The Digital Twin: A Potential Solution for the Personalized Diagnosis and Treatment of Musculoskeletal System Diseases
Abstract
1. Introduction
2. Biomechanics of Musculoskeletal System
3. Limitations of Conventional Biomechanical Methods
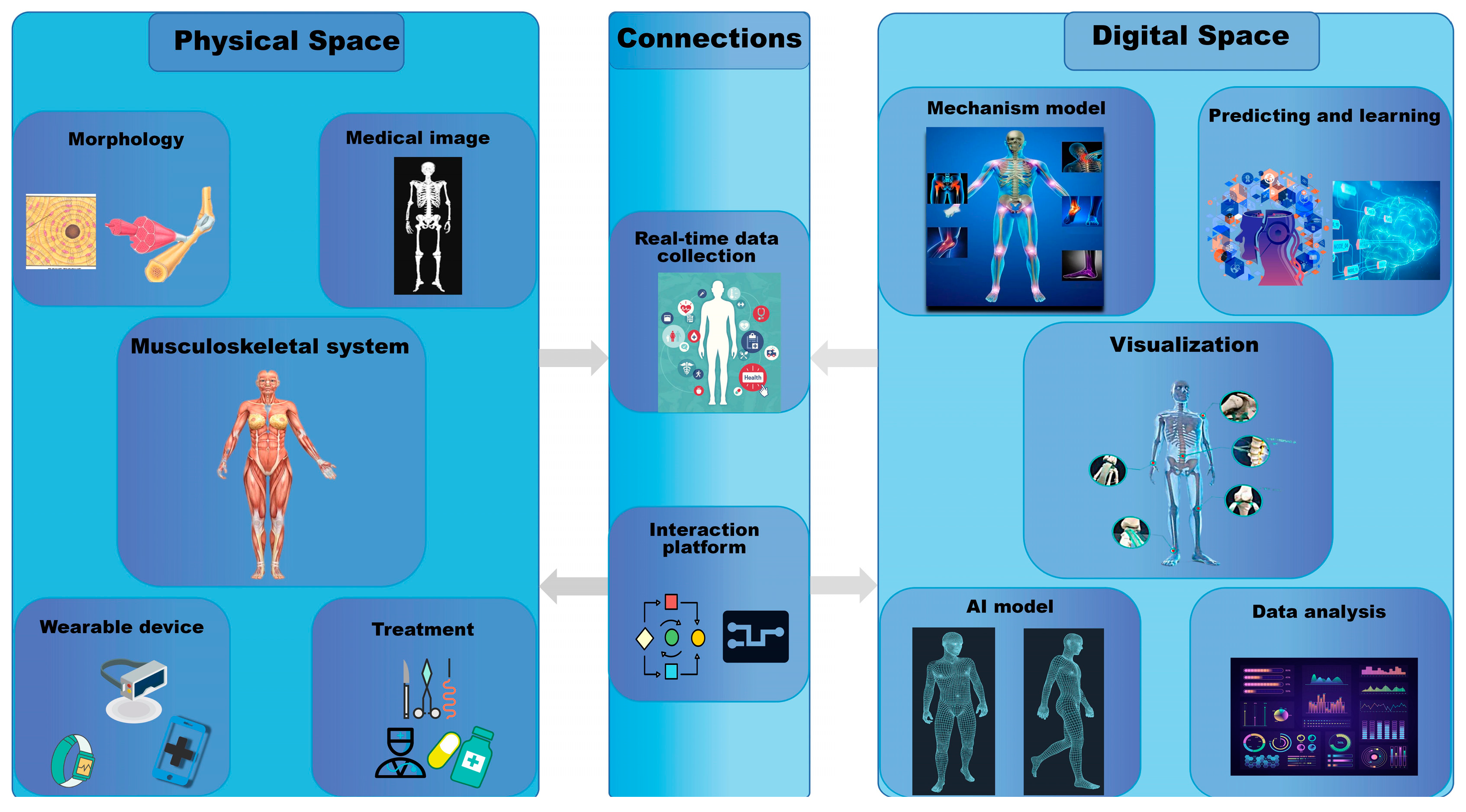
4. What Is Digital Twin?
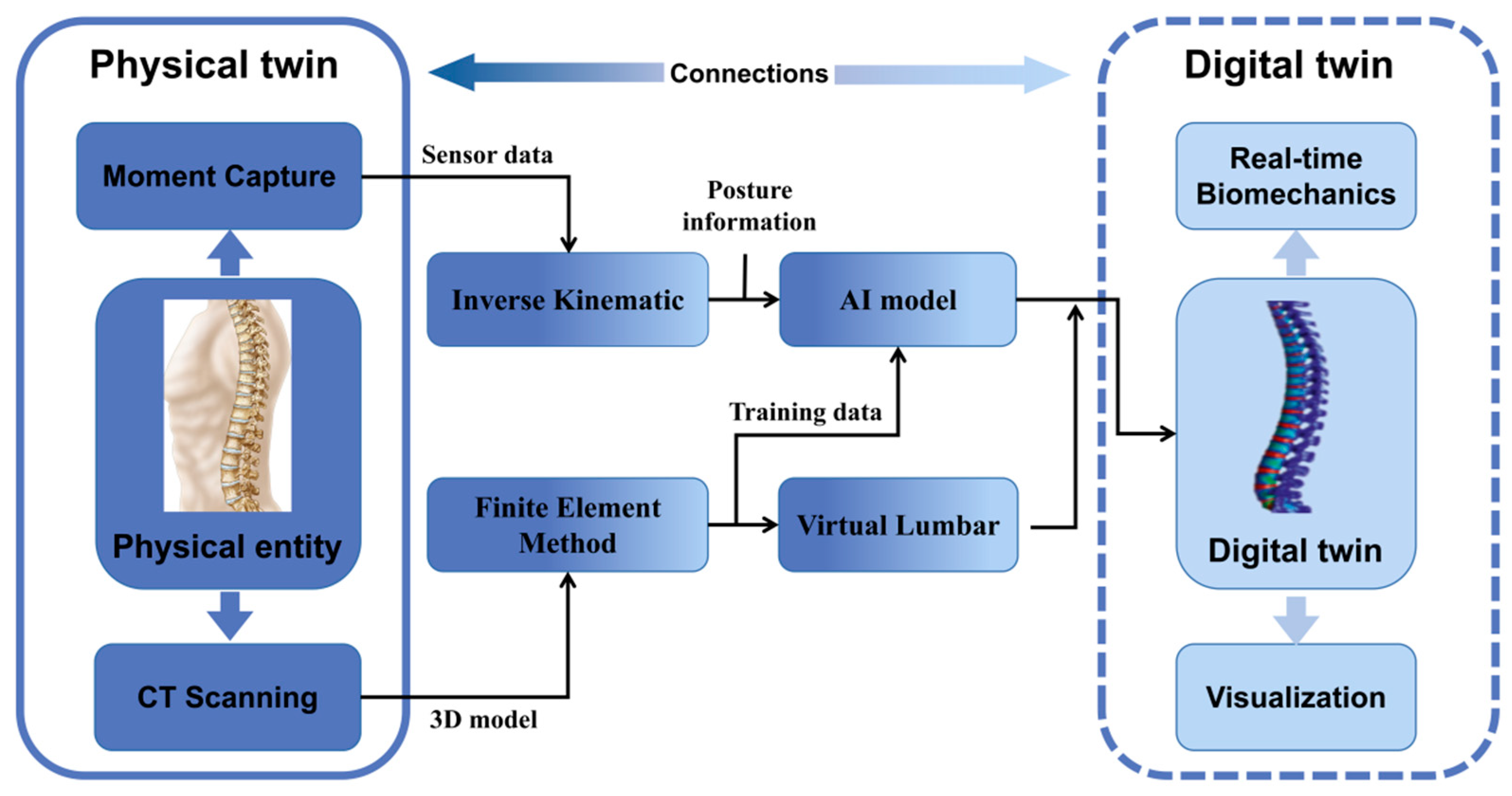
5. Current Applications of Digital Twin in the Musculoskeletal System
6. Disadvantages and Possible Improvements of DT
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronese, N.; Maggi, S. Epidemiology and social costs of hip fracture. Injury 2018, 49, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Cross, M.; Hill, C.; Smith, E.; Carson-Chahhoud, K.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Kaufman, J. Prevalence, Deaths, and Disability-Adjusted Life Years Due to Musculoskeletal Disorders for 195 Countries and Territories 1990–2017. Arthritis Rheumatol. 2021, 73, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Breen, A. Uneven intervertebral motion sharing is related to disc degeneration and is greater in patients with chronic, non-specific low back pain: An in vivo, cross-sectional cohort comparison of intervertebral dynamics using quantitative fluoroscopy. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2018, 27, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Glaessgen, E.; Stargel, D. The digital twin paradigm for future NASA and US Air Force vehicles. In Proceedings of the 53rd AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics and Materials Conference 20th AIAA/ASME/AHS Adaptive Structures Conference 14th AIAA, Honolulu, HI, USA, 23 April 2012; p. 1818. [Google Scholar]
- Tao, F.; Zhang, H.; Liu, A.; Nee, A.Y. Digital twin in industry: State-of-the-art. IEEE Trans. Ind. Inform. 2018, 15, 2405–2415. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, M.; Cheng, J.; Qi, Q. Digital twin workshop: A new paradigm for future workshop. Comput. Integr. Manuf. Syst. 2017, 23, 1–9. [Google Scholar]
- Sun, T.; He, X.; Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit. Health 2023, 9, 20552076221149651. [Google Scholar] [CrossRef]
- He, X.; Qiu, Y.; Lai, X.; Li, Z.; Shu, L.; Sun, W.; Song, X.J.D.T. Towards a shape-performance integrated digital twin for lumbar spine analysis. Digit. Twin 2021, 1, 8. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Song, X.; Shu, L.; Li, Z. The Digital Twin in Medicine: A Key to the Future of Healthcare? Front. Med. 2022, 9, 907066. [Google Scholar] [CrossRef]
- Pathria, M.N.; Chung, C.B.; Resnick, D.L. Acute and Stress-related Injuries of Bone and Cartilage: Pertinent Anatomy, Basic Biomechanics, and Imaging Perspective. Radiology 2016, 280, 21–38. [Google Scholar] [CrossRef]
- Lindsey, D.P.; Parrish, R.; Gundanna, M.; Leasure, J.; Yerby, S.A.; Kondrashov, D. Biomechanics of unilateral and bilateral sacroiliac joint stabilization: Laboratory investigation. J. Neurosurg. Spine 2018, 28, 326–332. [Google Scholar] [CrossRef]
- Nash, K.E.; Ong, K.G.; Guldberg, R.E. Implantable biosensors for musculoskeletal health. Connect. Tissue Res. 2022, 63, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.H.; Klein-Nulend, J.; Veldhuijzen, J.P. Mechanical stress and osteogenesis in vitro. J. Bone Miner. Res. 1992, 7 (Suppl. 2), S397–S401. [Google Scholar] [CrossRef]
- Chen, C.N.; Chang, H.I.; Yen, C.K.; Liu, W.L.; Huang, K.Y. Mechanical Stretch Induced Osteogenesis on Human Annulus Fibrosus Cells through Upregulation of BMP-2/6 Heterodimer and Activation of P38 and SMAD1/5/8 Signaling Pathways. Cells 2022, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
- Desmoulin, G.T.; Pradhan, V.; Milner, T.E. Mechanical Aspects of Intervertebral Disc Injury and Implications on Biomechanics. Spine (Phila Pa 1976) 2020, 45, E457–E464. [Google Scholar] [CrossRef]
- Vergroesen, P.P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef]
- Gellhorn, A.C.; Katz, J.N.; Suri, P. Osteoarthritis of the spine: The facet joints. Nat. Rev. Rheumatol. 2013, 9, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.; Adams, M.; Hutton, W. Disc space narrowing and the lumbar facet joints. J. Bone Jt. Surgery. Br. Vol. 1984, 66, 706–710. [Google Scholar] [CrossRef]
- Ke, S.; He, X.; Yang, M.; Wang, S.; Song, X.; Li, Z. The biomechanical influence of facet joint parameters on corresponding segment in the lumbar spine: A new visualization method. Spine J. Off. J. N. Am. Spine Soc. 2021, 21, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Welton, K.L.; Chahla, J.; LaPrade, R.F.; McCarty, E.C. Current Concepts of the Anterolateral Ligament of the Knee: Anatomy, Biomechanics, and Reconstruction. Am. J. Sports Med. 2018, 46, 1235–1242. [Google Scholar] [CrossRef]
- Stordeur, A.; Grange, S.; Servien, E.; Blache, Y.; Klasan, A.; Putnis, S.E.; Boyer, B.; Farizon, F.; Philippot, R.; Neri, T. Optimal Combination of Femoral Tunnel Orientation in Anterior Cruciate Ligament Reconstruction Using an Inside-out Femoral Technique Combined with an Anterolateral Extra-articular Reconstruction. Am. J. Sports Med. 2022, 50, 1205–1214. [Google Scholar] [CrossRef]
- Hu, B.W.; Lv, X.; Chen, S.F.; Shao, Z.W. Application of Finite Element Analysis for Investigation of Intervertebral Disc Degeneration: From Laboratory to Clinic. Curr. Med. Sci. 2019, 39, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, D.J.; Liu, Z.; Tang, B.; Zhong, Y.; Li, G.; Wan, Z. Motion characteristics of the lower lumbar spine in individuals with different pelvic incidence: An in vivo biomechanical study. Clin. Biomech. 2021, 88, 105419. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Bowen, L.; Kim, W.; Cai, L.; Schneider, S.E.; Nauman, E.A.; Neu, C.P. In vivo intervertebral disc deformation: Intratissue strain patterns within adjacent discs during flexion-extension. Sci. Rep. 2021, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- D'Lima, D.D.; Fregly, B.J.; Colwell, C.W., Jr. Implantable sensor technology: Measuring bone and joint biomechanics of daily life in vivo. Arthritis Res. Ther. 2013, 15, 203. [Google Scholar] [CrossRef]
- Phellan, R.; Hachem, B.; Clin, J.; Mac-Thiong, J.M.; Duong, L. Real-time biomechanics using the finite element method and machine learning: Review and perspective. Med. Phys. 2021, 48, 7–18. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Wang, L.; Du, Y.; Lu, T.J.; Xu, F. Engineering cell alignment in vitro. Biotechnol. Adv. 2014, 32, 347–365. [Google Scholar] [CrossRef]
- Zheng, H.D.; Sun, Y.L.; Kong, D.W.; Yin, M.C.; Chen, J.; Lin, Y.P.; Ma, X.F.; Wang, H.S.; Yuan, G.J.; Yao, M.; et al. Deep learning-based high-accuracy quantitation for lumbar intervertebral disc degeneration from MRI. Nat. Commun. 2022, 13, 841. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zhang, K.; Fung, K.M.; Thai, T.C.; Moore, K.; Mannel, R.S.; Liu, H.; Zheng, B.; Qiu, Y. Recent advances and clinical applications of deep learning in medical image analysis. Med. Image Anal. 2022, 79, 102444. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chang, C.W.; Li, C.T.; Chang, C.H.; Lin, C.F. Finite element analysis of plantar fascia during walking: A quasi-static simulation. Foot Ankle Int. 2015, 36, 90–97. [Google Scholar] [CrossRef]
- Genant, H.K.; Glüer, C.C.; Lotz, J.C. Gender differences in bone density, skeletal geometry, and fracture biomechanics. Radiology 1994, 190, 636–640. [Google Scholar] [CrossRef]
- Warnock, J.M.; Karayiannis, P.N.; Gallagher, N.E.; Hill, J.C.; Beverland, D.E. Are There Gender-Specific Errors in Restoration of Hip Biomechanics That Affect Outcome following Total Hip Arthroplasty? J. Arthroplast. 2020, 35, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.; Kopperdahl, D.L.; Lewis, C.E.; Saag, K.G.; Keaveny, T.M. Cost-Effectiveness of Osteoporosis Screening Using Biomechanical Computed Tomography for Patients With a Previous Abdominal CT. J. Bone Miner. Res. 2019, 34, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Keaveny, T.M.; Clarke, B.L.; Cosman, F.; Orwoll, E.S.; Siris, E.S.; Khosla, S.; Bouxsein, M.L. Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos. Int. 2020, 31, 1025–1048. [Google Scholar] [CrossRef] [PubMed]
- Grieves, M.; Vickers, J. Digital twin: Mitigating unpredictable, undesirable emergent behavior in complex systems. In Transdisciplinary Perspectives on Complex Systems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 85–113. [Google Scholar]
- Tao, F.; Zhang, M.; Liu, Y.; Nee, A.Y. Digital twin driven prognostics and health management for complex equipment. Cirp Ann. 2018, 67, 169–172. [Google Scholar] [CrossRef]
- Emmert-Streib, F.; Yli-Harja, O. What Is a Digital Twin? Experimental Design for a Data-Centric Machine Learning Perspective in Health. Int. J. Mol. Sci. 2022, 23, 13149. [Google Scholar] [CrossRef] [PubMed]
- Benson, M. Digital Twins for Predictive, Preventive Personalized, and Participatory Treatment of Immune-Mediated Diseases. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 410–416. [Google Scholar] [CrossRef]
- Coorey, G.; Figtree, G.A.; Fletcher, D.F.; Snelson, V.J.; Vernon, S.T.; Winlaw, D.; Grieve, S.M.; McEwan, A.; Yang, J.Y.H.; Qian, P.; et al. The health digital twin to tackle cardiovascular disease-a review of an emerging interdisciplinary field. NPJ Digit. Med. 2022, 5, 126. [Google Scholar] [CrossRef]
- Rahmim, A.; Brosch-Lenz, J.; Fele-Paranj, A.; Yousefirizi, F.; Soltani, M.; Uribe, C.; Saboury, B. Theranostic digital twins for personalized radiopharmaceutical therapies: Reimagining theranostics via computational nuclear oncology. Front. Oncol. 2022, 12, 1062592. [Google Scholar] [CrossRef]
- Pesapane, F.; Rotili, A.; Penco, S.; Nicosia, L.; Cassano, E. Digital Twins in Radiology. J. Clin. Med. 2022, 11, 6553. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; He, X.; Qiu, Y.; Song, X. Building a Trustworthy Product-Level Shape-Performance Integrated Digital Twin With Multifidelity Surrogate Model. J. Mech. Des. 2022, 144, 031703. [Google Scholar] [CrossRef]
- Rivera, L.F.; Jiménez, M.; Angara, P.; Villegas, N.M.; Tamura, G.; Müller, H.A. Towards continuous monitoring in personalized healthcare through digital twins. In Proceedings of the 29th Annual International Conference on Computer Science and Software Engineering, Toronto, ON, Canada, 4–6 November 2019; pp. 329–335. [Google Scholar]
- Corral-Acero, J.; Margara, F.; Marciniak, M.; Rodero, C.; Loncaric, F.; Feng, Y.; Gilbert, A.; Fernandes, J.F.; Bukhari, H.A.; Wajdan, A.; et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 2020, 41, 4556–4564. [Google Scholar] [CrossRef] [PubMed]
- Geris, L.; Lambrechts, T.; Carlier, A.; Papantoniou, I. The future is digital: In silico tissue engineering. Curr. Opin. Biomed. Eng. 2018, 6, 92–98. [Google Scholar] [CrossRef]
- Yang, D.; Karimi, H.R.; Kaynak, O.; Yin, S. Developments of digital twin technologies in industrial, smart city and healthcare sectors: A survey. Complex Eng. Syst. 2021, 1, 3. [Google Scholar] [CrossRef]
- Erol, T.; Mendi, A.F.; Doğan, D. The Digital Twin Revolution in Healthcare. In Proceedings of the 2020 4th International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT), Istanbul, Turkey, 22–24 October 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Hernigou, P.; Olejnik, R.; Safar, A.; Martinov, S.; Hernigou, J.; Ferre, B. Digital twins, artificial intelligence, and machine learning technology to identify a real personalized motion axis of the tibiotalar joint for robotics in total ankle arthroplasty. Int. Orthop. 2021, 45, 2209–2217. [Google Scholar] [CrossRef]
- Ahmadian, H.; Mageswaran, P.; Walter, B.A.; Blakaj, D.M.; Bourekas, E.C.; Mendel, E.; Marras, W.S.; Soghrati, S. A Digital Twin for Simulating the Vertebroplasty Procedure and its Impact on Mechanical Stability of Vertebra in Cancer Patients. Int. J. Numer. Method. Biomed. Eng. 2022, 38, e3600. [Google Scholar] [CrossRef]
- Ahmadian, H.; Mageswaran, P.; Walter, B.A.; Blakaj, D.M.; Bourekas, E.C.; Mendel, E.; Marras, W.S.; Soghrati, S. Toward an artificial intelligence-assisted framework for reconstructing the digital twin of vertebra and predicting its fracture response. Int. J. Numer. Method. Biomed. Eng. 2022, 38, e3601. [Google Scholar] [CrossRef]
- Hernigou, P.; Safar, A.; Hernigou, J.; Ferre, B. Subtalar axis determined by combining digital twins and artificial intelligence: Influence of the orientation of this axis for hindfoot compensation of varus and valgus knees. Int. Orthop. 2022, 46, 999–1007. [Google Scholar] [CrossRef]
- Aubert, K.; Germaneau, A.; Rochette, M.; Ye, W.; Severyns, M.; Billot, M.; Rigoard, P.; Vendeuvre, T. Development of Digital Twins to Optimize Trauma Surgery and Postoperative Management. A Case Study Focusing on Tibial Plateau Fracture. Front. Bioeng. Biotechnol. 2021, 9, 722275. [Google Scholar] [CrossRef]
- Bruynseels, K. When nature goes digital: Routes for responsible innovation. J. Responsible Innov. 2020, 7, 342–360. [Google Scholar] [CrossRef]
- Popa, E.O.; van Hilten, M.; Oosterkamp, E.; Bogaardt, M.-J. The use of digital twins in healthcare: Socio-ethical benefits and socio-ethical risks. Life Sci. 2021, 17, 6. [Google Scholar] [CrossRef]
- Huang, P.H.; Kim, K.H.; Schermer, M. Ethical Issues of Digital Twins for Personalized Health Care Service: Preliminary Mapping Study. J. Med. Internet Res. 2022, 24, e33081. [Google Scholar] [CrossRef]
- Sisodiya, S.M. Precision medicine and therapies of the future. Epilepsia 2021, 62 (Suppl. 2), S90–S105. [Google Scholar] [CrossRef]
- Coorey, G.; Figtree, G.A.; Fletcher, D.F.; Redfern, J. The health digital twin: Advancing precision cardiovascular medicine. Nat. Rev. Cardiol. 2021, 18, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Kamel Boulos, M.N.; Zhang, P. Digital Twins: From Personalised Medicine to Precision Public Health. J. Pers. Med. 2021, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Peirlinck, M.; Costabal, F.S.; Yao, J.; Guccione, J.M.; Tripathy, S.; Wang, Y.; Ozturk, D.; Segars, P.; Morrison, T.M.; Levine, S.; et al. Precision medicine in human heart modeling: Perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 2021, 20, 803–831. [Google Scholar] [CrossRef] [PubMed]
- Lauzeral, N.; Borzacchiello, D.; Kugler, M.; George, D.; Rémond, Y.; Hostettler, A.; Chinesta, F. A model order reduction approach to create patient-specific mechanical models of human liver in computational medicine applications. Comput. Methods Programs Biomed. 2019, 170, 95–106. [Google Scholar] [CrossRef]
- MacLean, A.L.; Harrington, H.A.; Stumpf, M.P.; Byrne, H.M. Mathematical and Statistical Techniques for Systems Medicine: The Wnt Signaling Pathway as a Case Study. Methods Mol. Biol. 2016, 1386, 405–439. [Google Scholar] [CrossRef]
- Gunasegaram, D.; Murphy, A.; Barnard, A.; DebRoy, T.; Matthews, M.; Ladani, L.; Gu, D. Towards developing multiscale-multiphysics models and their surrogates for digital twins of metal additive manufacturing. Addit. Manuf. 2021, 46, 102089. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chung, P.-S.; Hwang, M.-S. A Survey on Attribute-based Encryption Schemes of Access Control in Cloud Environments. Int. J. Netw. Secur. 2013, 15, 231–240. [Google Scholar]
- Liu, Y.; Zhang, L.; Yang, Y.; Zhou, L.; Ren, L.; Wang, F.; Liu, R.; Pang, Z.; Deen, M.J. A novel cloud-based framework for the elderly healthcare services using digital twin. IEEE Access 2019, 7, 49088–49101. [Google Scholar] [CrossRef]
- Armeni, P.; Polat, I.; De Rossi, L.M.; Diaferia, L.; Meregalli, S.; Gatti, A. Digital Twins in Healthcare: Is It the Beginning of a New Era of Evidence-Based Medicine? A Critical Review. J. Pers. Med. 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Masison, J.; Beezley, J.; Mei, Y.; Ribeiro, H.; Knapp, A.C.; Sordo Vieira, L.; Adhikari, B.; Scindia, Y.; Grauer, M.; Helba, B.; et al. A modular computational framework for medical digital twins. Proc. Natl. Acad. Sci. USA 2021, 118, e2024287118. [Google Scholar] [CrossRef] [PubMed]

| Method | Advantage | Disadvantage |
|---|---|---|
| Morphology | More accurate structural features can be presented based on anatomical and imaging techniques. | Invasive, ethical and safety issues. |
| Sensers | Quantitative evaluation of biomechanical changes in human body parameters through digital simulations. | Sensor volume and safety issues. |
| Animal model | Similar to the human body and avoids ethical barriers. | Low reproducibility of animal models and tissue cultures. |
| Finite element analysis | Reproducible, quantifiable, and non-invasive. | Low simulation accuracy and quasi-static analysis. |
| Digital twin | Reproducible, quantitative, personalized, and dynamic analysis. | Lack of standardization, high cost, and immature application. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Wang, J.; Suo, M.; Liu, X.; Huang, H.; Zhang, J.; Zhang, W.; Li, Z. The Digital Twin: A Potential Solution for the Personalized Diagnosis and Treatment of Musculoskeletal System Diseases. Bioengineering 2023, 10, 627. https://doi.org/10.3390/bioengineering10060627
Sun T, Wang J, Suo M, Liu X, Huang H, Zhang J, Zhang W, Li Z. The Digital Twin: A Potential Solution for the Personalized Diagnosis and Treatment of Musculoskeletal System Diseases. Bioengineering. 2023; 10(6):627. https://doi.org/10.3390/bioengineering10060627
Chicago/Turabian StyleSun, Tianze, Jinzuo Wang, Moran Suo, Xin Liu, Huagui Huang, Jing Zhang, Wentao Zhang, and Zhonghai Li. 2023. "The Digital Twin: A Potential Solution for the Personalized Diagnosis and Treatment of Musculoskeletal System Diseases" Bioengineering 10, no. 6: 627. https://doi.org/10.3390/bioengineering10060627
APA StyleSun, T., Wang, J., Suo, M., Liu, X., Huang, H., Zhang, J., Zhang, W., & Li, Z. (2023). The Digital Twin: A Potential Solution for the Personalized Diagnosis and Treatment of Musculoskeletal System Diseases. Bioengineering, 10(6), 627. https://doi.org/10.3390/bioengineering10060627








