Res. 2015, 23, 53–59. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsushita, T.; Tabata, Y.; Saito, T.; Matsumoto, T.; Nagai, K.; Kuroda, R.; Kurosaka, M. Intra-articular administration of gelatin hydrogels incorporating rapamycin–micelles reduces the development of experimental osteoarthritis in a murine model. Biomaterials 2014, 35, 9904–9911. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Sha´ban, M.; Radzi, M.A.A. Scaffolds for Cartilage Regeneration: To Use or Not to Use? In Bioinspired Biomaterials: Advances in Tissue Engineering and Regenerative Medicine. Advances in Experimental Medicine and Biology, 1st ed.; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Springer Singapore: Singapore, 2020; pp. 97–114. [Google Scholar]
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Shen, J. Sustainable, flexible and biocompatible hydrogels derived from microbial polysaccharides with tailorable structures for tissue engineering. Carbohydr. Polym. 2020, 237, 116160. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Dong, D.; Li, J.; Cui, M.; Wang, J.; Zhou, Y.; Luo, L.; Wei, Y.; Ye, L.; Sun, H.; Yao, F. In Situ “Clickable” Zwitterionic Starch-Based Hydrogel for 3D Cell Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 4442–4455. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, Y.; Luo, J. Synthesis and characterizations of zwitterionic copolymer hydrogels with excellent lubrication behavior. Tribol. Int. 2019, 143, 106026. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent Progress in Cartilage Lubrication. Adv. Mater. 2021, 33, e2005513. [Google Scholar] [CrossRef]
- Milner, P.E.; Parkes, M.; Puetzer, J.L.; Chapman, R.; Stevens, M.M.; Cann, P.; Jeffers, J.R. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018, 65, 102–111. [Google Scholar] [CrossRef]
- Bonyadi, S.Z.; Demott, C.J.; Grunlan, M.A.; Dunn, A.C. Cartilage-like tribological performance of charged double network hydrogels. J. Mech. Behav. Biomed. Mater. 2020, 114, 104202. [Google Scholar] [CrossRef]
- Rong, M.; Liu, H.; Scaraggi, M.; Bai, Y.; Bao, L.; Ma, S.; Ma, Z.; Cai, M.; Dini, D.; Zhou, F. High Lubricity Meets Load Capacity: Cartilage Mimicking Bilayer Structure by Brushing Up Stiff Hydrogels from Subsurface. Adv. Funct. Mater. 2020, 30, 202004062. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage–Bone Crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Zhou, X.; Haudenschild, A.K.; Sherlock, B.E.; Hu, J.C.; Leach, J.K.; Athanasiou, K.A.; Marcu, L. Detection of glycosaminoglycan loss in articular cartilage by fluorescence lifetime imaging. J. Biomed. Opt. 2018, 23, 126002. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent Advances in Understanding the Role of Cartilage Lubrication in Osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef]
- Lee, D.W.; Banquy, X.; Israelachvili, J.N. Stick-slip friction and wear of articular joints. Proc. Natl. Acad. Sci. USA 2013, 110, E567–E574. [Google Scholar] [CrossRef]
- Cooper, B.; Lawson, T.; Snyder, B.; Grinstaff, M. Reinforcement of articular cartilage with a tissue-interpenetrating polymer network reduces friction and modulates interstitial fluid load support. Osteoarthr. Cartil. 2017, 25, 1143–1149. [Google Scholar] [CrossRef]
- Cooper, B.G.; Stewart, R.C.; Burstein, D.; Snyder, B.D.; Grinstaff, M.W. A Tissue-Penetrating Double Network Restores the Mechanical Properties of Degenerated Articular Cartilage. Angew. Chem. Int. Ed. 2016, 55, 4226–4230. [Google Scholar] [CrossRef]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Electron Microscope Unit: Sample Preparation Techniques on Electron Microscopy. Available online: https://emunit.hku.hk/documents/SamplePreparationTechnique.pdf (accessed on 28 June 2022).
- Microscopy Core Facility: Biological Sample Fixation for SEM. Available online: https://research.usu.edu/microscopy/files/SEM-sample-prep4.pdf (accessed on 28 June 2022).
- Yandi, W.; Nagy, B.; Skallberg, A.; Uvdal, K.; Zimmermann, R.; Liedberg, B.; Ederth, T. Polyampholytic Poly(AEMA-co-SPMA) Thin Films and Their Potential for Antifouling Applications. ACS Appl. Polym. Mater. 2021, 3, 5361–5372. [Google Scholar] [CrossRef]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, Y.; Luo, J. Macroscale superlubricity achieved between zwitterionic copolymer hydrogel and sapphire in water. Mater. Des. 2020, 188, 108441. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mow, V.C.; Ratcliffe, A. Restoration of Injured or Degenerated Articular Cartilage. J. Am. Acad. Orthop. Surg. 1994, 2, 192–201. [Google Scholar] [CrossRef]
- Changoor, A.; Nelea, M.; Méthot, S.; Tran-Khanh, N.; Chevrier, A.; Restrepo, A.; Shive, M.; Hoemann, C.; Buschmann, M. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthr. Cartil. 2011, 19, 1458–1468. [Google Scholar] [CrossRef]
- Meng, W.; Gao, L.; Venkatesan, J.K.; Wang, G.; Madry, H.; Cucchiarini, M. Translational applications of photopolymerizable hydrogels for cartilage repair. J. Exp. Orthop. 2019, 6, 47. [Google Scholar] [CrossRef]
- Kim, J.; Lin, B.; Kim, S.; Choi, B.; Evseenko, D.; Lee, M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J. Biol. Eng. 2015, 9, 1. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Shimomura, K.; Sohn, J.; Fritch, M.R.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G.; Tuan, R.S. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Pascual-Garrido, C.; Aisenbrey, E.A.; Fontan, F.R.; Payne, K.; Bryant, S.J.; Goodrich, L.R. Photopolymerizable Injectable Cartilage Mimetic Hydrogel for the Treatment of Focal Chondral Lesions: A Proof of Concept Study in a Rabbit Animal Model. Am. J. Sports Med. 2018, 47, 212–221. [Google Scholar] [CrossRef]
- Bahney, C.; Lujan, T.; Hsu, C.; Bottlang, M.; West, J.; Johnstone, B. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur. Cells Mater. 2011, 22, 43–55. [Google Scholar] [CrossRef]
- Nikolić, L.; Stojanović, T.; Nikolić, V.; Urošević, M.; Ilić-Stojanović, S.; Dinić, A.; Gajić, I.; Savić, V.; Zdravković, A. Synthesis and characterisation of hydrogels based on starch and citric acid. Adv. Technol. 2020, 9, 50–57. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Aly, A.A.; Amr, A.; Soliman, A.A.F. Biocompatible hydrogel for cartilage repair with adjustable properties. Polym. Adv. Technol. 2019, 30, 2026–2033. [Google Scholar] [CrossRef]
- Tsegay, N.M.; Du, X.; Liu, S.-S.; Wang, C.-F.; Chen, S. Frontal polymerization for smart intrinsic self-healing hydrogels and its integration with microfluidics. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1412–1423. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Wahab, H.A.; Ismail, M.A.; Naser, A.; Abdelhai, F.; El-Damhougy, B.K.; Nady, N.; Meganid, A.S.; Alkhursani, S.A. Characterization of Starch-based three components of gamma-ray cross-linked hydrogels to be used as a soil conditioner. Mater. Sci. Eng. B 2020, 260, 114645. [Google Scholar] [CrossRef]
- de Lima, G.G.; Elter, J.K.; Chee, B.S.; Magalhães, W.L.E.; Devine, D.M.; Nugent, M.J.D.; de Sá, M.J.C. A tough and novel dual-response PAA/P(NiPAAM-co-PEGDMA) IPN hydrogels with ceramics by photopolymerization for consolidation of bone fragments following fracture. Biomed. Mater. 2019, 14, 054101. [Google Scholar] [CrossRef]
- Hua, Y.; Xia, H.; Jia, L.; Zhao, J.; Zhao, D.; Yan, X.; Zhang, Y.; Tang, S.; Zhou, G.; Zhu, L.; et al. Ultrafast, tough, and adhesive hydrogel based on hybrid photocrosslinking for articular cartilage repair in water-filled arthroscopy. Sci. Adv. 2021, 7, eabg0628. [Google Scholar] [CrossRef]
- Lotz, M.K.; Caramés, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat. Rev. Rheumatol. 2011, 7, 579–587. [Google Scholar] [CrossRef]
- Grogan, S.P.; D’lima, D.D. Joint aging and chondrocyte cell death. Int. J. Clin. Rheumatol. 2010, 5, 199–214. [Google Scholar] [CrossRef]
- Kowalski, M.A.; Fernandes, L.M.; Hammond, K.E.; Labib, S.; Drissi, H.; Patel, J.M. Cartilage-penetrating hyaluronic acid hydrogel preserves tissue content and reduces chondrocyte catabolism. J. Tissue Eng. Regen. Med. 2022, 16, 1138–1148. [Google Scholar] [CrossRef]
- Wilusz, R.; Zauscher, S.; Guilak, F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthr. Cartil. 2013, 21, 1895–1903. [Google Scholar] [CrossRef]
- Geever, L.M.; Cooney, C.C.; Lyons, J.; Kennedy, J.E.; Nugent, M.; Devery, S.; Higginbotham, C.L. Characterisation and controlled drug release from novel drug-loaded hydrogels. Eur. J. Pharm. Biopharm. 2008, 69, 1147–1159. [Google Scholar] [CrossRef]
- Bai, T.; Liu, S.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S. Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions. Biomaterials 2014, 35, 3926–3933. [Google Scholar] [CrossRef]
- Bielecka-Kowalska, A.; Czarny, P.; Wigner, P.; Synowiec, E.; Kowalski, B.; Szwed, M.; Krupa, R.; Toma, M.; Drzewiecka, M.; Majsterek, I.; et al. Ethylene glycol dimethacrylate and diethylene glycol dimethacrylate exhibits cytotoxic and genotoxic effect on human gingival fibroblasts via induction of reactive oxygen species. Toxicol. Vitr. 2018, 47, 8–17. [Google Scholar] [CrossRef]
- Huang, H.; Tan, Y.; Ayers, D.C.; Song, J. Anionic and Zwitterionic Residues Modulate Stiffness of Photo-Cross-Linked Hydrogels and Cellular Behavior of Encapsulated Chondrocytes. ACS Biomater. Sci. Eng. 2018, 4, 1843–1851. [Google Scholar] [CrossRef]
- Cui, X.; Breitenkamp, K.; Finn, M.; Lotz, M.; D’Lima, D.D.; Mir, T.A.; Nakamura, M.; Guo, T.; Lembong, J.; Zhang, L.G.; et al. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef]
- Chien, H.-W.; Yu, J.; Li, S.T.; Chen, H.-Y.; Tsai, W.-B. An in situ poly(carboxybetaine) hydrogel for tissue engineering applications. Biomater. Sci. 2017, 5, 322–330. [Google Scholar] [CrossRef]
- Bapary, M.A.J.; Takano, J.; Soma, S.; Sankai, T.; Bapary, M. Effect of blue light-emitting diode light and antioxidant potential in a somatic cell. Cell Biol. Int. 2019, 43, 1296–1306. [Google Scholar] [CrossRef]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef]
- Lim, K.S.; Abinzano, F.; Bernal, P.N.; Sanchez, A.A.; Atienza-Roca, P.; Otto, I.A.; Peiffer, Q.C.; Matsusaki, M.; Woodfield, T.; Malda, J.; et al. One-Step Photoactivation of a Dual-Functionalized Bioink as Cell Carrier and Cartilage-Binding Glue for Chondral Regeneration. Adv. Healthc. Mater. 2020, 9, e1901792. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Li, T.; Zhang, L.; Azhar, U.; Ma, J.; Zhai, C.; Zong, C.; Zhang, S. Cytocompatible and non-fouling zwitterionic hyaluronic acid-based hydrogels using thiol-ene “click” chemistry for cell encapsulation. Carbohydr. Polym. 2020, 236, 116021. [Google Scholar] [CrossRef]
- Patel, J.M.; Loebel, C.; Saleh, K.S.; Wise, B.C.; Bonnevie, E.D.; Miller, L.M.; Carey, J.L.; Burdick, J.A.; Mauck, R.L. Stabilization of Damaged Articular Cartilage with Hydrogel-Mediated Reinforcement and Sealing. Adv. Healthc. Mater. 2021, 10, e2100315. [Google Scholar] [CrossRef]
- Grenier, S.; Donnelly, P.E.; Gittens, J.; Torzilli, P.A. Resurfacing damaged articular cartilage to restore compressive properties. J. Biomech. 2015, 48, 122–129. [Google Scholar] [CrossRef]
- Stanco, D.; Urbán, P.; Tirendi, S.; Ciardelli, G.; Barrero, J. 3D bioprinting for orthopaedic applications: Current advances, challenges and regulatory considerations. Bioprinting 2020, 20, e00103. [Google Scholar] [CrossRef]
- O’connell, C.D.; Di Bella, C.; Thompson, F.; Augustine, C.; Beirne, S.; Cornock, R.; Richards, C.J.; Chung, J.; Gambhir, S.; Yue, Z.; et al. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016, 8, 015019. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef]
- Onofrillo, C.; Duchi, S.; O’connell, C.D.; Blanchard, R.; O’connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication 2018, 10, 045006. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köck, H.; Striegl, B.; Kraus, A.; Zborilova, M.; Christiansen, S.; Schäfer, N.; Grässel, S.; Hornberger, H. In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes. Bioengineering 2023, 10, 767. https://doi.org/10.3390/bioengineering10070767
Köck H, Striegl B, Kraus A, Zborilova M, Christiansen S, Schäfer N, Grässel S, Hornberger H. In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes. Bioengineering. 2023; 10(7):767. https://doi.org/10.3390/bioengineering10070767
Chicago/Turabian StyleKöck, Hannah, Birgit Striegl, Annalena Kraus, Magdalena Zborilova, Silke Christiansen, Nicole Schäfer, Susanne Grässel, and Helga Hornberger. 2023. "In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes" Bioengineering 10, no. 7: 767. https://doi.org/10.3390/bioengineering10070767
APA StyleKöck, H., Striegl, B., Kraus, A., Zborilova, M., Christiansen, S., Schäfer, N., Grässel, S., & Hornberger, H. (2023). In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes. Bioengineering, 10(7), 767. https://doi.org/10.3390/bioengineering10070767







