Abstract
Objectives: To assess the effectiveness of two consecutive intraarticular injections of PRP to treat knee osteoarthritis (KOA), discriminating between responders and impaired patients. Methods: This retrospective study included 73 consecutive patients who were referred for two intra-articular PRP injections (one week apart) for treating symptomatic moderate/severe KOA. Biological characterization of the PRP, including platelets, leukocytes and erythrocytes, was evaluated. Patient’s subjective symptoms were recorded before the treatment and 1 year after the second injection using pain VAS and WOMAC scores. Responders were defined by an improvement of 10 points on WOMAC. Results: At a 1-year follow up, we found 36 (49.3%) patients who fulfilled the criteria of responders, and 21 (28.8%) patients were impaired. A statistically and clinically significant global improvement of −29.2 ± 14.3 (p < 0.001) points in WOMAC score was observed 1 year after treatment in the responder group, with a higher response rate in patients with KL 2 (57.7%) compared to KL IV (28.6%). The percentage of patients with KL IV was higher in the impaired group (48.0%) compared to the responders (16.6%). As expected, the evaluation of the functionality of the knee in the impaired group indicates that it significantly worsened after one year from treatment (p = 0.027). However, the average pain score remained stable with no significant differences after 1 year (p = 0.843). No clinical complications or severe adverse events after the PRP injections were reported. Conclusion: The present study suggests that two intra-articular injections of 10 mL of very pure PRP provide pain and functional improvement in symptomatic KOA.
1. Introduction
Knee osteoarthritis (KOA) is a common disease associated with global knee pain and limiting flexion-related activities of daily living and exercise. It is caused by the loss of the cartilage typically in a combination of degeneration of the articular cartilage as well as abnormal biomechanical tracking of anterior, medial or, most closely, in the lateral knee parts [1,2]. Nevertheless, KOA is not limited to the cartilage and bone component, but rather affects the whole joint compartment, including the synovia and the ligaments [3]. From an epidemiology point of view, KOA prevalence is around 10% in men and 13% in women in the population over 60 years of age, which means that KOA is one of the main disability causes in old people [4]. Despite its prevalence, treatment of this painful disorder is challenging due to the variety of causes and the lack of knowledge on articular cartilage regeneration [5]. Symptomatic and conservative treatments are the main therapeutic modalities for patients with KOA. They include options such as antalgic and anti-inflammatory drugs, weight loss, physical therapy, bracing and intra-articular injections [6]. If these therapies fail, a select group of patients may require surgical intervention, such as patellofemoral replacement or complete knee arthroplasty [7,8]. A large effort has been put into finding conservative treatments able to delay natural osteoarthritis progression, with the aim of avoiding or postponing the need of important surgical interventions, such as total knee prostheses. Various clinical trials have shown that a single intra-articular injection of platelet-rich plasma (PRP) is an effective therapy for improving the clinical conditions of patients suffering from knee OA [9,10,11]. However, only a few reports have studied the effect of more than one injection of PRP [12,13,14,15,16]. In the present study, we examined retrospectively the effectiveness of two consecutive intraarticular injections of PRP to treat KOA, discriminating between responders and impaired patients.
2. Materials and Methods
2.1. Patients
The authors retrospectively reviewed 73 patients with knee OA from February 2018 to March 2020, who were aged between 20 and 85 years old and referred to our institution by sports medicine and orthopedic wards (secondary care) for intra-articular injection of PRP after failure of initial treatment with oral non-steroidal anti-inflammatory drugs and conservative physiotherapy treatment. Demographic, clinical and radiological variables such as age, sex, body mass index (BMI) and degree of radiological involvement were collected.
Inclusion criteria included patients with persistent non-traumatic KOA lasting more than 3 months, with pathologic X-ray images (at least grade II Kellgren and Lawrence), who underwent two consecutive intra-articular infiltrations of PRP and had a clinical assessment 1 year after treatment. Exclusion criteria were previous intra-articular treatment (i.e., corticoid or hyaluronic acid injections) or surgical treatment. Also, patients who received additional intra-articular treatment or surgical treatment during follow-up were excluded.
The study was registered with the “Agence Nationale de Sécurité du Médicament” (ANSM, registration number 2017-A01894-49) and in accordance with the Helsinki declaration about medical research. This retrospective study was approved by our Institutional Ethics Review Board (reference number 06-2019.2).
2.2. PRP Preparation and Infiltration
PRP treatments were 2 injections one week apart. The same two operators (AS or BD) specialized in musculoskeletal radiology performed PRP preparation, PRP injections and evaluations. For PRP preparation, Hy-Tissue PRP® 50 (Fidia Farmaceutici SpA, Abano Terme, Italy) was employed. In brief, 45 mL of peripheral blood was collected and anticoagulated with 5 mL of 3.8% sodium citrate. The 50 mL citrated blood was centrifuged at 1800 rpm for 8 min using Duografter® II (Fidia Farmaceutici SpA, Italy) and 10 mL of very pure PRP was obtained according to the manufacturer’s instructions. For quality control, 0.5 mL of PRP was used for analysis of platelets (PLT), red blood cells (RBC), and leukocytes (WBC) using an automated hematology analyzer (Stel3—Linear Chemical, Barcelona, Spain). Intra-articular injection of non-activated PRP was performed 30 min after obtention using a 21-gauge needle. Real-time ultrasound control and absence of resistance during injection were used to confirm intra-articular needle position. The patient was observed for two hours following the procedure to record any adverse event. The same procedure was repeated 1 week later. General activity involving physical exertion was forbidden for 1 month.
2.3. Clinical Data Assessment
Patients were examined in terms of pain and knee function. We have implemented in our routine service the systematic determination of the subjective scores VAS (Visual Analog Scale for pain) and WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index for global knee function evaluation) for all patients suffering from KOA. VAS and WOMAC tests were used to assess clinical outcomes by handing the respective questionnaires to every patient. Responders were defined as patients presenting an improvement of at least 10 points in WOMAC compared to baseline. According to Ehrich et al. (2000), a level of 10 points or more of improvement or decline on WOMAC is suggested as a cut-off representing a clinically significant difference (minimal perceptible clinical improvement or MPCI) [17]. Impaired patients were defined as patients presenting an impairment of at least 1 point compared to baseline in WOMAC total score.
2.4. Radiographic Assessment
Prior to therapy, patients had front and lateral radiographs taken. Kellgren–Lawrence grade was assessed by the same 2 experienced radiologists (AS and BD). A consensus meeting was held in all situations where one radiologist assessed the radiograph with one rating lower or higher than the other.
2.5. Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla, CA, USA. Discrete variables are described as a number and percentage and were compared between groups using the Fisher’s test. Continuous variables were described by means and standard deviations. Data symmetry was analyzed using D’Agostino and Pearson tests. Parametric tests (paired t-test) were used for normal distributions and the Wilcoxon matched-pairs signed-rank test (paired data) or Mann–Whitney test (unpaired data) for non-Gaussian distributions. All tests were two-sided. For all tests, p < 0.05 was considered significant.
3. Results
3.1. Patients
In this retrospective study, 73 patients with knee OA who received intraarticular injections of leukocyte-poor PRP (LP-PRP) from February 2018 to March 2020 in our center fulfilled all the defined inclusion/exclusion criteria. Among them, 37 were female (51%) and 36 were male (49%). The mean body mass index (BMI) was 26 ± 3.7 kg/m2. The mean WOMAC score at baseline was 38.3 ± 20.7. The mean pain (VAS) at baseline was 5.5 ± 2.1. Radiographic analysis revealed that 52 (71%) presented with grade II/III knee OA and 21 (29%) of patients presented with grade IV according to the classification of Kellgren and Laurence (KL).
3.2. Demographic Assessment: Comparison of Responders, Non-Responders and Impaired Patients
Of the total (n = 73), 36 (49.3%) patients fulfilled the criteria of responders, 37 (50.7%) of non-responders and 21 (28.8%) of impaired. Responders and impaired groups were similar in terms of age, sex ratio, BMI and baseline pain (Table 1). However, the WOMAC score was significatively higher at baseline in the responders compared to impaired (p = 0.021). The percentage of patients with KL IV was higher in the impaired group (48.0%) compared to the responders (16.6%). Considering only patients with KL II/III (n = 52), 30 (57.7%) patients fulfilled the criteria of responders, 22 (42.3%) of non-responders and 11 (21.2%) of impaired. Considering only patients with KL IV, (n = 21), 6 (28.6%) patients fulfilled the criteria of responders, 15 (71.4%) of non-responders and 10 (47.6%) of impaired.

Table 1.
Baseline clinical characteristics and demographics of responders and impaired patients.
3.3. Biological Characteristics of PRP Injected
Peripheral blood aspiration was standardized to 45 mL, always yielding a fixed volume of PRP (10 mL) after PPP (platelet-poor plasma) separation. PRP characterization and doses of the first and second infiltration for responder or impaired patient groups are summarized in Table 2.

Table 2.
Biological characteristics of PRP injected (N = 73) responders and impaired patients.
There were no significant differences in the PLT, RBC or WBC doses administered in each PRP injection. The preparation process leads to the obtention of a PRP with a high platelet composition (>95%) and a very good reproducibility regarding platelet purity (coefficient of variation <3%). The PRP obtained using this method is classified as ABA (leucocyte-poor (LP-PRP) based on the DEPA classification system [18].
3.4. Clinical Assessment
A statistically significant overall global improvement of −11.8 ± 21.3 (p < 0.001) points in WOMAC score was observed 1 year after treatment. This significative improvement was also observed in VAS pain change (−2.3 ± 2.6; p < 0.001) (Figure 1).
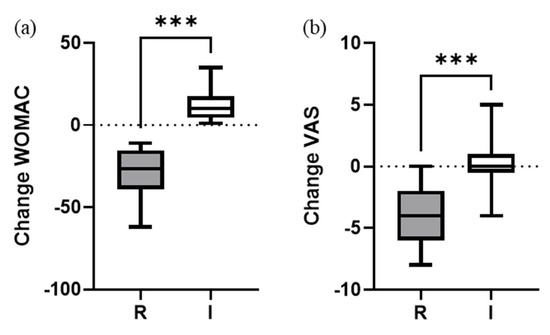
Figure 1.
Change of (a) WOMAC and (b) VAS pain scores 1 year after PRP treatment. Absolute variation from baseline. Box and whisker plots represent the median, the lower and upper quartile, and the min and max, respectively. Mann–Whitney test (two-tailed) was used to compare groups. *** (p < 0.001). R = responders; I = impaired.
The percentage of responder patients was similar to non-responders (49% vs. 51%, respectively). When comparing responder vs. impaired (Table 3), we found significant change improvements in WOMAC and VAS scores for responders (p < 0.001). As expected, the evaluation of the functionality of the knee in the impaired group showed that it significantly worsened one year after the treatment (p = 0.027). However, the average pain score remained stable with no significant differences after 1 year (p = 0.843). No clinical complications or severe adverse events after the PRP injections were reported.

Table 3.
Means at baseline and at 1-year follow up and significance of intergroup changes measured as VAS pain and WOMAC score. VAS: visual analog scale. WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index. Data are provided as mean ± SD (range). CI: confidence interval; 95% CI referred to change.
4. Discussion
This study allowed an overall assessment of the efficacy of two consecutive intraarticular infiltrations of PRP in our routine care. In brief, the administration of two intraarticular injections of a high volume of autologous pure PRP with an average platelet dose provided significant clinical benefits (responders) to 49.3% of patients with KOA with grade II to IV one year after treatment. This result could be attributed to the inclusion of patients with severe KOA. In fact, the proportion of responder patients with KOA was 57.6% and 28.6% for KL II/III and IV, respectively. Other authors also reported that severe forms of KOA respond less well to PRP treatments. Saita et al. (2021) [19] performed a retrospective study with a higher number of patients (517), assessing the effectiveness of three injections of PRP (LP-PRP; 4–5 mL, average PLT concentration = 475.4 × 106/mL) for treating KOA grades II/III/IV. The responder rate according to OMERACT-OARSI responder criteria at 12 months was 75.2%, 66.35, and 50.9% for patients with KOA grade II, III and IV, respectively, demonstrating that the severity of KOA was a predictor of poor PRP therapy outcomes. A different result was obtained by Chopin et al. (2023) [20] who studied the clinical benefits at 7 months after two consecutive intraarticular injections of PRP (3–6 mL, LP-PRP) on 197 patients with moderate–severe KOA (KL II/III/IV), showing no statistical differences between the number of responders vs. non responders in the function of the severity of the KOA.
A similar study was conducted by Bec et al. (2021) [21] who also targeted KOA grades II/III/IV but only found a responder rate (based on an increase in KOOS change in 10 points) of 45% at 6 months (34 responders/75 patients). This study differs from the current one on several points: (a) the final follow up was shorter (6 months), (b) the mean volume of pure PRP injected, dose of platelets and the number of interventions was lower compared to our protocol (6.8 mL PRP; 2.6 × 109 platelet dose, one single injection, respectively), and (c) the responders definition was not only based on WOMAC score but using the OMERACT OARSI criteria (Outcome Measures in Rheumatology, Osteoarthritis Research Society International) [22], which also considers pain and patients global assessment. Another similar study was conducted by Guillibert et al. [23] where the responder rate reached 80% based on a responder rate definition based on the KOOS score at a 6-month follow up. This study employed a very pure PRP volume injection more similar to our study (8.8 mL) but lower PLT dose (2.6 × 109) and only a single injection. Additionally, the study included only patients with mild to moderate KOA (KL II/III), excluding those with severe KO (KL IV). In our series, we employed a higher volume of very pure PRP in each knee injection (10 mL) based on the articular capacity of the knee to favor a better distribution of PRP throughout the joint [24,25]. We also consider that the inclusion of patients with KOA KL IV is important because it gives a broader idea about the effectiveness of the treatment in the early or late stages of osteoarthritis. The concept of very pure PRP was suggested by Magalon et al. [18], where it was considered that PRP is very pure when the relative composition of platelets is greater than 90%. The characterization of the PRP in our study showed that the very pure PRP consisted of a high volume of plasma with a high quantity of platelets and very low WBC and RBC content; the latter is known for being potentially harmful when injected into a knee [26,27,28]. It is remarkable that the dose of PLT used in our study was on average two times higher than the dose employed by Guillibert et al. and Beck et al. Even though up to now it has not been clearly demonstrated that platelet dose correlates with clinical effectiveness [29,30,31,32], we believe that this factor could be additionally relevant for obtaining a high rate of responders with a high level of functional improvement of the knee at 1 year after treatment (mean WOMAC change of 67%). However, Bansal et al. (2021) [33] employed a higher PLT dose (10 × 109; 10 mL PRP; one single intraarticular injection) for treating patients with KOA KL II/III, showing a small improvement in function and pain scores at a 1-year follow up (WOMAC: baseline: 55.0 (52–56); 1 year: 51.9 ± 7.4; IKDC: baseline: 53.6 ± 6.34; 1 year: 62.8 ± 6.24). It seems that one of the factors that could be more relevant for influencing the magnitude of clinical efficacy is the number of PRP infiltrations rather than the PLT dose. It has been reported in a recent meta-analysis of randomized controlled trials (RCTs) that multiple PRP cycles seems to be more effective than a single injection [34]. A different strategy that could have been used to improve the number of responding patients in severe KOA consists of a combined protocol of intraosseous and intraarticular PRP infiltrations [35,36]. This technique has shown a greater number of responders compared to the intra-articular injection technique [37]. As a retrospective study, the absence of a placebo or control group is an important limitation. Even though the patients included in this study suffered from chronic knee pain not responding to other known conservative therapies for more than a year, the effectiveness of the PRP therapy itself could have been attributed, at least in part, to the placebo effect. Nevertheless, a retrospective study can be considered acceptable for investigating the determinants of PRP therapy effectiveness, as well as for determining to what extent its effectiveness depends on KOA severity. The absence of a periodic follow up until 1 year without intermediate assessments could also be a source of bias. We have not included data on the correlation of platelet dose with clinical efficacy because (a) the platelet dose interval was quite narrow (Table 2) and (b) in our opinion, it is not possible to calculate a correlation between the change in VAS or WOMAC at a specific follow up time to a PLT dose since we had two consecutive interventions with two different PLT doses. Despite these limitations, the study provided further insights towards a better understanding of the biological activity of a high volume, very pure PRP injection for treating KOA.
5. Conclusions
The present study suggests that two intra-articular injections at a high volume of very pure PRP provides pain and functional improvements in moderate symptomatic KOA in 49% of the patients after 1 year of the treatment, apparently with better results in patients with grade II osteoarthritis, with a low percentage of impaired patients (29%). More studies will be required to confirm our findings and to elucidate the reasons why there are patients who do not respond or even worsen after receiving PRP treatment.
Author Contributions
Conceptualization, A.S. and B.D.; Data curation, A.S., P.-F.L., L.P., P.M., M.-H.M.-D. and B.D.; Methodology, A.S. and B.D.; Formal analysis, A.S. and B.D.; Visualization, A.S. and B.D.; Validation; A.S. and B.D.; Supervision, A.S. and B.D.; Writing—original draft, B.D.; and Writing—review and editing, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
The study was registered with the “Agence Nationale de Sécurité du Médicament” (ANSM, registration number 2017-A01894-49) and in accordance with the Helsinki declaration about medical research. This retrospective study was approved by our Institutional Ethics Review Board (reference number 06-2019.2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are available upon reasonable request addressed to the corresponding author.
Acknowledgments
The authors acknowledge Luis Vidal for carefully reading the manuscript.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
| PRP | Platelet-rich plasma |
| VAS | Visual Analogue Scale |
| WOMAC | Western Ontario, McMaster Universities Osteoarthritis Index |
| KOA | Knee osteoarthritis |
| MPCI | Minimal perceptible clinical improvement |
References
- Kobayashi, S.; Pappas, E.; Fransen, M.; Refshauge, K.; Simic, M. The prevalence of patellofemoral osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2016, 24, 1697–1707. [Google Scholar] [CrossRef]
- van Jonbergen, H.-P.W.; Poolman, R.W.; van Kampen, A. Isolated patellofemoral osteoarthritis. Acta Orthop. 2010, 81, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Joo, Y.-B. Patellofemoral osteoarthritis. Knee Surg. Relat. Res. 2012, 24, 193–200. [Google Scholar] [CrossRef]
- Lonner, J.H.; Bloomfield, M.R. The clinical outcome of patellofemoral arthroplasty. Orthop. Clin. N. Am. 2013, 44, 271–280. [Google Scholar] [CrossRef]
- Mont, M.A.; Haas, S.; Mullick, T.; Hungerford, D.S. Total knee arthroplasty for patellofemoral arthritis. J. Bone Jt. Surg. Am. 2002, 84, 1977–1981. [Google Scholar] [CrossRef]
- Laskin, R.S.; van Steijn, M. Total knee replacement for patients with patellofemoral arthritis. Clin. Orthop. Relat. Res. 1999, 367, 89–95. [Google Scholar]
- Hohmann, E.; Tetsworth, K.; Glatt, V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 955–967. [Google Scholar] [CrossRef]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef]
- Saraf, A.; Hussain, A.; Bishnoi, S.; Azam, G.; Habib, H. Serial Platelet-Rich Plasma Intra-articular Injections in Kellgren and Lawrence Grade IV Knee Joint Osteoarthritis: A Prospective Blinded Placebo-Controlled Interventional Study. Indian. J. Orthop. 2022, 56, 1722–1728. [Google Scholar] [CrossRef]
- Huda, N.; Islam, M.S.U.; Bishnoi, S.; Kumar, H.; Aggarwal, S.; Ganai, A.A. Role of Triple Injection Platelet-Rich Plasma for Osteoarthritis Knees: A 2 Years Follow-Up Study. Indian. J. Orthop. 2022, 56, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dhillon, M.S.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.T.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Leukocyte-poor PRP application for the treatment of knee osteoarthritis. Joints 2013, 1, 112–120. [Google Scholar] [PubMed]
- Vaquerizo, V.; Padilla, S.; Aguirre, J.J.; Begona, L.; Orive, G.; Anitua, E. Two cycles of plasma rich in growth factors (PRGF-Endoret) intra-articular injections improve stiffness and activities of daily living but not pain compared to one cycle on patients with symptomatic knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Ehrich, E.W.; Davies, G.M.; Watson, D.J.; Bolognese, J.A.; Seidenberg, B.C.; Bellamy, N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J. Rheumatol. 2000, 27, 2635–2641. [Google Scholar]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport. Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef]
- Saita, Y.; Kobayashi, Y.; Nishio, H.; Wakayama, T.; Fukusato, S.; Uchino, S.; Momoi, Y.; Ikeda, H.; Kaneko, K. Predictors of Effectiveness of Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4514. [Google Scholar] [CrossRef]
- Chopin, C.; Geoffroy, M.; Kanagaratnam, L.; Dorilleau, C.; Ecarnot, F.; Siboni, R.; Salmon, J.H. Prognostic Factors Related to Clinical Response in 210 Knees Treated by Platelet-Rich Plasma for Osteoarthritis. Diagnostics 2023, 13, 760. [Google Scholar] [CrossRef]
- Bec, C.; Rousset, A.; Brandin, T.; François, P.; Rabarimeriarijaona, S.; Dumoulin, C.; Heleu, G.; Grimaud, F.; Veran, J.; Magalon, G.; et al. A Retrospective Analysis of Characteristic Features of Responders and Impaired Patients to a Single Injection of Pure Platelet-Rich Plasma in Knee Osteoarthritis. J. Clin. Med. 2021, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; van der Heijde, D.; Altman, R.D.; Anderson, J.J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef]
- Guillibert, C.; Charpin, C.; Raffray, M.; Benmenni, A.; Dehaut, F.X.; El Ghobeira, G.; Giorgi, R.; Magalon, J.; Arniaud, D. Single Injection of High Volume of Autologous Pure PRP Provides a Significant Improvement in Knee Osteoarthritis: A Prospective Routine Care Study. Int. J. Mol. Sci. 2019, 20, 1327. [Google Scholar] [CrossRef]
- Rastogi, A.K.; Davis, K.W.; Ross, A.; Rosas, H.G. Fundamentals of Joint Injection. AJR Am. J. Roentgenol. 2016, 207, 484–494. [Google Scholar] [CrossRef]
- Huang, G.; Hua, S.; Yang, T.; Ma, J.; Yu, W.; Chen, X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018, 15, 3096–3102. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Hooiveld, M.J.; Roosendaal, G.; van den Berg, H.M.; Bijlsma, J.W.; Lafeber, F.P. Haemoglobin-derived iron-dependent hydroxyl radical formation in blood-induced joint damage: An in vitro study. Rheumatology 2003, 42, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.T.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single-versus double-spinning approach. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2082–2091. [Google Scholar] [CrossRef]
- Joshi Jubert, N.; Rodriguez, L.; Reverte-Vinaixa, M.M.; Navarro, A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop. J. Sports Med. 2017, 5, 2325967116689386. [Google Scholar] [CrossRef]
- Louis, M.L.; Magalon, J.; Jouve, E.; Bornet, C.E.; Mattei, J.C.; Chagnaud, C.; Rochwerger, A.; Veran, J.; Sabatier, F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared with Viscosupplementation. Arthroscopy 2018, 34, 1530–1540.e1532. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Goyal, T.; Verma, N.; Paswan, A.K.; Dubey, R.K. Intradiscal Platelet-Rich Plasma Injection for Discogenic Low Back Pain and Correlation with Platelet Concentration: A Prospective Clinical Trial. Pain Med. 2020, 21, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Vilchez-Cavazos, F.; Millán-Alanís, J.M.; Blázquez-Saldaña, J.; Álvarez-Villalobos, N.; Peña-Martínez, V.M.; Acosta-Olivo, C.A.; Simental-Mendía, M. Comparison of the Clinical Effectiveness of Single Versus Multiple Injections of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2019, 7, 2325967119887116. [Google Scholar] [CrossRef]
- Sanchez, M.; Delgado, D.; Sanchez, P.; Muinos-Lopez, E.; Paiva, B.; Granero-Molto, F.; Prosper, F.; Pompei, O.; Perez, J.C.; Azofra, J.; et al. Combination of Intra-Articular and Intraosseous Injections of Platelet Rich Plasma for Severe Knee Osteoarthritis: A Pilot Study. Biomed. Res. Int. 2016, 2016, 4868613. [Google Scholar] [CrossRef]
- Ríos Luna, A.; Fahandezh-Saddi Díaz, H.; Villanueva Martinez, M.; Prado, R.; Padilla, S.; Anitua, E. Office-Based Intraosseous Infiltrations of PRGF in Knee Osteoarthritis: Description of Technique. Arthrosc. Tech. 2022, 11, e917–e921. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Jorquera, C.; de Dicastillo, L.L.; Fiz, N.; Knörr, J.; Beitia, M.; Aizpurua, B.; Azofra, J.; Delgado, D. Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: A prospective observational study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720x221100304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).