Evaluation of Polymer-Coated Carbon Nanotube Flexible Microelectrodes for Biomedical Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
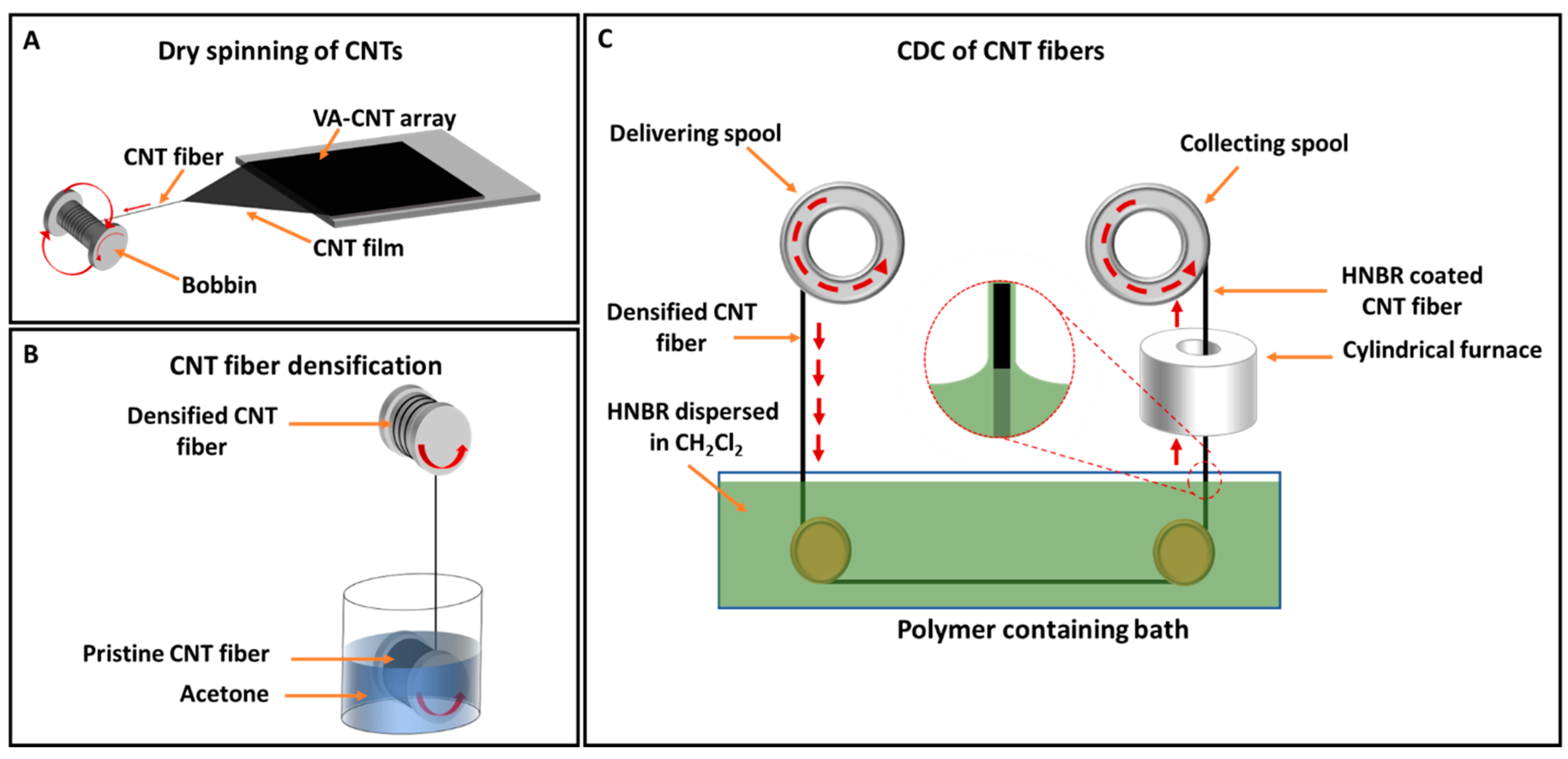
2.2. CNT Fiber Synthesis and Densification
2.3. Fabrication of HNBR-Coated CNT Fibers Using the CDC Approach
2.4. Polymer Coating Evaluation
2.5. Spectroscopic Analyses
2.5.1. X-ray Photoelectron Spectroscopy (XPS)
2.5.2. Fourier Transform Infrared (FT-IR) and Raman Spectroscopy
2.6. Fabrication of HNBR-Coated CNT Fiber Microelectrodes
2.7. Electrochemical Characterization
2.7.1. Electrical Insulating Properties of HNBR Coating
2.7.2. EIS Analysis
2.7.3. Cyclic Voltammetry (CV)
2.8. Long-Term Stability
2.9. In Vitro Cytotoxicity of HNBR Coating
2.9.1. Elution Test
2.9.2. Neuronal Cultures
3. Results and Discussion
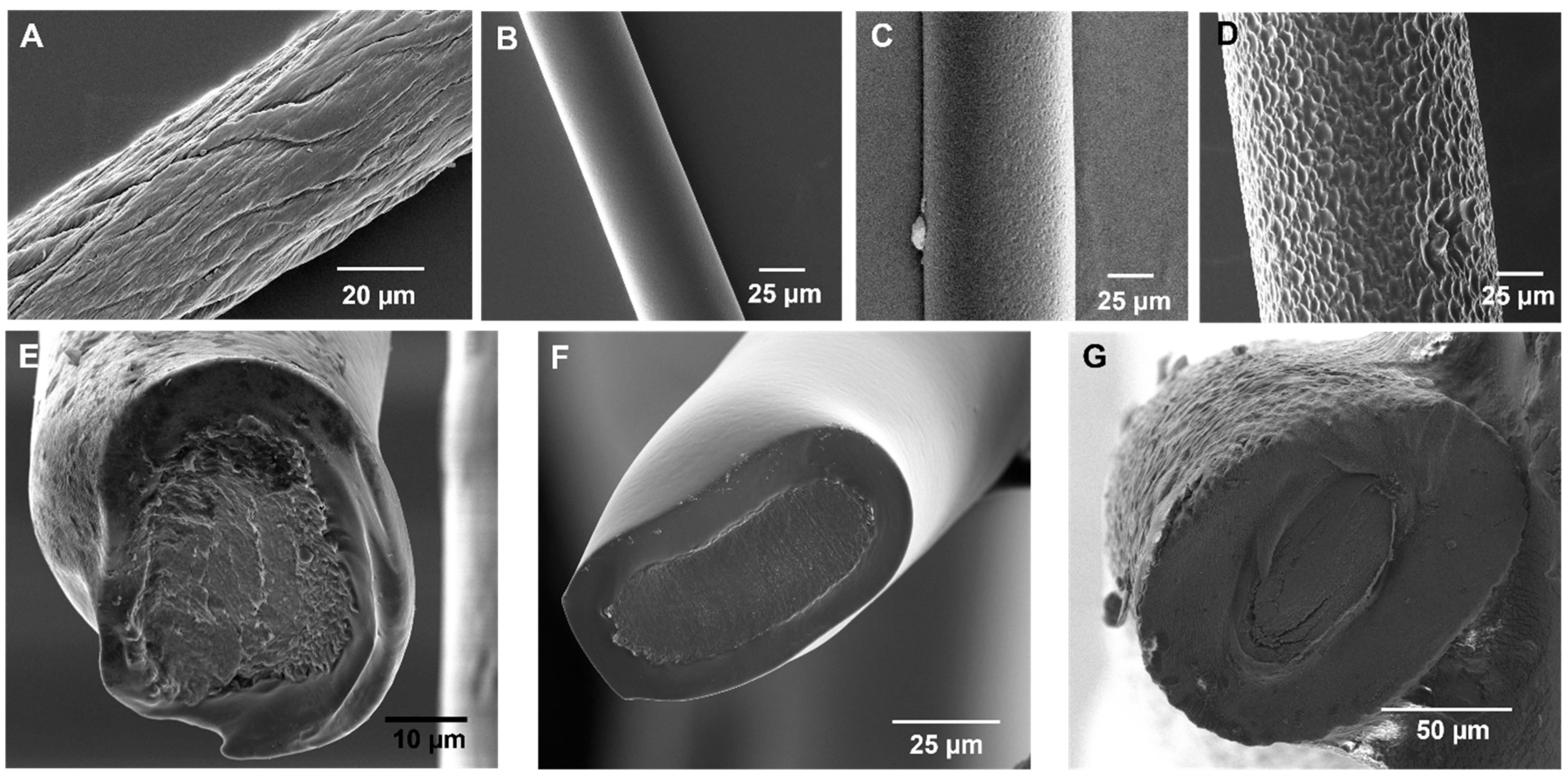
3.1. Optimization of the CDC Technique for Fabricating Flexible HNBR-Coated CNT Fibers
3.2. Spectroscopic Characterization of HNBR Coating
3.3. Cytotoxicity Evaluation of HNBR Coating
3.4. Electrochemical Analysis of HNBR-Coated CNT Fiber Microelectrodes
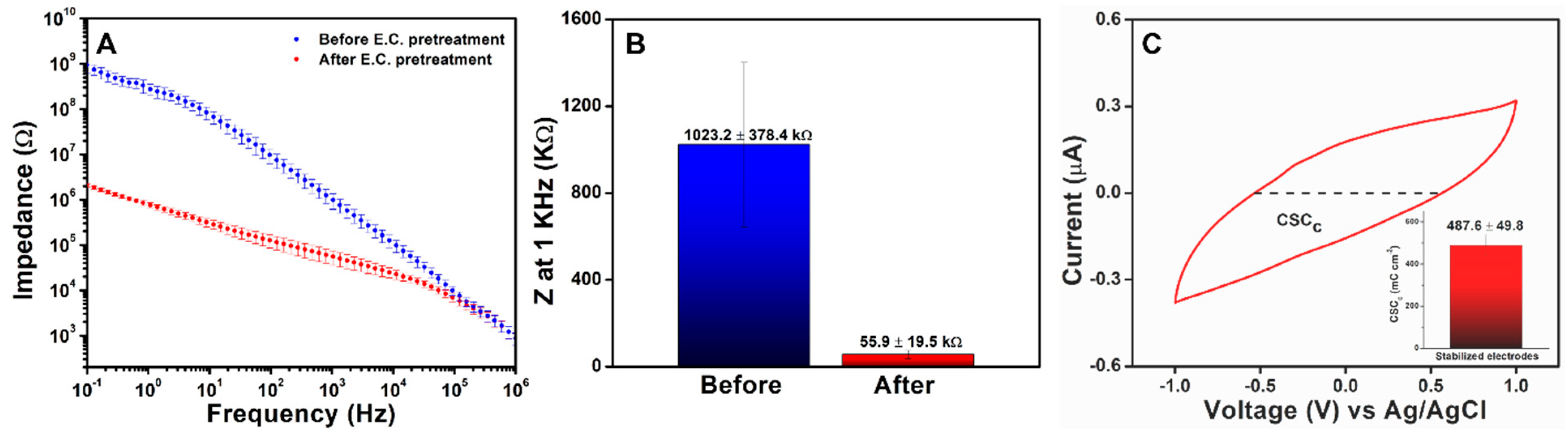
3.4.1. EIS Analysis
3.4.2. CSC and Water Window Analysis
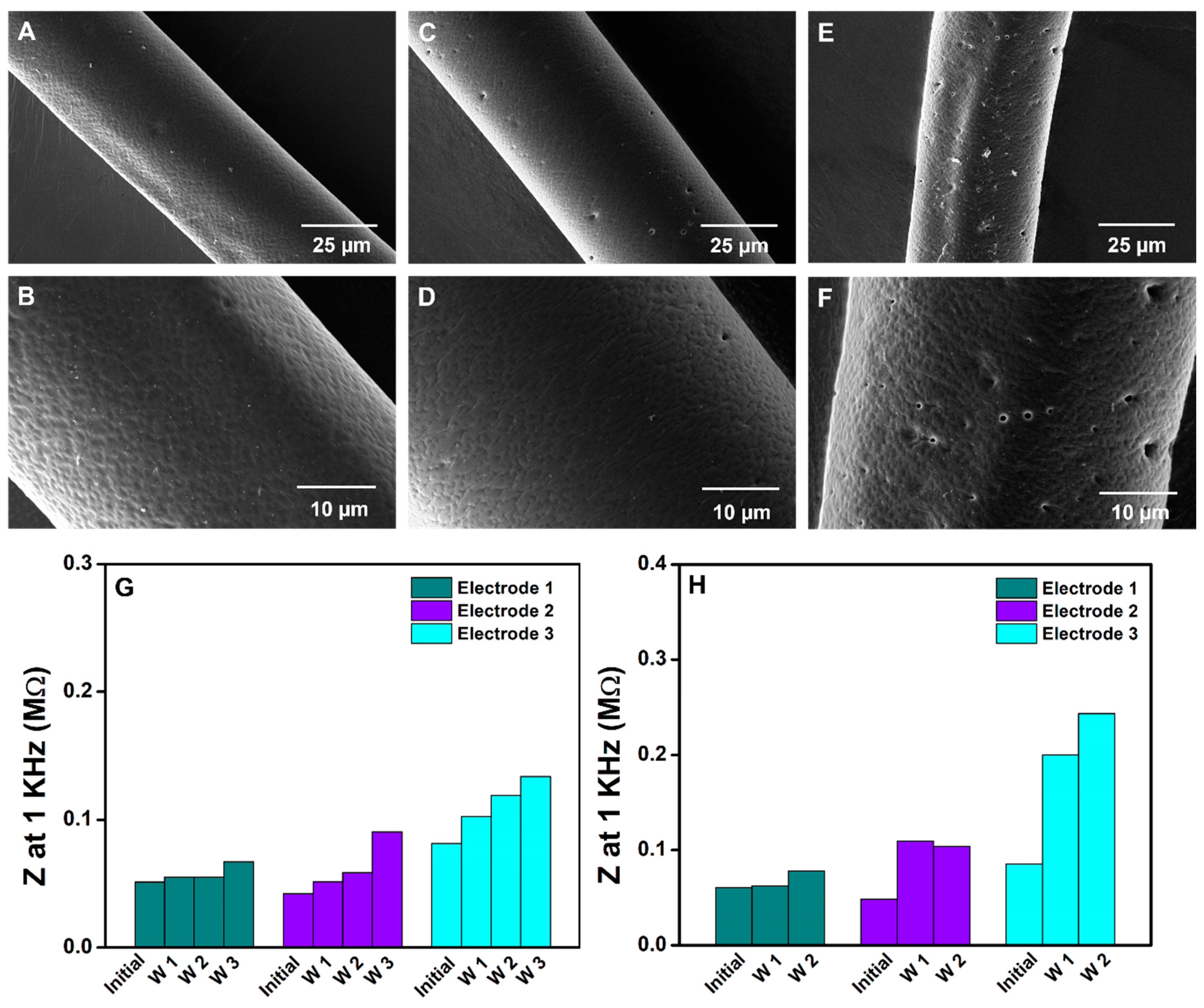
3.4.3. Aging Studies of HNBR-Coated CNT Fiber Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible Neural Electrodes for Long-Lasting Intracortical Recording. iScience 2020, 23, 101387. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, R.; He, L.; Feng, H.; Li, Y.; Li, Z. Recent Development of Implantable and Flexible Nerve Electrodes. Smart Mater. Med. 2020, 1, 131–147. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Fang, Y. Bioinspired Flexible Electronics for Seamless Neural Interfacing and Chronic Recording. Nanoscale Adv. 2020, 2, 3095–3102. [Google Scholar] [CrossRef]
- Golabchi, A.; Woeppel, K.M.; Li, X.; Lagenaur, C.F.; Cui, X.T. Neuroadhesive Protein Coating Improves the Chronic Performance of Neuroelectronics in Mouse Brain. Biosens. Bioelectron. 2020, 155, 112096. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, V.; Dyer, A.T.; Knott, E.; Xue, Q.S.; Nishida, T.; Reynolds, J.R.; Shaw, G.; Streit, W.; Sanchez, J.C. Coupling Biotic and Abiotic Metrics to Create a Testbed for Predicting Neural Electrode Performance. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3020–3023. [Google Scholar] [CrossRef]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure Mode Analysis of Silicon-Based Intracortical Microelectrode Arrays in Non-Human Primates. J. Neural Eng. 2013, 10, 066014. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A Review of Organic and Inorganic Biomaterials for Neural Interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef]
- Vitale, F.; Summerson, S.R.; Aazhang, B.; Kemere, C.; Pasquali, M. Neural Stimulation and Recording with Bidirectional, Soft Carbon Nanotube Fiber Microelectrodes. ACS Nano 2015, 9, 4465–4474. [Google Scholar] [CrossRef]
- Lu, L.; Fu, X.; Liew, Y.; Zhang, Y.; Zhao, S.; Xu, Z.; Zhao, J.; Li, D.; Li, Q.; Stanley, G.B.; et al. Soft and MRI Compatible Neural Electrodes from Carbon Nanotube Fibers. Nano Lett. 2019, 19, 1577–1586. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, T.; Zwang, T.J.; Hong, G.; Zhao, Y.; Viveros, R.D.; Fu, T.-M.; Gao, T.; Lieber, C.M. Bioinspired Neuron-like Electronics. Nat. Mater. 2019, 18, 510–517. [Google Scholar] [CrossRef]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated Ultraflexible Electrode Arrays for High-Density Intracortical Recording. Adv. Sci. 2018, 5, 1700625. [Google Scholar] [CrossRef] [PubMed]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon Nanotube Coating Improves Neuronal Recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef]
- Alba, N.A.; Du, Z.J.; Catt, K.A.; Kozai, T.D.Y.; Cui, X.T. In Vivo Electrochemical Analysis of a PEDOT/MWCNT Neural Electrode Coating. Biosensors 2015, 5, 618. [Google Scholar] [CrossRef]
- Kolarcik, C.L.; Catt, K.; Rost, E.; Albrecht, I.N.; Bourbeau, D.; Du, Z.; Kozai, T.D.Y.; Luo, X.; Weber, D.J.; Cui, X.T. Evaluation of Poly(3,4-Ethylenedioxythiophene)/Carbon Nanotube Neural Electrode Coatings for Stimulation in the Dorsal Root Ganglion. J. Neural Eng. 2015, 12, 16008. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Alvarez, N.T.; Miller, P.; Malik, R.; Haase, M.R.; Schulz, M.; Shanov, V.; Zhu, X. Mechanical Strength Improvements of Carbon Nanotube Threads through Epoxy Cross-Linking. Materials 2016, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, J.; Fu, T.M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-Dimensional Macroporous Nanoelectronic Networks as Minimally Invasive Brain Probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Yoshida Kozai, T.D.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall Implantable Composite Microelectrodes with Bioactive Surfaces for Chronic Neural Interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Ruhunage, C.K.; Dhawan, V.; McKenzie, T.J.; Hoque, A.; Rahm, C.E.; Nawarathne, C.P.; Ayres, N.; Cui, X.T.; Alvarez, N.T. Hydrophilic Micro- and Macroelectrodes with Antibiofouling Properties for Biomedical Applications. ACS Biomater. Sci. Eng. 2022, 8, 2920–2931. [Google Scholar] [CrossRef]
- Schneider, J.J. Vertically Aligned Carbon Nanotubes as Platform for Biomimetically Inspired Mechanical Sensing, Bioactive Surfaces, and Electrical Cell Interfacing. Adv. Biosyst. 2017, 1, 1700101. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gong, Y.; Li, W. A Review: Electrode and Packaging Materials for Neurophysiology Recording Implants. Front. Bioeng. Biotechnol. 2021, 8, 1515. [Google Scholar] [CrossRef] [PubMed]
- Takmakov, P.; Ruda, K.; Scott Phillips, K.; Isayeva, I.S.; Krauthamer, V.; Welle, C.G. Rapid Evaluation of the Durability of Cortical Neural Implants Using Accelerated Aging with Reactive Oxygen Species. J. Neural Eng. 2015, 12, 026003. [Google Scholar] [CrossRef] [PubMed]
- Gulino, M.; Kim, D.; Pané, S.; Santos, S.D.; Pêgo, A.P. Tissue Response to Neural Implants: The Use of Model Systems Toward New Design Solutions of Implantable Microelectrodes. Front. Neurosci. 2019, 13, 689. [Google Scholar] [CrossRef]
- Schander, A.; Stemmann, H.; Tolstosheeva, E.; Roese, R.; Biefeld, V.; Kempen, L.; Kreiter, A.K.; Lang, W. Design and Fabrication of Novel Multi-Channel Floating Neural Probes for Intracortical Chronic Recording. Sens. Actuators A Phys. 2016, 247, 125–135. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for BioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Review of Polymer MEMS Micromachining. J. Micromech. Microeng. 2016, 26, 013001. [Google Scholar] [CrossRef]
- Lee, S.W.; Min, K.S.; Jeong, J.; Kim, J.; Kim, S.J. Monolithic Encapsulation of Implantable Neuroprosthetic Devices Using Liquid Crystal Polymers. IEEE Trans. Biomed. Eng. 2011, 58, 2255–2263. [Google Scholar] [CrossRef]
- Min, K.S.; Lee, C.J.; Jun, S.B.; Kim, J.; Lee, S.E.; Shin, J.; Chang, J.W.; Kim, S.J. A Liquid Crystal Polymer-Based Neuromodulation System: An Application on Animal Model of Neuropathic Pain. Neuromodulation 2014, 17, 160–169. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jeong, J.; Kim, S.J. Emerging Encapsulation Technologies for Long-Term Reliability of Microfabricated Implantable Devices. Micromachines 2019, 10, 508. [Google Scholar] [CrossRef]
- Jeong, J.; Chou, N.; Kim, S. Long-Term Characterization of Neural Electrodes Based on Parylene-Caulked Polydimethylsiloxane Substrate. Biomed. Microdevices 2016, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent Progress on Parylene C Polymer for Biomedical Applications: A Review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Haggren, T.; Shah, A.; Autere, A.; Kakko, J.P.; Dhaka, V.; Kim, M.; Huhtio, T.; Sun, Z.; Lipsanen, H. Nanowire Encapsulation with Polymer for Electrical Isolation and Enhanced Optical Properties. Nano Res. 2017, 10, 2657–2666. [Google Scholar] [CrossRef]
- Chun, W.; Chou, N.; Cho, S.; Yang, S.; Kim, S. Evaluation of Sub-Micrometer Parylene C Films as an Insulation Layer Using Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2014, 77, 537–547. [Google Scholar] [CrossRef]
- Wahjudi, P.N.; Oh, J.H.; Salman, S.O.; Seabold, J.A.; Rodger, D.C.; Tai, Y.-C.; Thompson, M.E. Improvement of Metal and Tissue Adhesion on Surface-Modified Parylene C. J. Biomed. Mater. Res. Part A 2008, 89, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Sadhir, R.K.; James, W.J.; Yasuda, H.K.; Sharma, A.K.; Nichols, M.F.; Haln, A.W. The Adhesion of Glow-Discharge Polymers, Silastic and Parylene to Implantable Platinum Electrodes: Results of Tensile Pull Tests after Exposure to Isotonic Sodium Chloride. Biomaterials 1981, 2, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, T.; Beutel, H.; Schuettler, M.; Meyer, J.U. Micromachined, Polyimide-Based Devices for Flexible Neural Interfaces. Biomed. Microdevices 2000, 2, 283–294. [Google Scholar] [CrossRef]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for Neural Implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Joshi-Imre, A.; Black, B.J.; Abbott, J.; Kanneganti, A.; Rihani, R.; Chakraborty, B.; Danda, V.R.; Maeng, J.; Sharma, R.; Rieth, L.; et al. Chronic Recording and Electrochemical Performance of Amorphous Silicon Carbide-Coated Utah Electrode Arrays Implanted in Rat Motor Cortex. J. Neural Eng. 2019, 16, 046006. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Wang, S. Improved Mechanical Properties and Thermal Degradation of Low-Temperature Hydrogenated Acrylonitrile Butadiene Rubber Composites with Poly(Sodium Methacrylate) Nanowires. RSC Adv. 2016, 6, 64110–64120. [Google Scholar] [CrossRef]
- Cao, P.; Huang, C.; Zhang, L.; Yue, D. One-Step Fabrication of RGO/HNBR Composites via Selective Hydrogenation of NBR with Graphene-Based Catalyst. RSC Adv. 2015, 5, 41098–41102. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Ochmann, T.; Kienzle, N.; Ruff, B.; Haase, M.R.; Hopkins, T.; Pixley, S.; Mast, D.; Schulz, M.J.; Shanov, V. Polymer Coating of Carbon Nanotube Fibers for Electric Microcables. Nanomaterials 2014, 4, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Khorova, E.A.; Razdyakonova, G.I.; Khodakova, S.Y. Effect of the Structure of Hydrogenated Butadiene-Nitrile Rubber on the Resistance to Aggressive Media and High Temperatures. Procedia Eng. 2016, 152, 556–562. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Miller, P.; Haase, M.; Kienzle, N.; Zhang, L.; Schulz, M.J.; Shanov, V. Carbon Nanotube Assembly at Near-Industrial Natural-Fiber Spinning Rates. Carbon N. Y. 2015, 86, 350–357. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Chakrabarti, S.; Schulz, M.J.; Shanov, V. Spinning Yarn from Long Carbon Nanotube Arrays. J. Mater. Res. 2011, 26, 645–651. [Google Scholar] [CrossRef]
- Woeppel, K.M.; Cui, X.T. Nanoparticle and Biomolecule Surface Modification Synergistically Increases Neural Electrode Recording Yield and Minimizes Inflammatory Host Response. Adv. Healthc. Mater. 2021, 10, 2002150. [Google Scholar] [CrossRef]
- Evertz, A.; Schrein, D.; Olsen, E.; Hoffmann, G.-A.; Overmeyer, L. Dip Coating of Thin Polymer Optical Fibers. Opt. Fiber Technol. 2021, 66, 102638. [Google Scholar] [CrossRef]
- Jesswein, I.; Hirth, T.; Schiestel, T. Continuous Dip Coating of PVDF Hollow Fiber Membranes with PVA for Humidification. J. Memb. Sci. 2017, 541, 281–290. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Zhao, J.; Meng, F.; Xu, G.; Yong, Z.; Jia, J.; Zhang, Z.; Li, Q. Enhancement of Carbon Nanotube Fibres Using Different Solvents and Polymers. Compos. Sci. Technol. 2012, 72, 1402–1407. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Zhou, R.; Zhu, H.; Wang, J.; Liu, L.; Fan, S.; Jiang, K. Carbon Nanotube Yarns with High Tensile Strength Made by a Twisting and Shrinking Method. Nanotechnology 2010, 21, 045708. [Google Scholar] [CrossRef]
- Quéré, D. Fluid coating on a fiber. Annu. Rev. Fluid Mech. 1999, 31, 347–384. [Google Scholar] [CrossRef]
- White, D.A.; Tallmadge, J.A. A Gravity Corrected Theory for Cylinder Withdrawal. AIChE J. 1967, 13, 745–750. [Google Scholar] [CrossRef]
- Grosso, D. How to Exploit the Full Potential of the Dip-Coating Process to Better Control Film Formation. J. Mater. Chem. 2011, 21, 17033–17038. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Wang, R.; Brauer, M.H.; Zheng, K.; Li, W. Stability Performance Analysis of Various Packaging Materials and Coating Strategies for Chronic Neural Implants under Accelerated, Reactive Aging Tests. Micromachines 2020, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.; Schuettler, M.; Boehler, C.; Boretius, T.; Stieglitz, T. Thin Films and Microelectrode Arrays for Neuroprosthetics. MRS Bull. 2012, 37, 590–598. [Google Scholar] [CrossRef]
- Jackson, K.D.O.; Loadman, M.J.R.; Jones, C.H.; Ellis, G. Fourier Transform Raman Spectroscopy of Elastomers: An Overview. Spectrochim. Acta Part A Mol. Spectrosc. 1990, 46, 217–226. [Google Scholar] [CrossRef]
- Schneider, B.; Štokr, J.; Schmidt, P.; Mihailov, M.; Dirlikov, S.; Peeva, N. Stretching and Deformation Vibrations of CH2, C(CH3) and O(CH3) Groups of Poly(Methyl Methacrylate). Polymer 1979, 20, 705–712. [Google Scholar] [CrossRef]
- Cornell, S.W.; Koenig, J.L. The Raman Spectra of Polybutadiene Rubbers. Macromolecules 1969, 2, 540–545. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Zhang, Z.; Yang, H.; Nie, X. One-Step Synthesis of End-Functionalized Hydrogenated Nitrile-Butadiene Rubber by Combining the Functional Metathesis with Hydrogenation. ChemistryOpen 2020, 9, 374–380. [Google Scholar] [CrossRef]
- Bai, C.; Gong, Z.; An, L.; Qiang, L.; Zhang, J.; Yushkov, G.; Nikolaev, A.; Shandrikov, M.; Zhang, B. Adhesion and Friction Performance of DLC/Rubber: The Influence of Plasma Pretreatment. Friction 2021, 9, 627–641. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Zao, W.; Feng, H.; Hou, Y.; Huo, A. High-Performance Nitrile Butadiene Rubber Composites with Good Mechanical Properties, Tunable Elasticity, and Robust Shape Memory Behaviors. Ind. Eng. Chem. Res. 2020, 59, 15936–15947. [Google Scholar] [CrossRef]
- Shao, C.; Mao, Y.; Shang, P.; Li, Q.; Wu, C. Improved Tensile Strength of Acrylonitrile–Butadiene Rubber/Anhydrous Copper Sulfate Composites Prepared by Coordination Cross-Linking. Polym. Bull. 2019, 76, 1435–1452. [Google Scholar] [CrossRef]
- Sang, J.; Aisawa, S.; Muraoka, H.; Mori, K.; Hirahara, H. Direct Functionalizing of Acrylonitrile-Butadiene Rubber Surfaces through Different Peroxide Curing. React. Funct. Polym. 2020, 146, 104446. [Google Scholar] [CrossRef]
- Martínez, L.; Álvarez, L.; Huttel, Y.; Méndez, J.; Román, E.; Vanhulsel, A.; Verheyde, B.; Jacobs, R. Surface Analysis of NBR and HNBR Elastomers Modified with Different Plasma Treatments. Vacuum 2007, 81, 1489–1492. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a Key Functional Requirement of Next-Generation Medical Devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility Assessment of Biomaterials Used in Orthopedic Devices: An Overview (Review). Exp. Ther. Med. 2021, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dubin, R.A.; Callegari, G.C.; Kohn, J.; Neimark, A.V. Carbon Nanotube Fibers Are Compatible with Mammalian Cells and Neurons. IEEE Trans. Nanobiosci. 2008, 7, 11–14. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hu, Y.; Wan, J.; Gao, Q.; Wang, Y.; Xie, S.; Qiu, L.; Wang, C.; Zheng, G.; Wang, B.; et al. Biocompatible Carbon Nanotube Fibers for Implantable Supercapacitors. Carbon N. Y. 2017, 122, 162–167. [Google Scholar] [CrossRef]
- Yan, J.S.; Orecchioni, M.; Vitale, F.; Coco, J.A.; Duret, G.; Antonucci, S.; Pamulapati, S.S.; Taylor, L.W.; Dewey, O.S.; Di Sante, M.; et al. Biocompatibility Studies of Macroscopic Fibers Made from Carbon Nanotubes: Implications for Carbon Nanotube Macrostructures in Biomedical Applications. Carbon N. Y. 2021, 173, 462–476. [Google Scholar] [CrossRef]
- Schander, A.; Stemmann, H.; Claussen, K.C.; Kreiter, A.K.; Lang, W. In-Vitro and In-Vivo Longevity Evaluation of Free-Floating Intracortical Silicon-Stiffened Neural Probes. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 799–802. [Google Scholar] [CrossRef]
- Brown, B.; Parker, C.B.; Stoner, B.R.; Grill, W.M.; Glass, J.T. Electrochemical Charge Storage Properties of Vertically Aligned Carbon Nanotube Films: The Activation-Enhanced Length Effect. J. Electrochem. Soc. 2011, 158, K217. [Google Scholar] [CrossRef]
- Takmakov, P.; Zachek, M.K.; Keithley, R.B.; Walsh, P.L.; Donley, C.; McCarty, G.S.; Wightman, R.M. Carbon Microelectrodes with a Renewable Surface. Anal. Chem. 2010, 82, 2020–2028. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Tian, L.; Patil, A.C.; Peng, S.; Yang, I.H.; Thakor, N.V.; Ramakrishna, S. Neural Interfaces Engineered via Micro- and Nanostructured Coatings. Nano Today 2017, 14, 59–83. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical Stimulation of Excitable Tissue: Design of Efficacious and Safe Protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Cisnal, A.; Ihmig, F.R.; Fraile, J.C.; Turiel, J.P.; Muñoz-Martinez, V. Application of a Novel Measurement Setup for Characterization of Graphene Microelectrodes and a Comparative Study of Variables Influencing Charge Injection Limits of Implantable Microelectrodes. Sensors 2019, 19, 2725. [Google Scholar] [CrossRef]
- Luo, X.; Weaver, C.L.; Zhou, D.D.; Greenberg, R.; Cui, X.T. Highly Stable Carbon Nanotube Doped Poly(3,4-Ethylenedioxythiophene) for Chronic Neural Stimulation. Biomaterials 2011, 32, 5551–5557. [Google Scholar] [CrossRef] [PubMed]
- Keren, L.B.; Hanein, Y. Carbon Nanotube Based Multi Electrode Arrays for Neuronal Interfacing: Progress and Prospects. Front. Neural Circuits 2012, 6, 122. [Google Scholar] [CrossRef]
- Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured Platinum Grass Enables Superior Impedance Reduction for Neural Microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef]
- Cogan, S.F.; Plante, T.D.; Ehrlich, J. Sputtered Iridium Oxide Films (SIROFs) for Low-Impedance Neural Stimulation and Recording Electrodes. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 4153–4156. [Google Scholar] [CrossRef]
- Zhang, H.; Shih, J.; Zhu, J.; Kotov, N.A. Layered Nanocomposites from Gold Nanoparticles for Neural Prosthetic Devices. Nano Lett. 2012, 12, 3391–3398. [Google Scholar] [CrossRef]
- Abidian, M.R.; Corey, J.M.; Kipke, D.R.; Martin, D.C. Conducting-Polymer Nanotubes Improve Electrical Properties, Mechanical Adhesion, Neural Attachment, and Neurite Outgrowth of Neural Electrodes. Small 2010, 6, 421–429. [Google Scholar] [CrossRef]
- Caldwell, R.; Street, M.G.; Sharma, R.; Takmakov, P.; Baker, B.; Rieth, L. Characterization of Parylene-C Degradation Mechanisms: In Vitro Reactive Accelerated Aging Model Compared to Multiyear in Vivo Implantation. Biomaterials 2020, 232, 119731. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Jing, Y.; Tu, J.; Shi, X.; Kadlcak, J.; Yong, Z.; Liu, S.; Liu, G. Investigation on the Mechanical Properties and Oil Resistance of Sulfur Cured Nitrile Rubber/Hydrogenated Nitrile Butadiene Rubber Blends. Polym. Eng. Sci. 2021, 61, 3050–3059. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruhunage, C.; Dhawan, V.; Nawarathne, C.P.; Hoque, A.; Cui, X.T.; Alvarez, N.T. Evaluation of Polymer-Coated Carbon Nanotube Flexible Microelectrodes for Biomedical Applications. Bioengineering 2023, 10, 647. https://doi.org/10.3390/bioengineering10060647
Ruhunage C, Dhawan V, Nawarathne CP, Hoque A, Cui XT, Alvarez NT. Evaluation of Polymer-Coated Carbon Nanotube Flexible Microelectrodes for Biomedical Applications. Bioengineering. 2023; 10(6):647. https://doi.org/10.3390/bioengineering10060647
Chicago/Turabian StyleRuhunage, Chethani, Vaishnavi Dhawan, Chaminda P. Nawarathne, Abdul Hoque, Xinyan Tracy Cui, and Noe T. Alvarez. 2023. "Evaluation of Polymer-Coated Carbon Nanotube Flexible Microelectrodes for Biomedical Applications" Bioengineering 10, no. 6: 647. https://doi.org/10.3390/bioengineering10060647
APA StyleRuhunage, C., Dhawan, V., Nawarathne, C. P., Hoque, A., Cui, X. T., & Alvarez, N. T. (2023). Evaluation of Polymer-Coated Carbon Nanotube Flexible Microelectrodes for Biomedical Applications. Bioengineering, 10(6), 647. https://doi.org/10.3390/bioengineering10060647







