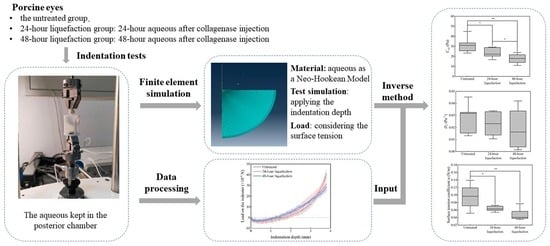
An Inverse Method to Determine Mechanical Parameters of Porcine Vitreous Bodies Based on the Indentation Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Indentation Tests
2.2. Construction of the Finite Element Model
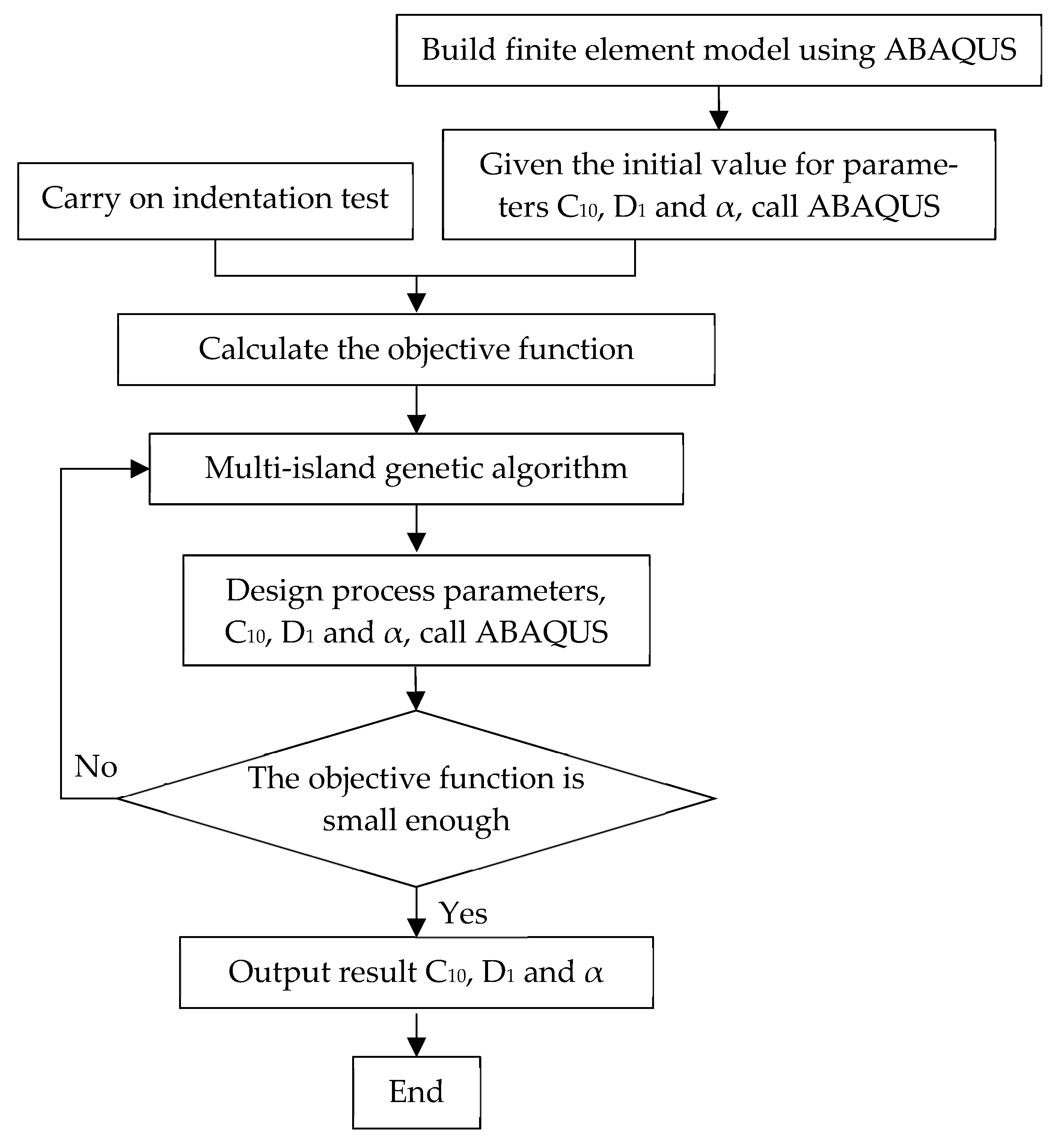
2.3. The Inverse Method to Determine the Mechanical Properties
2.4. Statistical Analysis
3. Results
3.1. Experimental Results
3.2. Identification Results of the Mechanical Parameters
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milston, R.; Madigan, M.C.; Sebag, J. Vitreous Floaters: Etiology, Diagnostics, and Management. Surv. Ophthalmol. 2016, 61, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; Maxwell, C.J.; Swindle-Reilly, K.E. Macro- and Microscale Properties of the Vitreous Humor to Inform Substitute Design and Intravitreal Biotransport. Curr. Eye Res. 2021, 46, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.N. Structural Macromolecules and Supramolecular Organisation of the Vitreous Gel. Prog. Retin. Eye Res. 2000, 19, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Cohen, N. The Effects of Aging on the Mechanical Properties of the Vitreous. J. Biomech. 2021, 119, 110310. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.N.; Holmes, D.F.; Kadler, K.E.; McLeod, D.; Bos, K.J. Age-Related Changes on the Surface of Vitreous Collagen Fibrils. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1041–1046. [Google Scholar] [CrossRef]
- Zhang, Q.; Filas, B.A.; Roth, R.; Heuser, J.; Ma, N.; Sharma, S.; Panitch, A.; Beebe, D.C.; Shui, Y.-B. Preservation of the Structure of Enzymatically-Degraded Bovine Vitreous Using Synthetic Proteoglycan Mimics. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8153–8162. [Google Scholar] [CrossRef]
- Lumi, X.; Hawlina, M.; Glavač, D.; Facskó, A.; Moe, M.C.; Kaarniranta, K.; Petrovski, G. Ageing of the Vitreous: From Acute Onset Floaters and Flashes to Retinal Detachment. Ageing Res. Rev. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- Filas, B.A.; Zhang, Q.; Okamoto, R.J.; Shui, Y.-B.; Beebe, D.C. Enzymatic Degradation Identifies Components Responsible for the Structural Properties of the Vitreous Body. Investig. Ophthalmol. Vis. Sci. 2014, 55, 55–63. [Google Scholar] [CrossRef]
- Tram, N.K.; Swindle-Reilly, K.E. Rheological Properties and Age-Related Changes of the Human Vitreous Humor. Front. Bioeng. Biotechnol. 2018, 6, 199. [Google Scholar] [CrossRef]
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Transl. Vis. Sci. Technol. 2019, 8, 56. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Geng, X.; Huo, H.; Dong, Y.; Tang, M.; Tang, Z.; Qiao, X.; Fan, Y. A Simple Indentation Technique for Identifying Localized Liquefaction of the Vitreous Body. J. Biomech. 2021, 129, 110795. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Litt, M.; Buchsbaum, G. Rheology of the Vitreous Body. Part I: Viscoelasticity of Human Vitreous. Biorheology 1992, 29, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Litt, M.; Buchsbaum, G. Rheology of the Vitreous Body: Part 2. Viscoelasticity of Bovine and Porcine Vitreous. Biorheology 1994, 31, 327–338. [Google Scholar] [CrossRef]
- Swindle, K.E.; Hamilton, P.D.; Ravi, N. In Situ Formation of Hydrogels as Vitreous Substitutes: Viscoelastic Comparison to Porcine Vitreous. J. Biomed. Mater. Res. A 2008, 87, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.S.; Park, J.; Kornfield, J.A.; Karageozian, H. Rheological Properties of the Vitreous and the Role of Hyaluronic Acid. J. Biomech. 2008, 41, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Kashani, P.; Hubschman, J.-P.; Sassoon, D.; Kavehpour, H.P. Rheology of the Vitreous Gel: Effects of Macromolecule Organization on the Viscoelastic Properties. J. Biomech. 2011, 44, 419–423. [Google Scholar] [CrossRef]
- Colter, J.; Williams, A.; Moran, P.; Coats, B. Age-Related Changes in Dynamic Moduli of Ovine Vitreous. J. Mech. Behav. Biomed. Mater. 2015, 41, 315–324. [Google Scholar] [CrossRef]
- Silva, A.F.; Alves, M.A.; Oliveira, M.S.N. Rheological Behaviour of Vitreous Humour. Rheol. Acta 2017, 56, 377–386. [Google Scholar] [CrossRef]
- Uesugi, K.; Sakaguchi, H.; Hayashida, Y.; Hayashi, R.; Baba, K.; Suganuma, Y.; Yokoi, H.; Tsujikawa, M.; Nishida, K. A Self-Assembling Peptide Gel as a Vitreous Substitute: A Rabbit Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4068–4075. [Google Scholar] [CrossRef]
- Huang, D.; Chen, Y.-S.; Xu, Q.; Hanes, J.; Rupenthal, I.D. Effects of Enzymatic Degradation on Dynamic Mechanical Properties of the Vitreous and Intravitreal Nanoparticle Mobility. Eur. J. Pharm. Sci. 2018, 118, 124–133. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Brown, M.B.; Cook, M.T.; Chau, D.Y.S. Diffusion through the ex vivo Vitreal Body-Bovine, Porcine, and Ovine Models Are Poor Surrogates for the Human Vitreous. Int. J. Pharm. 2018, 550, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Aboulatta, A.; Abass, A.; Makarem, A.; Eliasy, A.; Zhou, D.; Chen, D.; Liu, X.; Elsheikh, A. Experimental Evaluation of the Viscoelasticity of Porcine Vitreous. J. R. Soc. Interface 2021, 18, 20200849. [Google Scholar] [CrossRef] [PubMed]
- Zimberlin, J.A.; McManus, J.J.; Crosby, A.J. Cavitation Rheology of the Vitreous: Mechanical Properties of Biological Tissue. Soft Matter 2010, 6, 3632–3635. [Google Scholar] [CrossRef]
- Yoon, S.; Aglyamov, S.; Karpiouk, A.; Emelianov, S. Correspondence: Spatial Variations of Viscoelastic Properties of Porcine Vitreous Humors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Rangchian, A.; Hubschman, J.-P.; Kavehpour, H.P. Time Dependent Degradation of Vitreous Gel under Enzymatic Reaction: Polymeric Network Role in Fluid Properties. J. Biomech. 2020, 109, 109921. [Google Scholar] [CrossRef]
- Pokki, J.; Ergeneman, O.; Sevim, S.; Enzmann, V.; Torun, H.; Nelson, B.J. Measuring Localized Viscoelasticity of the Vitreous Body Using Intraocular Microprobes. Biomed. Microdevices 2015, 17, 85. [Google Scholar] [CrossRef]
- Shah, N.S.; Beebe, D.C.; Lake, S.P.; Filas, B.A. On the Spatiotemporal Material Anisotropy of the Vitreous Body in Tension and Compression. Ann. Biomed. Eng. 2016, 44, 3084–3095. [Google Scholar] [CrossRef]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In Vivo Measurements of the Elastic Mechanical Properties of Human Skin by Indentation Tests. Med. Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef]
- Rho, J.Y.; Zioupos, P.; Currey, J.D.; Pharr, G.M. Microstructural Elasticity and Regional Heterogeneity in Human Femoral Bone of Various Ages Examined by Nano-Indentation. J. Biomech. 2002, 35, 189–198. [Google Scholar] [CrossRef]
- An, Y.H.; Zhang, J.; Kang, Q.; Friedman, R.J. Mechanical Properties of Rat Epiphyseal Cancellous Bones Studied by Indentation Testing. J. Mater. Sci. Mater. Med. 1997, 8, 493–495. [Google Scholar] [CrossRef]
- Li, L.; Maccabi, A.; Abiri, A.; Juo, Y.Y.; Zhang, W.; Chang, Y.J.; Saddik, G.N.; Jin, L.; Grundfest, W.S.; Dutson, E.P.; et al. Characterization of Perfused and Sectioned Liver Tissue in a Full Indentation Cycle Using a Visco-Hyperelastic Model. J. Mech. Behav. Biomed. Mater. 2019, 90, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Chai, H. On the Mechanical Properties of Tooth Enamel under Spherical Indentation. Acta Biomater. 2014, 10, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Lanir, Y.; Dikstein, S.; Hartzshtark, A.; Manny, V. In-vivo Indentation of Human Skin. J. Biomech. Eng. 1990, 112, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, M.H.; Wildman, R.D.; Ashcroft, I.A.; Ruiz, P.D. A Hybrid Approach to Determining Cornea Mechanical Properties in vivo Using a Combination of Nano-Indentation and Inverse Finite Element Analysis. J. Mech. Behav. Biomed. Mater. 2013, 27, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, J.E.; Amini, R.; Simha, N.K.; Barocas, V.H. Anterior-posterior Asymmetry in Iris Mechanics Measured by Indentation. Exp. Eye Res. 2011, 93, 475–481. [Google Scholar] [CrossRef]
- Makarova, N.; Sokolov, I. Cell Mechanics Can Be Robustly Derived from AFM Indentation Data Using the Brush Model: Error Analysis. Nanoscale 2022, 14, 4334–4347. [Google Scholar] [CrossRef]
- Hollister, J.C.P.; Rodriguez, M.; Hosseini, H.; Papour, A.; Hubschman, J.-P.; Kavehpour, H.P. Ultrasonic Vitrectomy Performance Assessment Using Micro-Extensional Rheology. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Henann, D.L. Finite-Element Modeling of Soft Solids with Liquid Inclusions. Extrem. Mech. Lett. 2016, 9, 147–157. [Google Scholar] [CrossRef]
- Spilker, R.L.; Suh, J.K.; Mow, V.C. A Finite Element Analysis of the Indentation Stress-Relaxation Response of Linear Biphasic Articular Cartilage. J. Biomech. Eng. 1992, 114, 191–201. [Google Scholar] [CrossRef]
- Fu, Y.B.; Chui, C.K. Modelling and Simulation of Porcine Liver Tissue Indentation Using Finite Element Method and Uniaxial Stress-Strain Data. J. Biomech. 2014, 47, 2430–2435. [Google Scholar] [CrossRef]
- Yang, Y.; Li, K.; Sommer, G.; Yung, K.L.; Holzapfel, G.A. Mechanical Characterization of Porcine Liver Properties for Computational Simulation of Indentation on Cancerous Tissue. Math. Med. Biol. 2020, 37, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Yung, K.-L.; Hung, T.W.R.; Yu, K.-M. Analyzing Liver Surface Indentation for in vivo Refinement of Tumor Location in Minimally Invasive Surgery. Ann. Biomed. Eng. 2021, 49, 1402–1415. [Google Scholar] [CrossRef] [PubMed]
- Delalleau, A.; Gwendal, J.; Lagarde, J.M.; Zahouani, H.; Bergheau, J.M. Characterization of the Mechanical Properties of Skin by Inverse Analysis Combined with the Indentation Test. J. Biomech. 2006, 39, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.T.M.; Zhang, M.; Leung, A.K.L.; Fan, Y.B. Three-dimensional Finite Element Analysis of the Foot During Standing. J. Biomech. 2005, 38, 1045–1054. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Li, L.; Yu, C.; Zhao, Y.; Si, H. Modelling tri-cortical pedicle screw fixation in thoracic vertebrae under osteoporotic condition: A finite element analysis based on computed tomography. Comput. Methods Programs Biomed. 2020, 187, 105035. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Yoon, J.; Chae, S. Finite element analysis of knee and ankle joint during gait based on motion analysis. Med. Eng. Phys. 2019, 63, 33–41. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Y.; Anwar, A.; Zhang, Y.; Wang, L. Determination of three-dimensional corrective force in adolescent idiopathic scoliosis and biomechanical finite element analysis. Front. Bioeng. Biotechnol. 2020, 8, 963. [Google Scholar] [CrossRef]
- Liu, Y.; Mollaeian, K.; Ren, J. Finite element modeling of living cells for AFM indentation-based biomechanical characterization. Micron 2019, 116, 108–115. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, L.Z.; Wang, C.; Sun, G.Y.; Liu, S.Y.; Fan, Y.B. Mechanism of traumatic retinal detachment in blunt impact: A finite element study. J. Biomech. 2013, 46, 1321–1327. [Google Scholar] [CrossRef]
- Damiano, R.J.; Ma, D.; Xiang, J.; Siddiqui, A.H.; Snyder, K.V.; Meng, H. Finite element modeling of endovascular coiling and flow diversion enables hemodynamic prediction of complex treatment strategies for intracranial aneurysm. J. Biomech. 2015, 48, 3332–3340. [Google Scholar] [CrossRef]
- Bo, X.; Wang, W.; Zan, C.; Liu, Z.C. Compression-distraction reduction surgical verification and optimization to treat the basilar invagination and atlantoaxial dislocation: A finite element analysis. Biomed. Eng. OnLine 2016, 15 (Suppl. S2), 383–397. [Google Scholar] [CrossRef] [PubMed]
- van Deemter, M.; Pas, H.H.; Kuijer, R.; van der Worp, R.J.; Hooymans, J.M.M.; Los, L.I. Enzymatic Breakdown of Type II Collagen in the Human Vitreous. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Stitzel, J.D.; Duma, S.M.; Cormier, J.M.; Herring, I.P. A Nonlinear Finite Element Model of the Eye with Experimental Validation for the Prediction of Globe Rupture. Stapp Car Crash J. 2002, 46, 81–102. [Google Scholar] [CrossRef]
- Pavlatos, E.; Ma, Y.; Clayson, K.; Pan, X.; Liu, J. Regional Deformation of the Optic Nerve Head and Peripapillary Sclera During IOP Elevation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3779–3788. [Google Scholar] [CrossRef]
- Jia, X.; Yu, J.; Liao, S.-H.; Duan, X.-C. Biomechanics of the Sclera and Effects on Intraocular Pressure. Int. J. Ophthalmol. 2016, 9, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Sigal, I.A.; Flanagan, J.G.; Ethier, C.R. Factors Influencing Optic Nerve Head Biomechanics. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4189–4199. [Google Scholar] [CrossRef]
- Hua, Y.; Voorhees, A.P.; Sigal, I.A. Cerebrospinal Fluid Pressure: Revisiting Factors Influencing Optic Nerve Head Biomechanics. Investig. Ophthalmol. Vis. Sci. 2018, 59, 154–165. [Google Scholar] [CrossRef]
- Hua, Y.; Tong, J.; Ghate, D.; Kedar, S.; Gu, L. Intracranial Pressure Influences the Behavior of the Optic Nerve Head. J. Biomech. Eng. 2017, 139, 031003. [Google Scholar] [CrossRef]
- Gandin, A.; Murugesan, Y.; Torresan, V.; Ulliana, L.; Citron, A.; Contessotto, P.; Battilana, G.; Panciera, T.; Ventre, M.; Netti, A.P.; et al. Simple yet Effective Methods to Probe Hydrogel Stiffness for Mechanobiology. Sci. Rep. 2021, 11, 22668. [Google Scholar] [CrossRef]
- Long, R.; Hall, M.S.; Wu, M.; Hui, C.-Y. Effects of Gel Thickness on Microscopic Indentation Measurements of Gel Modulus. Biophys. J. 2011, 101, 643–650. [Google Scholar] [CrossRef]
- Zrínyi, M.; Stirling, T. A Novel Method to Determine the Elastic Modulus of Extremely Soft Materials. Soft Matter 2015, 11, 4180–4188. [Google Scholar] [CrossRef]
- Long, R.; Hui, C.-Y. Crack Buckling in Soft Gels under Compression. Acta Mech. Sin. 2012, 28, 1098–1105. [Google Scholar] [CrossRef]
- Rivlin, R.S.; Taylor, G.I. Large Elastic Deformations of Isotropic Materials. I. Fundamental Concepts. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1997, 240, 459–490. [Google Scholar] [CrossRef]
- Ballit, A.; Dao, T.-T. Recurrent Neural Network to Predict Hyperelastic Constitutive Behaviors of the Skeletal Muscle. Med. Biol. Eng. Comput. 2022, 60, 1177–1185. [Google Scholar] [CrossRef]
- Akatsu, T.; Akimoto, Y.; Sasaki, R.; Shinoda, Y.; Wakai, F. Numerical Analysis of Point-Sharp Indentation-Load Relaxation Simulated Using the Finite-Element Method to Characterize the Power-Law Creep Deformation of a Visco-Elastoplastic Solid. Int. J. Solids Struct. 2022, 238, 111417. [Google Scholar] [CrossRef]
- Ahn, B.; Kim, J. Measurement and Characterization of Soft Tissue Behavior with Surface Deformation and Force Response under Large Deformations. Med. Image Anal. 2010, 14, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, P.; Martin, C.P.; Vancea, I.; Moriarty, P.J.; Krasnogor, N. A Genetic Algorithm Approach to Probing the Evolution of Self-Organized Nanostructured Systems. Nano Lett. 2007, 7, 1985–1990. [Google Scholar] [CrossRef]
- Ghosh, P.; Bagchi, M.C. QSAR Modeling for Quinoxaline Derivatives Using Genetic Algorithm and Simulated Annealing Based Feature Selection. Curr. Med. Chem. 2009, 16, 4032–4048. [Google Scholar] [CrossRef]
- Khorram, B.; Yazdi, M. A New Optimized Thresholding Method Using Ant Colony Algorithm for MR Brain Image Segmentation. J. Digit. Imaging 2019, 32, 162–174. [Google Scholar] [CrossRef]
- Zhang, K.; Qian, X.; Mei, X.; Liu, Z. An Inverse Method to Determine the Mechanical Properties of the Iris in vivo. Biomed. Eng. Online 2014, 13, 66. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, K.; Liu, Z. A Method to Determine the Mechanical Properties of the Retina Based on an Experiment in vivo. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), S287–S297. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, H.; Yang, Y.; Zhang, J.; Yang, F.; Yan, D.; Yang, H.; Wang, Y. Optimization of Energy Management Strategy for Extended Range Electric Vehicles Using Multi-Island Genetic Algorithm. J. Energy Storage 2023, 61, 106802. [Google Scholar] [CrossRef]
- Los, L.I.; van der Worp, R.J.; van Luyn, M.J.; Hooymans, J.M. Age-Related Liquefaction of the Human Vitreous Body: LM and TEM Evaluation of the Role of Proteoglycans and Collagen. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2828–2833. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Winzen, U.; Bishop, P.N.; Eller, A. Regulation of Eye Size by the Retinal Basement Membrane and Vitreous Body. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Green, W.; Sebag, J. Vitreoretinal Interface. In Retina, 4th ed.; Elsevier Inc.: Philadelphia, PA, USA, 2006; Volume 3, pp. 1921–1989. [Google Scholar] [CrossRef]






| Group | C10 (Pa) | D1 (×10−2 Pa−1) | α (N/m) |
|---|---|---|---|
| Untreated group | 31.57 ± 6.91 | 2.79 ± 1.10 | 0.095 ± 0.0296 |
| 24 h liquefaction group | 23.89 ± 4.95 | 2.33 ± 0.96 | 0.0632 ± 0.00647 |
| 48 h liquefaction group | 17.65 ± 4.35 | 1.93 ± 1.53 | 0.0466 ± 0.0150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, H.; Zhang, K.; Zhang, H.; Qian, X. An Inverse Method to Determine Mechanical Parameters of Porcine Vitreous Bodies Based on the Indentation Test. Bioengineering 2023, 10, 646. https://doi.org/10.3390/bioengineering10060646
Zu H, Zhang K, Zhang H, Qian X. An Inverse Method to Determine Mechanical Parameters of Porcine Vitreous Bodies Based on the Indentation Test. Bioengineering. 2023; 10(6):646. https://doi.org/10.3390/bioengineering10060646
Chicago/Turabian StyleZu, Haicheng, Kunya Zhang, Haixia Zhang, and Xiuqing Qian. 2023. "An Inverse Method to Determine Mechanical Parameters of Porcine Vitreous Bodies Based on the Indentation Test" Bioengineering 10, no. 6: 646. https://doi.org/10.3390/bioengineering10060646
APA StyleZu, H., Zhang, K., Zhang, H., & Qian, X. (2023). An Inverse Method to Determine Mechanical Parameters of Porcine Vitreous Bodies Based on the Indentation Test. Bioengineering, 10(6), 646. https://doi.org/10.3390/bioengineering10060646