Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs
Abstract
1. Introduction
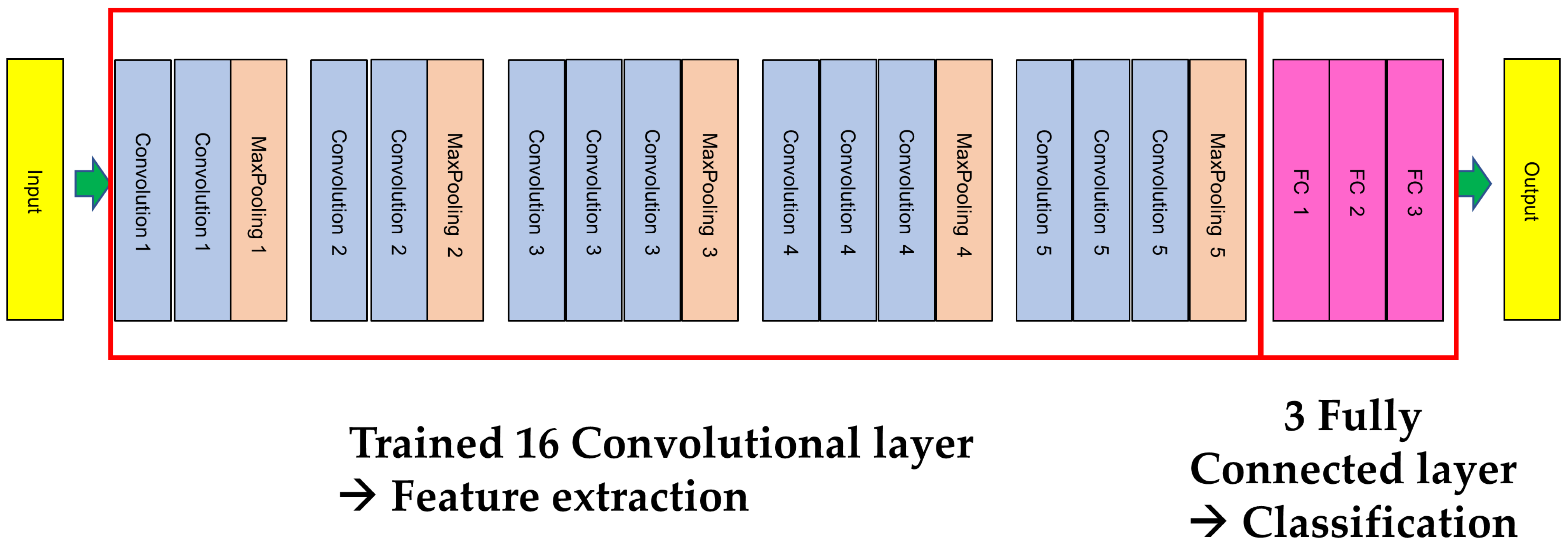
2. Materials and Methods
3. Statistical Analysis
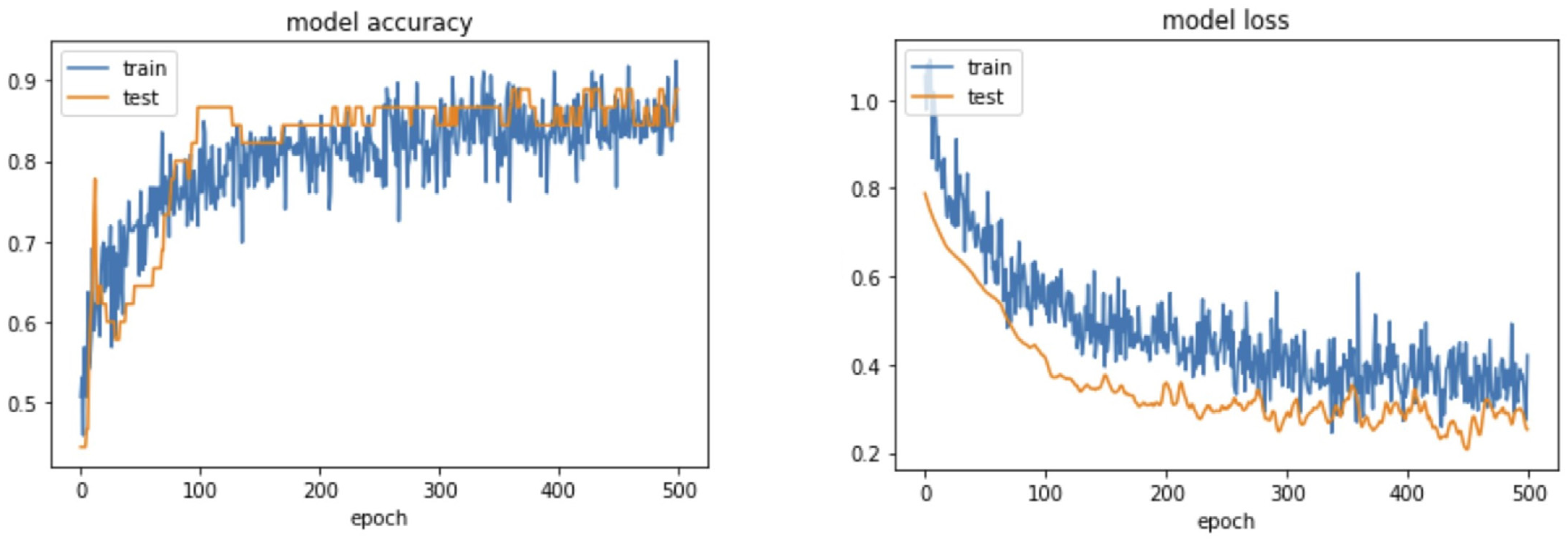
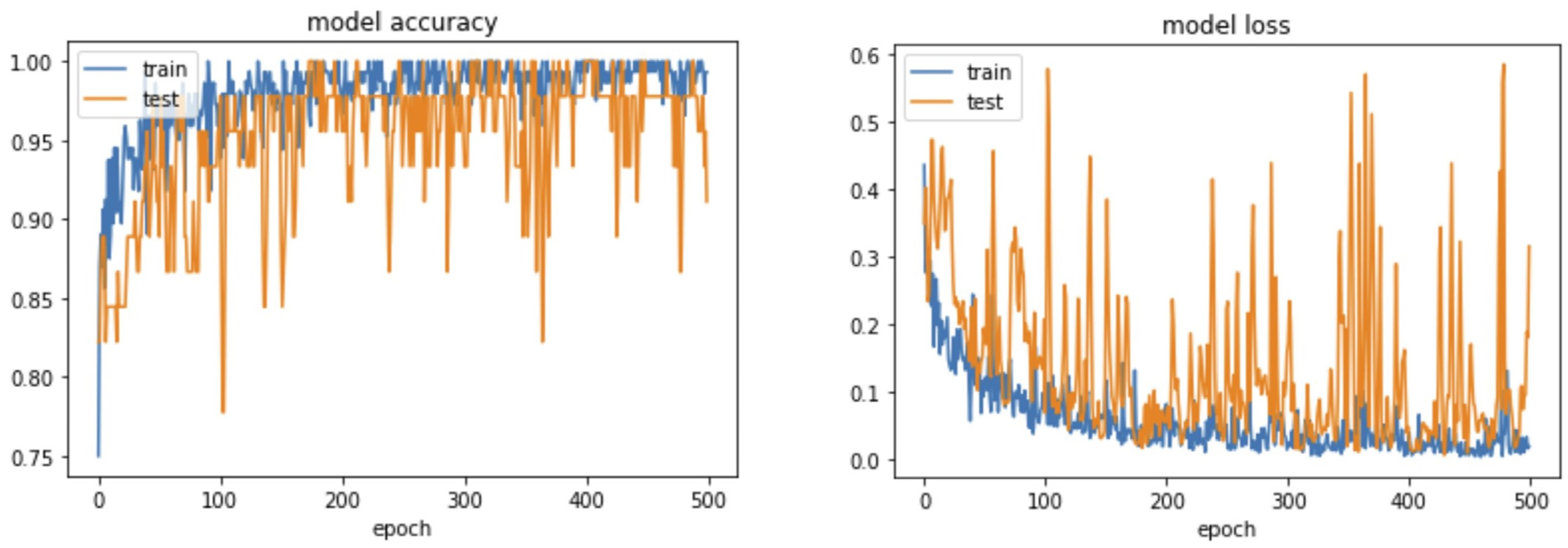
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Shin, W.C.; Song, M.K.; Han, H.S.; Lee, M.C.; Ro, D.H. Which orally administered antithrombotic agent is most effective for preventing venous thromboembolism after total knee arthroplasty? A propensity score-matching analysis. Knee Surg. Relat. Res. 2021, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, V.; Sood, M.; Kumar, S.; Sood, N.; Kumar, P.; Padhi, P.P. Does Risk Mitigation Reduce 90-Day Complications in Patients Undergoing Total Knee Arthroplasty?: A Cohort Study. Clin. Orthop. Surg. 2022, 14, 56–68. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, K.B.; Kim, J.I.; Park, G.T.; Cho, Y.C. Risk factors for deep vein thrombosis even using low-molecular-weight heparin after total knee arthroplasty. Knee Surg. Relat. Res. 2021, 33, 29. [Google Scholar] [CrossRef]
- Lee, J.M.; Ha, C.; Jung, K.; Choi, W. Clinical Results after Design Modification of Lospa Total Knee Arthroplasty System: Comparison between Posterior-Stabilized (PS) and PS Plus Types. Clin. Orthop. Surg. 2022, 14, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Patrick, N.J.; Man, L.L.C.; Wai-Wang, C.; Tim-Yun, O.M.; Wing, C.K.; Hing, C.K.; Yin, C.K.; Ki-Wai, H.K. No difference in long-term functional outcomes or survivorship after total knee arthroplasty with or without computer navigation: A 17-year survivorship analysis. Knee Surg. Relat. Res. 2021, 33, 30. [Google Scholar] [CrossRef]
- Song, S.J.; Kim, K.I.; Suh, D.U.; Park, C.H. Comparison of Patellofemoral-Specific Clinical and Radiographic Results after Total Knee Arthroplasty Using a Patellofemoral Design-Modified Prosthesis and Its Predecessor. Clin. Orthop. Surg. 2021, 13, 175–184. [Google Scholar] [CrossRef]
- Takamura, D.; Iwata, K.; Sueyoshi, T.; Yasuda, T.; Moriyama, H. Relationship between early physical activity after total knee arthroplasty and postoperative physical function: Are these related? Knee Surg. Relat. Res. 2021, 33, 35. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Ranawat, C.S.; Flynn, W.F., Jr.; Deshmukh, R.G. Impact of modern technique on long-term results of total condylar knee arthroplasty. Clin. Orthop. Relat. Res. 1994, 309, 131–135. [Google Scholar]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Cram, P.; Lu, X.; Kates, S.L.; Singh, J.A.; Li, Y.; Wolf, B.R. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA 2012, 308, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Geary, M.B.; Macknet, D.M.; Ransone, M.P.; Odum, S.D.; Springer, B.D. Why Do Revision Total Knee Arthroplasties Fail? A Single-Center Review of 1632 Revision Total Knees Comparing Historic and Modern Cohorts. J. Arthroplasty 2020, 35, 2938–2943. [Google Scholar] [CrossRef]
- Na, B.R.; Kwak, W.K.; Lee, N.H.; Song, E.K.; Seon, J.K. Trend Shift in the Cause of Revision Total Knee Arthroplasty over 17 Years. Clin. Orthop. Surg. 2023, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- French, T.H.; Russell, N.; Pillai, A. The diagnostic accuracy of radionuclide arthrography for prosthetic loosening in hip and knee arthroplasty. BioMed Res. Int. 2013, 2013, 693436. [Google Scholar] [CrossRef]
- Signore, A.; Sconfienza, L.M.; Borens, O.; Glaudemans, A.; Cassar-Pullicino, V.; Trampuz, A.; Winkler, H.; Gheysens, O.; Vanhoenacker, F.; Petrosillo, N.; et al. Consensus document for the diagnosis of prosthetic joint infections: A joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Barnsley, L.; Barnsley, L. Detection of aseptic loosening in total knee replacements: A systematic review and meta-analysis. Skelet. Radiol. 2019, 48, 1565–1572. [Google Scholar] [CrossRef]
- Khalily, C.; Whiteside, L.A. Predictive value of early radiographic findings in cementless total hip arthroplasty femoral components: An 8- to 12-year follow-up. J. Arthroplasty 1998, 13, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.O.; Williams, T.H.; Samuel, A.; Ogonda, L.; Wimhurst, J.A. Reliability of the radiological assessments of radiolucency and loosening in total hip arthroplasty using PACS. HIP Int. 2011, 21, 577–582. [Google Scholar] [CrossRef]
- Jamshidi, A.; Pelletier, J.P.; Martel-Pelletier, J. Machine-learning-based patient-specific prediction models for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 49–60. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. The current role of the virtual elements of artificial intelligence in total knee arthroplasty. EFORT Open Rev. 2022, 7, 491–497. [Google Scholar] [CrossRef]
- Hanis, T.M.; Islam, M.A.; Musa, K.I. Diagnostic Accuracy of Machine Learning Models on Mammography in Breast Cancer Classification: A Meta-Analysis. Diagnostics 2022, 12, 1643. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.M.; Talley, P.C.; Chang, C.S. The accuracy of machine learning approaches using non-image data for the prediction of COVID-19: A meta-analysis. Int. J. Med. Inform. 2022, 164, 104791. [Google Scholar] [CrossRef] [PubMed]
- Borjali, A.; Chen, A.F.; Muratoglu, O.K.; Morid, M.A.; Varadarajan, K.M. Detecting mechanical loosening of total hip replacement implant from plain radiograph using deep convolutional neural network. arXiv 2019, arXiv:1912.00943. [Google Scholar]
- Lau, L.C.M.; Chui, E.C.S.; Man, G.C.W.; Xin, Y.; Ho, K.K.W.; Mak, K.K.K.; Ong, M.T.Y.; Law, S.W.; Cheung, W.H.; Yung, P.S.H. A novel image-based machine learning model with superior accuracy and predictability for knee arthroplasty loosening detection and clinical decision making. J. Orthop. Transl. 2022, 36, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Loppini, M.; Gambaro, F.M.; Chiappetta, K.; Grappiolo, G.; Bianchi, A.M.; Corino, V.D.A. Automatic Identification of Failure in Hip Replacement: An Artificial Intelligence Approach. Bioengineering 2022, 9, 288. [Google Scholar] [CrossRef]
- Rahman, T.; Khandakar, A.; Islam, K.R.; Soliman, M.M.; Islam, M.T.; Elsayed, A.; Qiblawey, Y.; Mahmud, S.; Rahman, A.; Musharavati, F. HipXNet: Deep Learning Approaches to Detect Aseptic Loos-Ening of Hip Implants Using X-ray Images. IEEE Access 2022, 10, 53359–53373. [Google Scholar] [CrossRef]
- Shah, R.F.; Bini, S.A.; Martinez, A.M.; Pedoia, V.; Vail, T.P. Incremental inputs improve the automated detection of implant loosening using machine-learning algorithms. Bone Jt. J. 2020, 102-B, 101–106. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Rahman, T.; Khandakar, A.; Al-Madeed, S.; Zughaier, S.M.; Doi, S.A.; Hassen, H.; Islam, M.T. An early warning tool for predicting mortality risk of COVID-19 patients using machine learning. Cogn. Comput. 2021, 1–16. [Google Scholar] [CrossRef]
- Rahman, T.; Khandakar, A.; Qiblawey, Y.; Tahir, A.; Kiranyaz, S.; Kashem, S.B.A.; Islam, M.T.; Al Maadeed, S.; Zughaier, S.M.; Khan, M.S. Exploring the effect of image enhancement techniques on COVID-19 detection using chest X-ray images. Comput. Biol. Med. 2021, 132, 104319. [Google Scholar] [CrossRef]
- Lee, K.S.; Jung, S.K.; Ryu, J.J.; Shin, S.W.; Choi, J. Evaluation of Transfer Learning with Deep Convolutional Neural Networks for Screening Osteoporosis in Dental Panoramic Radiographs. J. Clin. Med. 2020, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Hosny, K.M.; Kassem, M.A.; Foaud, M.M. Classification of skin lesions using transfer learning and augmentation with Alex-net. PLoS ONE 2019, 14, e0217293. [Google Scholar] [CrossRef]
- Khan, N.M.; Abraham, N.; Hon, M. Transfer learning with intelligent training data selection for prediction of Alzheimer’s disease. IEEE Access 2019, 7, 72726–72735. [Google Scholar] [CrossRef]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional neural networks for medical image analysis: Full training or fine tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef]
- Yosinski, J.; Clune, J.; Bengio, Y.; Lipson, H. How transferable are features in deep neural networks? Adv. Neural Inf. Process. Syst. 2014, 27, 3320–3328. [Google Scholar] [CrossRef]
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 655–663. [Google Scholar] [CrossRef]
- Bieganowski, T.; Buchalter, D.B.; Singh, V.; Mercuri, J.J.; Aggarwal, V.K.; Rozell, J.C.; Schwarzkopf, R. Bone loss in aseptic revision total knee arthroplasty: Management and outcomes. Knee Surg. Relat. Res. 2022, 34, 30. [Google Scholar] [CrossRef]
- Gupta, P.; Czerwonka, N.; Desai, S.S.; deMeireles, A.J.; Trofa, D.P.; Neuwirth, A.L. The current utilization of the patient-reported outcome measurement information system (PROMIS) in isolated or combined total knee arthroplasty populations. Knee Surg. Relat. Res. 2023, 35, 3. [Google Scholar] [CrossRef]
- Khanasuk, Y.; Ngarmukos, S.; Tanavalee, A. Does the intramedullary femoral canal plug reduce blood loss during total knee arthroplasty? Knee Surg. Relat. Res. 2022, 34, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Martinez Martos, S.; Dai, Y.; Beller, E.M. The femoral intercondylar notch is an accurate landmark for the resection depth of the distal femur in total knee arthroplasty. Knee Surg. Relat. Res. 2022, 34, 32. [Google Scholar] [CrossRef] [PubMed]
- Shon, O.J.; Kim, G.B. Does the degree of intraoperatively identified cartilage loss affect the outcomes of primary total knee arthroplasty without patella resurfacing? A prospective comparative cohort study. Knee Surg. Relat. Res. 2022, 34, 36. [Google Scholar] [CrossRef]
- Claassen, L.; Ettinger, M.; Plaass, C.; Daniilidis, K.; Calliess, T.; Ezechieli, M. Diagnostic value of bone scintigraphy for aseptic loosening after total knee arthroplasty. Technol. Health Care 2014, 22, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sterner, T.; Pink, R.; Freudenberg, L.; Jentzen, T.; Quitmann, H.; Bockisch, A.; Löer, F. The role of [18F]fluoride positron emission tomography in the early detection of aseptic loosening of total knee arthroplasty. Int. J. Surg. 2007, 5, 99–104. [Google Scholar] [CrossRef]
- Mayer-Wagner, S.; Mayer, W.; Maegerlein, S.; Linke, R.; Jansson, V.; Müller, P.E. Use of 18F-FDG-PET in the diagnosis of endoprosthetic loosening of knee and hip implants. Arch. Orthop. Trauma Surg. 2010, 130, 1231–1238. [Google Scholar] [CrossRef]
- Soffer, S.; Ben-Cohen, A.; Shimon, O.; Amitai, M.M.; Greenspan, H.; Klang, E. Convolutional Neural Networks for Radiologic Images: A Radiologist’s Guide. Radiology 2019, 290, 590–606. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Park, S.H.; Jung, Y.-J. Artificial intelligence based medical imaging: An Overview. J. Radiol. Sci. Technol. 2020, 43, 195–208. [Google Scholar]
- Lalehzarian, S.P.; Gowd, A.K.; Liu, J.N. Machine learning in orthopaedic surgery. World J. Orthop. 2021, 12, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.S.; Prevedello, L.M.; Kalpathy-Cramer, J.; Mamonov, A.B.; Bilbily, A.; Cicero, M.; Pan, I.; Pereira, L.A.; Sousa, R.T.; Abdala, N.; et al. The RSNA Pediatric Bone Age Machine Learning Challenge. Radiology 2019, 290, 498–503. [Google Scholar] [CrossRef]
- Meena, T.; Roy, S. Bone Fracture Detection Using Deep Supervised Learning from Radiological Images: A Paradigm Shift. Diagnostics 2022, 12, 2420. [Google Scholar] [CrossRef]
- Saeed, S.U.; Fu, Y.; Stavrinides, V.; Baum, Z.M.C.; Yang, Q.; Rusu, M.; Fan, R.E.; Sonn, G.A.; Noble, J.A.; Barratt, D.C.; et al. Image quality assessment for machine learning tasks using meta-reinforcement learning. Med. Image Anal. 2022, 78, 102427. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-W.; Nguyen, V.D.; Vonikakis, V.; Winkler, S. Deep learning for emotion recognition on small datasets using transfer learning. In Proceedings of the 2015 ACM on International Conference on Multimodal Interaction, Seattle, WA, USA, 9–13 November 2015; pp. 443–449. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar] [CrossRef]
- Vrbančič, G.; Podgorelec, V. Transfer learning with adaptive fine-tuning. IEEE Access 2020, 8, 196197–196211. [Google Scholar] [CrossRef]
- Kandel, I.; Castelli, M. How deeply to fine-tune a convolutional neural network: A case study using a histopathology dataset. Appl. Sci. 2020, 10, 3359. [Google Scholar] [CrossRef]
- Chang, M.J.; Ro, D.H.; Kim, T.W.; Lee, Y.S.; Han, H.S.; Chang, C.B.; Kang, S.B.; Lee, M.C. Worse outcome of debridement, antibiotics, and implant retention in acute hematogenous infections than in postsurgical infections after total knee arthroplasty: A multicenter study. Knee Surg. Relat. Res. 2022, 34, 38. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Kim, J.M.; Han, H.S. Decision-making factors and their thresholds for total knee arthroplasty in lateral tibiofemoral osteoarthritis patients: A retrospective cohort study. Knee Surg. Relat. Res. 2022, 34, 41. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, G.W.; Lee, C.Y.; Song, E.K.; Seon, J.K. No Difference in Clinical Outcomes and Survivorship for Robotic, Navigational, and Conventional Primary Total Knee Arthroplasty with a Minimum Follow-up of 10 Years. Clin. Orthop. Surg. 2023, 15, 82–91. [Google Scholar] [CrossRef]
- Ryu, J.J.; Kim, Y.H.; Choi, C.H. The additional tibial stem extension is not mandatory for the stability of 5 mm metal block augmented tibial prosthesis construct in primary total knee arthroplasty: 5-year minimum follow-up results. Knee Surg. Relat. Res. 2023, 35, 5. [Google Scholar] [CrossRef]
- Cho, J.; Lee, K.; Shin, E.; Choy, G.; Do, S. How much data is needed to train a medical image deep learning system to achieve necessary high accuracy? arXiv 2015, arXiv:1511.06348. [Google Scholar]



| Fixed (n = 399) | Loosened (n = 100) | p-Value | |
|---|---|---|---|
| Demographics before PSM | |||
| Age (years) * | 69.7 ± 6.8 | 70.4 ± 8.3 | 0.430 |
| Gender (female, %) | 353 (88.5%) | 80 (80.0%) | 0.032 |
| BMI (kg/m2) | 26.1 ± 3.4 | 26.3 ± 3.4 | 0.430 |
| Operation side (left, %) | 203 (50.9%) | 37 (37.0%) | 0.013 |
| ASA grade | 0.073 | ||
| 1 | 45 (11.3%) | 8 (8.0%) | |
| 2 | 347 (87.2%) | 87 (87.0%) | |
| 3 | 6 (1.5%) | 5 (5.0%) | |
| Demographics after PSM | |||
| Age (years) * | 70.9 ± 6.7 | 70.4 ± 8.3 | 0.603 |
| Gender (female, %) | 80 (80.0%) | 80 (80.0%) | 1.000 |
| BMI (kg/m2) | 26.5 ± 3.6 | 26.3 ± 3.4 | 0.649 |
| Operation side (left, %) | 37 (37.0%) | 37 (37.0%) | 1.000 |
| ASA grade | 0.238 | ||
| 1 | 7 (7.0%) | 8 (7.0%) | |
| 2 | 92 (92.0%) | 87 (87.0%) | |
| 3 | 1 (1.0%) | 5 (5.0%) |
| Performance Criteria | Transfer Learning Model 1 | Transfer Learning Model 2 |
|---|---|---|
| Accuracy | 87.5% | 97.5% |
| Sensitivity | 75.0% | 100% |
| Specificity | 100% | 95.0% |
| Positive predictive value | 100% | 95.2% |
| Negative predictive value | 80.0% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Cho, R.-K.; Yang, S.-C.; Hur, J.-H.; In, Y. Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs. Bioengineering 2023, 10, 632. https://doi.org/10.3390/bioengineering10060632
Kim M-S, Cho R-K, Yang S-C, Hur J-H, In Y. Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs. Bioengineering. 2023; 10(6):632. https://doi.org/10.3390/bioengineering10060632
Chicago/Turabian StyleKim, Man-Soo, Ryu-Kyoung Cho, Sung-Cheol Yang, Jae-Hyeong Hur, and Yong In. 2023. "Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs" Bioengineering 10, no. 6: 632. https://doi.org/10.3390/bioengineering10060632
APA StyleKim, M.-S., Cho, R.-K., Yang, S.-C., Hur, J.-H., & In, Y. (2023). Machine Learning for Detecting Total Knee Arthroplasty Implant Loosening on Plain Radiographs. Bioengineering, 10(6), 632. https://doi.org/10.3390/bioengineering10060632








